Mild or moderate aortic stenosis is managed conservatively in infants and children because most paediatric cardiologists believe it is well-tolerated and are reluctant to employ treatment strategies such as balloon angioplasty or aortic valve replacement that carry significant risks. Yet, it remains unclear whether myocardial injury exists during the earliest stages of left-heart obstruction. In adults, there is accumulating evidence that diffuse myocardial fibrosis does not regress after aortic valve replacement, despite otherwise favourable myocardial remodelling.Reference Azevedo, Nigri and Higuchi 1 – Reference Flett, Sado and Quarta 3 Accordingly, it may be important to treat afterload-induced heart disease during its earliest phases. As maladaptive cardiac growth and interstitial fibrosis result from underlying abnormalities of the microcirculation,Reference Marcus, Harrison and Chilian 4 we hypothesised that children with mild or moderate aortic stenosis would have abnormal myocardial perfusion, despite having no clinical symptoms, no evidence of fibrosis, and normal-appearing coronary arteries.
Recently, quantitative myocardial perfusion using cardiac magnetic resonance imaging has proven to provide excellent spatial and temporally resolved information in adults.Reference Constantine, Shan, Flamm and Sivananthan 5 , Reference Wang, Jerosch-Herold, Jacobs, Shahar and Folsom 6 In the present study, we take advantage of this new technique to quantify resting and adenosine-induced hyperaemic myocardial blood flow using adenosine-infusion cardiac magnetic resonance imaging in haemodynamically normal children and compared them to a group with mild or moderate aortic stenosis.
Methods
The protocol was approved by the Oregon Health & Science University’s institutional review board. Written and dated informed consent was obtained from all parents or guardians of all the patients, and informed assent was obtained from all children and adolescents older than 9 years.
Study subjects (Table 1)
In the present study, two groups of children – all greater than 3 months of age – were enrolled. Caveats by the human subjects committee precluded enrolling children without cardiac findings. Therefore, one group included children with haemodynamically trivial congenital heart malformations – the control group. The second group included those with unrepaired mild or moderate aortic stenosis, defined as Doppler-determined mean pressure gradients of <40 Torr. Overall, the study population consisted of 31 individuals, none of whom had any signs or symptoms of haemodynamically significant heart disease, nine were referred for concerns unrelated to cardiac haemodynamics, and 22 consented to participate solely in order to contribute to this research effort (see results Patient Characteristics for additional details).
Table 1 Patient characteristics, diagnoses, aortic valve Doppler of experimental groups.

AI=aortic insufficiency; Ang=balloon angioplasty; ASD=atrial septal defect (<2 mm); BAV=bicuspid aortic valve; cMR=cardiovascular magnetic resonance imaging; Hx of Coarct.=repair at one year of age with no echo evidence of residual obstruction; LVOT=left ventricular outflow tract; Nl coronary eval=normal coronary anatomy demonstrated by MRI; PS=mild pulmonary stenosis (Doppler velocity <2 m/second); VSD=small ventricular septal defect
*p<0.05 by Student’s t-test. Other non-significant comparisons include the following: BSA, SV, CI, LV Vol, HR, systolic/diastolic BPs, and RPP (both resting and hyperaemic)
Children were excluded from the study if they had a history of asthma, requiring bronchodilators within the previous year, were taking carbamazepine, dipyridamole, or verapamil, which may cause possible drug–drug interactions, or had consumed caffeine-containing substances within a 24-hour period before the study, which may reduce adenosine efficacy. Those with a history of kidney dysfunction had their glomerular filtration rate calculated and were excluded from the study if the estimated glomerular filtration rate was <30 ml/minute.
