In the normal foetus, highly oxygenated blood from the placenta enters the foetal abdomen via the umbilical vein. The umbilical vein courses towards the liver and joins the portal sinus, which is the confluence of the portal veins and the venous duct (Fig 1). The venous duct is a linear vessel that appears smaller or more constricted compared with the adjacent portal vein structures, and connects to the inferior caval vein near the junction of the hepatic veins to the right atrium. The venous duct is a vital component of the foetal venous circulation and can be considered a “bypass” or resistance structure connecting the umbilical vein directly to the inferior vena cava without flowing through the hepatic circulation. Approximately 20–30% of umbilical venous blood flow is shunted through the venous duct at any given time and is also dynamic in response to in utero regulatory measures.Reference Kiserud, Rasmussen and Skulstad 1 As the blood in the venous duct has not undergone oxygen extraction in the liver, oxygen levels are higher than the blood from the inferior caval vein and hepatic veins. Flow acceleration from the venous duct likely contributes to the streaming action of the oxygen-rich umbilical venous flow preferentially through the foramen ovale towards the left atrium and ventricle to supply cerebral and coronary artery circulations.Reference Edelstone and Rudolph 2
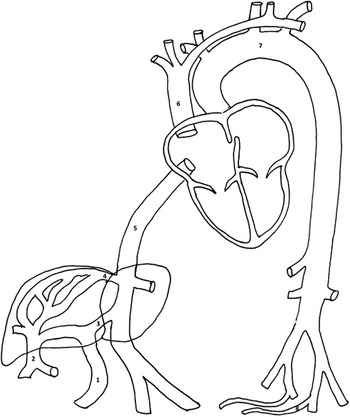
Figure 1 Diagram of foetal circulation. 1=umbilical vein; 2=portal vein; 3=venous duct; 4=hepatic vein; 5=inferior caval vein; 6=superior caval vein; 7=aorta; 8=umbilical arteries.
Agenesis of the venous duct results in direct drainage of the umbilical vein either into the hepatic vasculature or directly into the heart or systemic venous system. First described in 1888 by Paltauf et al, agenesis of the venous duct was previously considered a rare anomaly associated with a poor prognosis. However, with the advent of advanced foetal imaging, agenesis of the venous duct appears to be more common with a variable prognosis. Two subtypes have been described based on the drainage site, extrahepatic or intrahepatic. Extrahepatic umbilical venous drainage bypasses the liver and involves direct connections between the umbilical vein and the right atrium, inferior caval vein, or other systemic venous structures. Intrahepatic drainage involves direct connections between the umbilical vein and the portal sinus or hepatic sinusoids.Reference Berg, Kamil and Geipel 3 Agenesis of the venous duct can lead to intrauterine demise secondary to foetal hydrops or may be fairly well tolerated even through postnatal life, depending on the specific circumstances.Reference Paltauf 4 We report two additional cases of agenesis of the venous duct and provide a contemporary review of the literature with a total of 174 identified cases.
Case report 1
A 33-year-old, gravida 7 para 3 was referred to our Foetal Cardiac Programme at 31 4/7 weeks gestation for evaluation of cardiomegaly and tricuspid regurgitation. The pregnancy was also complicated by pre-eclampsia, poorly controlled diabetes mellitus, and intrauterine growth retardation. Foetal karyotype and microarray testing were normal. The foetal echocardiogram demonstrated the absence of the venous duct with the insertion of the umbilical vein directly into the right atrium (Fig 2). The hepatic veins and umbilical vein proximal to the insertion into the right atrium were dilated. Moderate cardiomegaly was present with a cardiothoracic area ratio of 0.48, and there was mild-to-moderate tricuspid valve insufficiency with good biventricular systolic function. There was no evidence of foetal hydrops. Plans for delivery at our institution were made; however, the mother developed preterm labour at 35 weeks, resulting in delivery at an outside facility. The initial course was notable for respiratory distress treated with intubation and surfactant. Neonatal echocardiography demonstrated a structurally normal heart with evidence of severe pulmonary hypertension. The infant was treated with nitric oxide with no clinical improvement and was transferred to our institution for further care.
