Introduction
Dispersal is a mechanism through which an organism can increase its fitness through movement to a habitat better suited for reproduction (Bowler and Benton Reference Bowler and Benton2005). The process of dispersal can be energetically costly (Zera and Harshman Reference Zera and Harshman2001; Harshman and Zera Reference Harshman and Zera2007; Zera Reference Zera, Whitman and Ananthakrishnan2009) and may reduce the resources available for subsequent reproduction (Hanski et al. Reference Hanski, Saastamoinen and Ovaskainen2006). This is particularly important for taxa that rely heavily on energy acquired as a juvenile to fuel adult flight (Thomas Reference Thomas and Quellet1988). Energy use during insect flight decreases subsequent reproductive output through a reduction in the size or number of eggs in many species (Isaacs and Byrne Reference Isaacs and Byrne1998; Fox and Czesak Reference Fox and Czesak2000; Elkin and Reid Reference Elkin and Reid2005; Gu et al. Reference Gu, Hughes and Dorn2006; Zhang et al. Reference Zhang, Wu, Wyckhuys and George2009; Gibbs and Dyck Reference Gibbs and Dyck2010; Guerra Reference Guerra2011; Elliott and Evenden Reference Elliott and Evenden2012; Steenman et al. Reference Steenman, Lehmann and Lehmann2013; Duthie et al. Reference Duthie, Abbott and Nason2014). However, compensation of energy used in flight can also occur by postdispersal feeding (Niitepõld and Boggs Reference Niitepõld and Boggs2015).
Bark and ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) are interesting taxa for studying physiological trade-offs between reproduction and flight, because adults undergo an obligatory flight to locate suitable hosts for brood production (Wood Reference Wood1982). Aerial dispersal can occur over long distances through flight aided by wind (Jackson et al. Reference Jackson, Straussfogel, Lindgren, Mitchell and Murphy2008) or over short distances through self-sustained flight (Robertson et al. Reference Robertson, Nelson and Boots2007). Dispersal distance is linked to both beetle physiology (Atkins Reference Atkins1966, Reference Atkins1969; Thompson and Bennett Reference Thompson and Bennett1971; Jactel Reference Jactel1993; Williams and Robertson Reference Williams and Robertson2008; Chen et al. Reference Chen, Li, Bu and Tian2011; Evenden et al. Reference Evenden, Whitehouse and Sykes2014) and the number and distribution of suitable host trees on the landscape (Robertson et al. Reference Robertson, Nelson and Boots2007).
The mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Curculionidae: Scolytinae), is a native bark beetle that colonises pine (Pinaceae) species in western North America. Its principal host is lodgepole pine (Pinus contorta var. latifolia Engelmann). The mountain pine beetle has killed trees over an area of 18 million ha during the most recent outbreak, which began in the mid-1990s in western North America (Safranyik et al. Reference Safranyik, Carroll, Régnière, Langor, Riel and Shore2010). During this outbreak, the range of mountain pine beetle reached the northern Rocky Mountains in Canada due to favourable climatic factors and long-distance dispersal aided by the wind (Jackson et al. Reference Jackson, Straussfogel, Lindgren, Mitchell and Murphy2008; de la Giroday et al. Reference de la Giroday, Carroll, Lindgren and Aukema2011, Reference de la Giroday, Carroll and Aukema2012). In its expanded range, the mountain pine beetle has successfully colonised a novel host, jack pine (P. banksiana Lambert), on the western edge of the boreal forest in Alberta, Canada (Cullingham et al. Reference Cullingham, Cooke, Dang, Davis, Cooke and Coltman2011). The defensive chemical profile (Clark et al. Reference Clark, Pitt, Carroll, Lindgren and Huber2014; Lusebrink et al. Reference Lusebrink, Erbilgin and Evenden2016) and the nutritional content of jack pine are different from that of the historic host, lodgepole pine (Ishangulyyeva et al. Reference Ishangulyyeva, Najar, Curtis and Erbilgin2016; Lusebrink et al. Reference Lusebrink, Erbilgin and Evenden2016). These differences between jack and lodgepole pine may alter the colonisation process (Erbilgin et al. Reference Erbilgin, Ma, Whitehouse, Shan, Najar and Evenden2014) and change the reproductive success of the mountain pine beetle in its expanded range.