Study design
All the individuals were sedated with propofol under the supervision of a paediatric cardiac anaesthesiologist. These studies were performed under deep sedation, maintaining a natural airway and spontaneous respiration. No individual was intubated. Vital signs were monitored throughout the study period, including continuous pulse oximetry, heart rate, and end-tidal CO2. Blood pressure and heart rate were recorded before rest perfusion data acquisition and every 60 seconds during adenosine infusion (140 µg/kg/minute). The surface ECG was monitored continuously. The adenosine infusion lasted for three minutes before initiating the perfusion scan. Adenosine infusion was stopped after the first pass of the contrast bolus, which could be followed with 1- to 2-second latency time in a display window of the scanner console. Perfusion data (ml/g/minute) were acquired from two left ventricular short-axis slices after administration of 0.03 mmol/kg of gadolinium contrast (GE HealthCare AS, Oslo, Norway), first at rest and then ~15 minutes later during adenosine infusion. The 15-minute interval between resting and hyperaemic perfusion imaging allowed for adequate contrast washout. During the 15-minute interval, a unique plane was determined through the short axis of the left ventricle, and a series of parallel cine images were obtained in order to determine left ventricular ejection fraction and mass. Following the adenosine infusion studies, a second dose of gadolinium was administered (0.1 mmol/kg) and a standard protocolReference Kramer, Barkhausen, Flamm, Kim and Nagel 7 was followed to detect delayed enhancement.
Pulse sequences and image analysis
Images were obtained on a 3-T Philips Intera magnetic resonance scanner. Single-shot turbo gradient-echo sequences with saturation recovery magnetisation preparation for T1-weighting were performed, TR/TE/flip angle=3.0/1.44 ms/20°, with a spatial resolution of <2×2 mm. Temporal resolution of this dynamic contrast-enhanced study was equal to heartbeat duration. Gradient echo cine pulse sequences, without steady-state free precession to avoid artefacts, were performed for multiple short-axis slices in order to determine ejection fraction and left ventricular mass. Images were visually assessed for regions of focal gadolinium late enhancement.
Endocardial and epicardial contours of two left ventricular short-axis slices were traced manually following each study using a dedicated cardiac magnetic resonance analysis software (ViewForum; Philips Medical Systems, Eindhoven, the Netherlands), as previously described.Reference Jerosch-Herold, Seethamraju, Swingen, Wilke and Stillman 8 The left ventricular myocardium was divided into six segments, using the insertion point of the right ventricular wall on the interventricular septum as a reference point for segmentation (Fig 1a). This method corresponds to the established American Heart Association’s segmentation of the left ventricular wall for perfusion studies.Reference Jerosch-Herold, Seethamraju, Swingen, Wilke and Stillman 8 Change in average segmental signal intensity during contrast transit was used to quantify absolute myocardial blood flow in millilitres per gram of myocardium per minute (ml/g/minute) by deconvolution of the tissue curves (Fig 1b), using the arterial input in the left ventricle, and assuming a specific density of myocardial tissue of 1.05 g/ml.Reference Jerosch-Herold, Seethamraju, Swingen, Wilke and Stillman 8 Myocardial perfusion reserve was defined as hyperaemic myocardial blood flow divided by resting myocardial blood flow. The left ventricle ejection fraction and end-diastolic mass were calculated using previously described algorithms.

Figure 1 ( a ) User traced endocardial and epicardial contours, and regions of interest in the centre of the left ventricular cavity (1=anterior, 2=lateral, 3=posterior, 4=inferior, 5=inferior septal, and 6=anterior septal). ( b ) Signal intensity curves for the blood pool within the left ventricular cavity (LV) and the interstitial compartments of six segments from two short-axis slices of the myocardium (Myo).