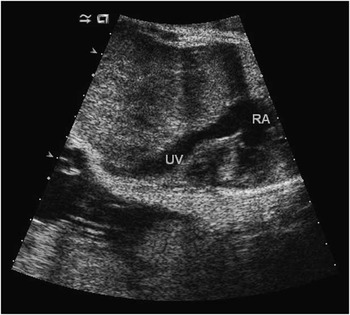
Figure 2 Foetal echocardiogram demonstrating insertion of the umbilical vein directly into the right atrium.
At the time of transfer, physical examination was notable for hepatomegaly. Echocardiography demonstrated evidence of severe pulmonary artery hypertension with an estimated right ventricular systolic pressure of 71 mmHg derived by the tricuspid valve regurgitation jet. Because of the known association between agenesis of the venous duct, and portosystemic shunt, a liver CT angiogram was performed. This demonstrated findings consistent with a Type 1 portosystemic shunt, which was subsequently confirmed by Interventional Radiology (Fig 3a). The vascular shunt was occluded using an Amplatzer vascular occlusion device (Fig 3b). Follow-up liver ultrasounds demonstrated evidence of additional hepatic shunts and an anomalous hepatic vein was found draining to the right atrium. This structure was occluded with coil and liquid glue embolisation (Figs 4a and 4b). The baby was discharged home at 5 months of age with tracheostomy collar, gastrostomy tube, and pulmonary vasodilator therapy.

Figure 3 ( a ) Angiography of the portal venous system in the anterior–posterior projection demonstrating a catheter in the right portal vein from the right internal jugular vein access, with contrast injection draining directly into the right atrium through a large shunt. ( b ) Angiography of the portosystemic shunt in the anterior–posterior projection demonstrating a vascular sheath in the shunt inferior to the right atrium after embolisation of the shunt using an Amplatzer II vascular plug.

Figure 4 ( a ) Angiography of the hepatic circulation in the anterior–posterior projection demonstrating a catheter in an anomalous hepatic vessel, from right internal jugular vein access, with contrast injection draining into the right atrium through the persistently patent portosystemic shunt. ( b ) Angiography of the hepatic circulation in the anterior–posterior projection demonstrating successful coil embolisation of the anomalous hepatic drainage, with subsequent ultrasound guided percutaneous “direct puncture” of the Amplatzer II plug and glue embolisation of plug interstices to complete the shunt occlusion (not pictured).
Case report 2
A 22-year-old gravida 5 para 0 was referred to our Foetal Cardiac Programme at 30 weeks’ gestational age for suspected cardiac disease. The pregnancy was also complicated by intrauterine growth retardation. Foetal echocardiography demonstrated agenesis of the venous duct with infrahepatic connection of the umbilical vein directly into the inferior caval vein (Fig 5). Intracardiac anatomy and function were otherwise normal.

Figure 5 Colour doppler from foetal echocardiogram demonstrating anomalous drainage of an umbilical vein (UV) into the right atrium. IVC=inferior vena cava.
Elective C-section was performed at 36 3/7 weeks’ gestational secondary to continued intrauterine growth retardation and breech presentation. The baby did not require extensive resuscitation. Postnatal echocardiography demonstrated normal ventricular chamber size and ventricular function. There was no echocardiographic evidence for significant pulmonary hypertension. With the known history of agenesis of the venous duct and potential for a portosystemic shunt, a liver ultrasound was performed and demonstrated a type I portosystemic shunt in which the main portal vein drained directly into the inferior caval vein. Postnatal genetics evaluation demonstrated a 6p25.3p22.3 duplication consisting of a 16.8 megabase duplication interval containing 155 genes. This duplication was located on chromosome 22p11.2. The baby was discharged home at 8 days of age and has been asymptomatic at 5 months of age.
Discussion
The venous circulation of the foetal liver comprises a portal and hepatic system. Development of the hepatic and portal circulations involves interactions between the umbilical veins that originate from the placenta, the vitelline veins that originate from the yolk sac, and the cardinal veins that originate from among the pleuropericardial folds (Fig 6). At roughly the fifth week of gestation, the vitelline veins are interrupted and eventually become incorporated into the developing liver as part of the hepatic sinusoids. As liver enlargement continues, connections between the hepatic sinusoids and umbilical veins begin to develop. There is regression of the entire right umbilical vein and the cranial portion of the left umbilical vein, which serves as the direct connection between the umbilical veins and the foetal heart. While these changes occur in the umbilical venous system, the vitelline veins undergo simultaneous changes as well. The right cranial vitelline vein initially enlarges and then forms the right hepatocardiac channel, whereas the left vitelline vein regresses. The right hepatocardiac channel contributes to the inferior caval vein as it forms its hepatic portion. This evolving process leads to the presence of the venous duct that forms from the enlarging anastomosis between the left umbilical vein and the right hepatocardiac channel. The venous duct then serves to direct placental blood flow from the left umbilical vein through the liver, bypassing the hepatic sinusoids. Next, the right caudal vitelline vein channels join to form the portal vein. A portion of the left portal vein is formed by the left umbilical vein and this connection is maintained in the foetus such that blood flow is directed from the left umbilical vein into the portal sinus, through the venous duct, and into the inferior caval vein. Blood not shunted through the venous duct makes its way through the left portal vein, perfusing the left lobe of the liver, and returns via the left hepatic veins to the inferior caval vein.Reference Sadler and Langman 5 Any deviations from the complex process of foetal venous development can lead to abnormalities of the venous system, including an absent venous duct.Reference Moss and Allen 6 , Reference Rychik 7 The genetic cause of agenesis of the venous duct is not known.
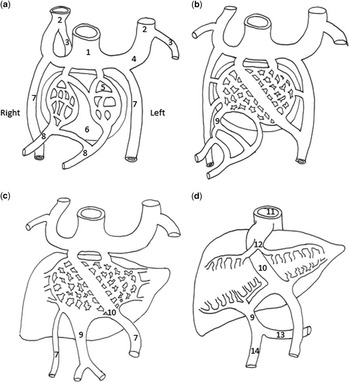
Figure 6 ( a ) Demonstrates the portal venous system at 4 weeks’ gestation, ( b ) at 5 weeks, ( c ) at 6 weeks, and ( d ) at 3 months. 1=sinus venosus; 2=anterior cardinal vein; 3=posterior cardinal vein; 4=common cardinal vein; 5=hepatic sinusoid; 6=liver; 7=umbilical vein; 8=vitelline vein; 9=portal vein; 10=venous duct; 11=inferior caval vein; 12=hepatic vein (vitelline vein); 13=splenic vein; 14=superior mesenteric vein (see text).
Historically, the extrahepatic type of agenesis of the venous duct has been considered to have a less favourable prognosis related to the development of cardiomegaly and foetal hydrops. Agenesis of the portal venous system has also been described in the extrahepatic type, which can lead to postnatal liver dysfunction. The intrahepatic type has been linked to foetal hydrops, as well as developmental abnormalities of the portal system, including portal hypertension.Reference Berg, Kamil and Geipel 3 Overall, clinical outcomes are largely linked to the presence of other anatomic or chromosome abnormalities. In a recent series from Thomas et al, the prognosis for the foetus with isolated agenesis of the venous duct was good regardless of the drainage location. The authors propose an algorithm for assessment and monitoring of the foetus with agenesis of the venous duct, which involved close prenatal monitoring for the development of foetal hydrops and postnatal evaluations to rule out portal vein agenesis in patients with extrahepatic drainage and portosystemic shunts in patients with intrahepatic drainage.Reference Thomas, Petersen, Cincotta, Lee-Tannock and Gardener 8 Our cases support the need to consider the presence of portosystemic shunts in each type, as both of our patients were diagnosed prenatally with extrahepatic umbilical venous connections and had postnatally confirmed portosystemic shunts.