Statistics
For all continuous variables, the lower quartile, median, and upper quartile were calculated. Pearson’s χ2 test, Student’s t-test, and Wilcoxon’s test were used as appropriate for bivariate comparisons. To compare myocardial blood flow within and between groups, a linear mixed effects statistical model was used. The dependent variable, myocardial blood flow, was adjusted in the fixed part of the model for gender, left ventricle mass, or stenosis severity, as well as differences in the rate pressure product, in the case of resting myocardial blood flow. Hyperaemic myocardial blood flow was adjusted simultaneously for resting myocardial blood flow as previously described.Reference Petersen, Jerosch-Herold and Hudsmith 9 Although no specific comparisons were made between individual patients, comparisons were made between pre-defined groups of patients – that is, control versus aortic stenosis. Confidence intervals of 95% were calculated. The model took into account inter-subject variance – aortic stenosis versus control – and intra-subject variance – resting/adenosine, slice, and region. Interaction terms between aortic stenosis/control and resting/adenosine to assess whether the effect of adenosine was different in control and aortic stenosis patients were included. Significance was assumed at p<0.05 or when the 95% confidence interval failed to overlap. All data analysess were performed using R statistical software version 2.12.1. 10
Results
Patient characteristics (Table 1)
Adenosine-infusion cardiac magnetic resonance imaging studies were performed in 31 children – 20 haemodynamically normal controls and 11 with aortic stenosis. None of the children were thought to have any symptoms referable to the cardiovascular system. All the children had normal-appearing coronary arteries and normal cardiac function as assessed by routine echocardiography. Some had trivial congenital heart disease such as a small ventricular septal defect, with Doppler-determined blood flow velocity >4 m/second, a vascular ring, a patent foramen ovale, or mild pulmonary stenosis, with Doppler-determined blood flow velocity <2 m/second.
The control group (n=20) consisted of children referred for the study and ultimately shown to have normal hearts or children with trivial congenital heart malformations. Among all, seven controls were referred for magnetic resonance imaging (MRI) because of concerns unrelated to cardiac haemodynamics: question of vascular ring with structurally normal heart and normal cardiac function in four, two children with question of abnormal coronary artery origin prompted by echocardiography were proven to have normal coronary anatomy, and chest pain in one who had a normal exercise stress test was ultimately given a non-cardiac diagnosis. The remaining 13 controls did not have clinical indications for the study but consented to participate after being approached by the investigators. All 13 participants had clinical evidence and echocardiography indicating haemodynamically insignificant cardiac malformations – tiny ventricular septal defect in seven, tiny atrial septal defect in two, trivial pulmonary stenosis in two, and a 13-year-old boy with coarctation repair at one year of age who had echo evidence of trileaflet aortic valve and no residual obstruction by echocardiography. The aortic stenosis group (n=11) included those with unrepaired mild or moderate aortic stenosis, defined as Doppler-determined mean pressure gradients of <40 Torr. These participants had no complaints, symptoms, or signs of heart disease, and after discussion with the investigators consented to participate in this research.
Median age and sex were not different between the groups (aortic stenosis 8.4 versus control 10.6 years, 82 versus 55% males). Mean pressure gradient between the left ventricle and the ascending aorta was higher in the aortic stenosis group compared with controls (24 versus 3 mmHg, p<0.001). The left ventricular wall mass index was higher in the aortic stenosis group compared with controls (65 versus 50 g/m2, p<0.05, Table 2). Ejection fraction was also higher in the aortic stenosis group compared with controls (75 versus 70%, p<0.05).
Table 2 cMR characteristics of experimental groups.

BSA=body surface area; CI=cardiac index; cMR=cardiovascular magnetic resonance imaging; CO=cardiac output; cRPP=change between resting and hyperaemic rate pressure products; EF=ejection fraction; h=hyperaemic; LVMass=left ventricle mass; LVVol=left ventricle volume; r=resting; SV=stroke volume; %tile=blood pressure percentiles
* Pearson’s test
** Median
*** Lower quartile
**** Upper quartile for continuous variables
***** Wilcoxon’s test
Physiological response to adenosine infusion
Adenosine infusion was well tolerated in all patients. In seven patients, less than five seconds of self-limited apnoea was observed moments after discontinuing the adenosine infusion. No intervention was required in any instance. In total, the adenosine infusion part of the study took ~30–45 minutes to be completed. Furthermore, during the 15-minute interval between rest and adenosine infusion, additional image acquisition was possible. When the control and aortic stenosis groups were compared, adenosine-induced hyperaemia had mild effects on blood pressure and heart rate, but the differences between groups were not different (Table 2). During adenosine infusion, the median heart rate increased from 83 to 109 beats per minute (p<0.001), and systolic blood pressure decreased from the median value of 89 to 70 mmHg (p<0.001). Blood pressures in both the resting and hyperaemic states were converted to percentile ranks based on age, gender, and height.Reference Roccella 11 The decrease in blood pressure percentile was significant within both control and aortic stenosis groups, but the difference between the groups was not significant. We noted that the percentile ranks for blood pressure were all lower than the normal range. We ascribed this observation to the fact that all the patients were under deep sedation during the studies. The change in the rate pressure product (RPP) was not different between the groups (median 316 versus 333, p=0.4). Focal regions of gadolinium late enhancement were not observed in either group.