Importantly, our cases demonstrate the variability in clinical course for this anomaly. Case 1 describes a child who has survived postnatally, but who has developed significant subsequent comorbidities, whereas case 2 demonstrates a child who was able to be discharged from the hospital at 1 week of age without intervention. In case 1, the signs of refractory pulmonary hypertension without a cause led to a consideration for further imaging to evaluate for a portosystemic shunt. Pulmonary hypertension has been well described in patients with portosystemic shunts as well as in patients with other congenital high cardiac output lesions, such as the vein of Galen malformation.Reference Hendson, Emery, Phillipos, Bhargava, Olley and Lemke 9 , Reference Ohno, Muneuchi and Ihara 10 The pathophysiology of pulmonary hypertension in portosystemic shunts is not well defined. Impaired hepatic metabolism of vasoactive substances has been proposed as a contributor to elevated pulmonary vasoconstriction.Reference Ohno, Muneuchi and Ihara 10 In addition, prenatal exposure to elevated cardiac output states resulting in greater than normal foetal pulmonary blood flow may impact normal pulmonary vascular development. Case 1 highlights the importance of obtaining a thorough liver examination to assess for portosystemic shunts in all infants with a prenatal diagnosis of agenesis of the venous duct, especially in the setting of a patient with persistent pulmonary hypertension. Case 2 is an example of a child with agenesis of the venous duct and a portosystemic shunt who did well and required minimal medical intervention. However, continued follow-up for this child is critical in order to monitor the development of liver dysfunction or regression of the portosystemic shunt.
Others have described the need for postnatal intervention on a persistent portosystemic shunt in a patient prenatally diagnosed with agenesis of the venous duct. Acherman et al described a series of six prenatally diagnosed cases of agenesis of the venous duct. This series included one case of intrahepatic type drainage occurring via a venous channel between the portal vein and right atrium, which was associated with the postnatal persistence of a portosystemic shunt requiring intervention.Reference Acherman, Evans and Galindo 11
We conducted a contemporary review of the literature that identified 36 case series with 172 reported cases of agenesis of the venous duct for a total of 37 case series with 174 reported cases, including this current study.Reference Kiserud, Rasmussen and Skulstad 1 , Reference Berg, Kamil and Geipel 3 , Reference Thomas, Petersen, Cincotta, Lee-Tannock and Gardener 8 , Reference Acherman, Evans and Galindo 11 – Reference Hille, Chaoui, Renz and Hecher 43 Several of these studies reported single patients, whereas the largest of these series consisted of 26 patients. Table 1 summarises data from each individual study, whereas Table 2 summarises data from all 174 cases. Among all the cases reported to date, 12 different umbilical vein drainage sites have been reported. The most common site was the right atrium that represented the drainage site of the umbilical vein in 36% of reported patients. Other noted drainage sites include the superior vena cava, the left atrium, and the azygos vein, each of which consisted 0.5% of all patients.
Table 1 Summary characteristics for each individual case organised by study.