Myocardial blood flow
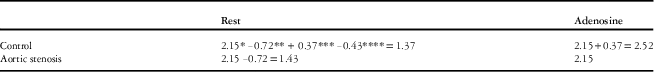
Normal resting and adenosine-induced hyperaemic flows obtained from 20 haemodynamically normal children (controls) are shown in Table 4. Overall, we found that the main effect of the adenosine treatment was significant, as myocardial blood flow went up in both the groups, and the main effects of group were also significant – that is, the change caused by adenosine-induced hyperaemia as well as the absolute value of maximal myocardial blood flow were different between the aortic stenosis and control groups. The aortic stenosis group’s adenosine-induced myocardial blood flow change compared with the resting state was significantly smaller than the decrease from adenosine infusion to rest in controls (Tables 3a and 4) (−0.72 ml/g/minute; 95% confidence interval: −0.50,−0.93 versus −1.14, 0.95, 1.34, p<0.0001). In addition, the hyperaemic flow adjusted for resting flow (Fig 2) was significantly lower in the aortic stenosis group compared with controls (2.15 ml/g/minute; interquartile range 1.5, 2.3 versus 2.51 (1.8, 3.3), p<0.001). When the model was analysed without adjusting resting flow for rate pressure product, the results were the same. In addition, when gender, left ventricle wall mass, or severity of stenosis was included in the model, the results were unchanged.

Figure 2 Children with mild/moderate aortic stenosis (AS) have significantly (p<0.001) lower hyperaemic myocardial blood flow (hMBF) than haemodynamically normal children. Dots represent resting MBF-adjusted values from each slice and region of the heart (AS, n=132 and control, n=240). When the identical model was re-analysed, where resting flows were not rate pressure product-adjusted, the results were the same.
A mixed linear effects model was created to account for both between and within-patient variation allowing for differences between groups – that is, aortic stenosis and control – and within-group treatments – resting, hyperaemia – the six myocardial regions, and the two slices – apical and basal. Initially all possible interactions were analysed. Significant interactions were identified between group and treatment (p<0.0001) and between treatment and region (p=0.0006); the interactions between group and region, between group and slice, and all higher-order interactions were not significant.
As shown in Tables 3a, 3b, and 4, adenosine-induced flows were significantly decreased in the aortic stenosis group compared with controls (0.37 ml/g/minute, CI 0.11, 0.62, p=0.0067), using the anterior region and the apical slice as reference. This effect can be generalised to all regions as there was no significant interaction between regional flow and the aortic stenosis and control groups. Rest flows in the aortic stenosis group were unchanged compared with controls (−0.06 ml/g/minute, 95% confidence interval: −0.31, 0.19), again using the anterior region and the apical slice as reference. The inferior and inferior septal regions, compared with the anterior region, demonstrated decreased flow during hyperaemia (p<0.01), but this effect was not different between the groups. In addition, the basal slice consistently had lower flow than the apical slice (−0.10 ml/g/minute, 95% confidence interval: −0.02,−0.17, p=0.0121), but again there was no difference identified between the control and aortic stenosis groups.
Table 3a AI-cMR-determined regional quantitative regional myocardial blood flow comparing children with aortic stenosis and haemodynamically normal hearts (group) at rest or during adenosine-induced hyperaemia (treatment).

* Baseline is for AS in hypaeremic state in the anterior region and apical slice. The prediction of flow (ml/g/minute) can be accomplished by summing baseline flow and the main effects (group, treatment, region, and slice) and the interaction terms (group and treatment and/or treatment and region)
Table 3b Summing main effects and interaction terms from Table 3a to predict myocardial blood flow at rest and during adenosine-induced hyperaemia in control and aortic stenosis (AS) groups.
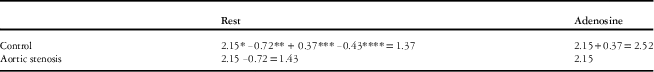
All values are expressed in ml/g/minute. Non-significant main effects or interactions should not be included in the prediction model
* Baseline reference flow is AS in hyperaemic state, anterior region, apical slice
** Main effect-treatment in resting state
*** Main effect-group in controls
**** Interaction term-control group and resting treatment
Table 4 Resting and hyperemic flows in aortic stenosis and control groups

* all values are ml/gm/min (95% confidence limits)
Discussion
The major finding of this study is that asymptomatic children with mild or moderate aortic stenosis have significantly decreased myocardial perfusion in response to adenosine infusion compared with healthy children. In adults with suspected coronary artery disease, the ability of adenosine-infusion cardiac magnetic resonance imaging to predict significant cardiac events is established.Reference Jahnke, Nagel and Gebker 12 The fact that children with mild or moderate aortic stenosis remain asymptomatic for many years does not exclude the possibilities that latent disease exists in these children or that significant injury occurs during the period of latency. The observation of decreased myocardial blood flow in response to adenosine stress in young aortic stenosis patients suggests that, in children with minimally after-loaded hearts, there is a disorder of the microcirculation that could be the substrate for later development of diffuse myocardial fibrosis.Reference Flett, Sado and Quarta 3
This report also provides the first quantitative information about resting and adenosine-induced regional myocardial blood flow in healthy children with haemodynamically normal hearts, data that will be applicable to other studies in children who require normative comparisons. It is notable that hyperaemic flow was relatively decreased in the inferior wall segments of both control and aortic stenosis group individuals. The same observation has been made in asymptomatic adults.Reference Chareonthaitawee, Kaufmann, Rimoldi and Camici 13 , Reference Rosen, Lima and Nasir 14 Although the mechanism for these differences is unclear, it is interesting that Puchalski et alReference Puchalski, Williams and Askovich 15 also observed regional wall motion abnormalities and gadolinium delayed enhancement in the inferior myocardial regions of asymptomatic children with Duchenne muscular dystrophy. They concluded that these findings heralded the onset of global disease in these children. Taken together, these data suggest that the posterior cardiac segments may be particularly vulnerable to ischaemic injury.
There is an increasing body of literature suggesting that microvascular dysfunction is the proximate cause of acute myocardial fibrosis.Reference Petersen, Jerosch-Herold and Hudsmith 9 Recent evidence suggests that diffuse myocardial fibrosis identified before aortic valve replacement may be the best predictor of long-term poor outcome.Reference Weidemann, Herrmann and Stork 2 Thus, early treatments that target precursors of cardiac fibrosis such as pathological angiogenesis or coronary vascular reactivity may prove useful. Our data suggest that myocardial abnormalities may occur in the setting of even mild or moderate aortic stenosis and begin as early as childhood. Whether this disorder of the microcirculation is a consequence of afterload-induced injury or a primary developmental phenomenon, independent of the severity of left-heart obstruction, deserves further investigation.