AIVC=absence of inferior vena cava; ASD=atrial septal defect; AV=azygos vein; AVC=atrioventricular septal defect; Cm=cardiomegaly; CoA=coarctation of the aorta; CS=coronary sinus; DORV=double outlet right ventricle; Dx=dextrocardia; EA=Ebstein anomaly; HA=hepatic artery; HLHS=hypoplastic left heart syndrome; HV=hepatic vein; IV=iliac vein; IVC=inferior vena cava; LA=left atrium; LSVC=left superior vena cava; PA=pulmonary atresia; PS=portal sinus; PV=portal vein; PVS=pulmonary vein stenosis; RA=right atrium; RV=renal vein; SVC=superior vena cava; TA=tricuspid atresia; TrA=truncus arteriosus; VSD=ventricular septal defect
Table 2 Summary characteristics for all cases combined

AIVC=absence of inferior vena cava; ASD=atrial septal defect; AV=azygos vein; AVC=atrioventricular canal; Cm=cardiomegaly; CoA=coarctation of the aorta; CS=coronary sinus; DORV=double outlet right ventricle; Dx=dextrocardia; EA=Ebstein anomaly; HA=hepatic artery; HLHS=hypoplastic left heart syndrome; HV=hepatic vein; IV=iliac vein; IVC=inferior vena cava; LA=left atrium; LSVC=left superior vena cava; PA=pulmonary atresia; PS=portal sinus; PV=portal vein; PVS=pulmonary vein stenosis; RA=right atrium; RV=renal vein; SVC=superior vena cava; TA=tricuspid atresia; TrA=truncus arteriosus; VSD=ventricular septal defect
The types of associated cardiac abnormalities diagnosed prenatally or postnatally are also quite variable. The most common cardiac abnormality was cardiomegaly that was identified in 22% of patients. The most common structural defect was an isolated ventricular septal defect found in 6% of patients. More complex cardiac defects were found in an additional 9% of patients, and included a wide variety of abnormalities. The high prevalence of associated cardiac abnormalities warrants careful cardiovascular evaluation of these patients prenatally and postnatally.
Portosystemic shunts were identified postnatally in 9% of all reported patients. A type 1 portosystemic shunt consists of a portal vein that drains entirely into the inferior caval vein, right atrium, or another drainage site, without an intrahepatic portion. A type 2 portosystemic shunt consists of a hypoplastic intrahepatic portal vein with a side-to-side anastomosis either to the inferior caval vein or another drainage site.Reference Jaeggi, Fouron and Hornberger 29 Interestingly, the clinical outcome varied for all patients in the review and did not seem to be influenced by the presence of, or the type of, portosystemic shunt. Similarly, we report two patients prenatally diagnosed with agenesis of the venous duct with extrahepatic drainage, and who were found postnatally to have a type 1 portosystemic shunt but with divergent clinical courses. Acherman et al speculate that congenital portosystemic venous shunts may be directly associated with unrecognised agenesis of the venous duct in the foetus, and our findings would suggest a similar relationship. Children with portosystemic shunts may be at risk for pulmonary hypertension, hepatopulmonary syndrome, hepatic encephalopathy, focal nodular hyperplasia, hepatoblastoma, and hepatocellular carcinoma.Reference Loomba, Telega and Gudausky 32 , Reference Marois, van Heerden, Carpenter and Sheedy 44 – Reference Yoshidome and Edwards 47 Early intervention on portosystemic shunts may lead to reduction in this risk.Reference Wakamoto, Manabe, Kobayashi and Hayashi 48 – Reference Otake, Kobayashi and Hashimoto 51
Of the reported patients, 24% had identifiable chromosomal abnormalities. This figure likely represents an underestimate as some of these patients simply underwent a karyotype without any specific microarray analysis. Reported chromosomal abnormalities included well-defined syndromes such as Noonan’s syndrome, Turner’s syndrome, and Down syndrome, as well as chromosomal rearrangements of non-specific significance. Of note, two patients also had the presence of ring chromosomes.
Information regarding the outcome of pregnancy was available for 171 patients with 69% of pregnancies resulting in a live birth. Of the 53 foetuses that did not survive to a live birth, 14 suffered from foetal demise, whereas 39 were electively terminated. Risk for spontaneous abortions appears to be related to the presence of hydrops and chromosomal abnormalities. Pregnancies electively terminated were also more likely to be with foetuses diagnosed with a chromosomal abnormality.
Conclusion
We report two cases of agenesis of the venous duct, both of which were extrahepatic in nature and associated with postnatal extrahepatic portosystemic shunts. These cases demonstrate the marked variability in clinical course, and the need to fully evaluate the liver anatomy and physiology when a prenatal diagnosis of agenesis of the venous duct is made. The prognosis of isolated agenesis of the venous duct can be very good; however, prenatal monitoring is warranted. All patients should undergo postnatal cardiac evaluations as well as assessments of the hepatic vasculature.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.