Studies of the immature myocardial microcirculation in humans are limited, because until the advent of cardiac magnetic resonance imaging there has not been a safe, non-invasive, and quantitative method to address this question. There is evidence from experimental animal models suggesting that maximal coronary blood flow is decreased in the after-loaded myocardium.Reference Wicker and Tarazi 16 , Reference Hoffman 17 In addition, Rakusan et alReference Hoffman 17 , Reference Rakusan, Flanagan, Geva, Southern and Van Praagh 18 studied pressure-overloaded left ventricular hypertrophy in post-mortem human hearts. In those studies, children with aortic stenosis had proportional capillary angiogenesis, meaning that capillary density was directly related to the degree of myocyte hypertrophy. On the other hand, in post-mortem hearts from adults with aortic stenosis, hypertrophy appeared to be associated with failure of compensatory capillary growth. Consistent with Rakusan et al’s studies in children with aortic stenosis, we found that resting blood flow per gram of tissue is preserved in the non-hyperaemic state; however, we found decreased adenosine-induced hyperaemia in the mildly/moderately after-loaded heart, an observation that would be impossible to make in a post-mortem study. Whether impaired hyperaemic blood flow in these asymptomatic children represents failure to recruit a potential vascular bed, or abnormal vascular reactivity perhaps caused by downregulation of the adenosine receptor, is unclear.Reference Feldman, Cheksis-Feiner, Hamad and Chan 19 Our findings lay the groundwork for future studies in animals and humans aimed at understanding the developmental relationships between angiogenesis, myocardial fibrosis, and myocardial dysfunction in children. Furthermore, the potential benefit of currently available therapies such as angiotensin or mineralocorticoid inhibitors, or the value of novel therapies that might prevent early fibrosisReference Funabiki, Onishi and Dohi 20 – Reference Young, Lam and Rickard 24 in the mildly after-loaded heart, are completely unknown.
Limitations
These studies were carried out using a 3-T magnet in order to maximise signal strength while quantifying myocardial blood flow. In none of these studies were we able to visually appreciate perfusion differences between the control group and aortic stenosis patients, nor were we able to identify evidence of regional gadolinium late enhancement. It is possible that regional differences will become more apparent with newer 3 T scanners that avoid previously described acquisition artefacts,Reference Dietrich, Reiser and Schoenberg 25 or with newer quantitative techniques.Reference Flett, Sado and Quarta 3 Importantly, we could not find a linear correlation between quantitative regional blood flow and stenosis severity or left ventricular mass. Thus, the clinical applicability of adenosine-infusion cardiac magnetic resonance imaging to risk stratify abnormal myocardial perfusion in children is probably limited at present because of the variable flows that resulted in overlap between the aortic stenosis and control individuals. Refined techniques and better scanners may solve this limitation. It is also possible that perfusion abnormalities may, in part, occur independently of the severity of aortic stenosis and other, perhaps modifiable, factors may play a role. Finally, HoffmanReference Hoffman 17 demonstrated in animal models that maximal coronary flow is dependent on perfusion pressure, and therefore defined coronary flow reserve over a range of pressures. This was not possible in our studies; however, we were able to show that the change in diastolic blood pressure or the change in the rate pressure product before and after inducing maximal dilation with adenosine was not different between the two experimental groups. Thus, it is likely that both the groups were studied at a similar level of coronary perfusion pressure.
Conclusion
In this report, we provide evidence that there may be significant abnormalities of the microcirculation in asymptomatic infants and children with even mild or moderate aortic stenosis. Whether myocardial perfusion abnormalities exist in other forms of left ventricle afterload in pre-clinical phases such as in early systemic hypertension or mild cardiomyopathy awaits future study. Very little is known about the adaptive and maladaptive responses, as they relate to myocardial perfusion in infants and children affected by cardiac hypertrophy, chronic hypoxaemia, and the effect of prolonged exposure to heart failure-inducing neuroendocrine hormones. As angiogenesis is a key component of the heart’s adaptation to ischaemia,Reference Berry, Balachandran, L’Allier, Lesperance, Bonan and Oldroyd 26 quantitative adenosine-infusion cardiac magnetic resonance imaging holds the unique promise of being able to monitor this process in infants and children.
Acknowledgements
The authors thank Angela Zimmerman, MD, for anaesthesia support during MRI studies, Robin Shaughnessy MD, Seshadri Balaji, MD, PhD, and Kent L. Thornburg PhD for their helpful comments, and Krista Bever for technical assistance.
Financial Support
Medical Research Foundation of Oregon (R.W. & M.S.)
Conflicts of Interest
There are no relevant conflicts of interest for any of the authors.
Ethical Standards
The authors assert that all the procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (Federal regulation 45CFR46, the federal policy for the protection of human subjects [the common rule]) and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the Oregon Health & Science University Institutional Review Board.