Like other bark beetles, adult mountain pine beetles feed on the natal host before emergence (Elkin and Reid Reference Elkin and Reid2005) and may use stored energy during the obligatory flight period before brood production. Evenden et al. (Reference Evenden, Whitehouse and Sykes2014) found that lipids, at least in part, power beetle flight, as measured on laboratory flight mills. The energy deficit that results from flight may be partially offset by adult feeding during colonisation of the new host (Elkin and Reid Reference Elkin and Reid2005). However, flight could indirectly affect reproduction because activities required for successful host colonisation after flight are energetically costly (Reid et al. Reference Reid, Sekhon and LaFramboise2017). The male-produced aggregation pheromone, exo-brevicomin, is synthesised in the fat body (Song et al. Reference Song, Gorzalski, Nguyen, Liu, Jeffrey, Blomquist and Tittiger2014), and the pheromone titre may be reduced in beetles with less fat following dispersal. Reduced fat reserves may also directly reduce the reproductive potential and offspring fitness of mountain pine beetles. Female mountain pine beetles with low energy reserves produce small eggs (Elkin and Reid Reference Elkin and Reid2005), which may result in small offspring that are more susceptible to overwintering mortality than large offspring (Lachowsky and Reid Reference Lachowsky and Reid2014). As differential overwintering mortality between the sexually size-dimorphic mountain pine beetles contributes to the typical female-biased adult sex ratio in this species (Lachowsky and Reid Reference Lachowsky and Reid2014), it is possible that maternal energy used during flight could influence offspring sex ratio and affect population dynamics of this species. Although previous rearing experiments in many pine species have found variable effects of host characteristics on the reproductive output of mountain pine beetles (Amman Reference Amman1982; Langor Reference Langor1989; Cerezke Reference Cerezke1995; Cale et al. Reference Cale, Taft, Najar, Hughes, Sweeney and Erbilgin2015; Esch et al. Reference Esch, Langor and Spence2016; Lusebrink et al. Reference Lusebrink, Erbilgin and Evenden2016), none have explored the effect of dispersal activity of adults on subsequent reproduction in different pine hosts.
Herein, we hypothesise that obligatory dispersal by flight for host colonisation implicates a trade-off with reproduction in the mountain pine beetle. We examine the influence of flight on subsequent reproductive capacity and offspring fitness of mountain pine beetles in two pine hosts. We predict that the energy use during flight will reduce the reproductive output of mountain pine beetles. We further test the hypothesis that beetle physiological state influences reproductive capacity differentially in different pine hosts. We predict that beetle condition will be more important for reproduction in lodgepole pine than jack pine. Differences in defensive and nutritional chemistry of the two hosts may interact with investment in reproduction to affect the number and quality of the offspring.
Materials and methods
Beetles
Mountain pine beetle-infested lodgepole pine bolts (n = five/site) were obtained from three sites near Grande Prairie, Alberta, Canada (54º30.344’N, 118º46.643’W; 54º33.809’N, 118º42.501’W; 54º39.069’N, 118º44.287’W) in October 2011. One approximately 50-cm bolt was cut from each tree from 1 m above the soil surface. The cut ends of each bolt were sealed with paraffin wax and housed at 5 °C for four–six months to expose beetles to an overwinter cold period. Uninfested lodgepole and jack pine bolts were obtained from sites near Edson and Lac La Biche, Alberta, respectively, in May 2012. Four uninfested bolts approximately 70-cm long) (Table 1) were obtained from each of three trees at a single site for each of the pine species. Bolts were transported to the laboratory at the University of Alberta (Edmonton, Alberta, Canada) where the ends were sealed with paraffin wax before storage at 5 °C until use.
Table 1. Characteristics of lodgepole and jack pine bolts infested with control and flown mountain pine beetles. Beetle establishment characteristics include the number of beetle pairs introduced and those that successfully entered each bolt. The number of resulting galleries per infested bolt and the average gallery length for each bolt are presented.

Parental beetle flight treatment
Infested bolts were removed from cold storage and placed at 24 °C in separate 121-L bins made of opaque plastic and fitted with glass emergence jars starting in April 2012. Removal of bolts from cold storage was staggered to manage the number of beetles emerging at a given time. The emergent adult beetles were separated by sex (Lyon Reference Lyon1958) and stored at 4 °C in microcentrifuge tubes (2 mL) with a piece of paper to provide a surface to which beetles could cling (Evenden et al. Reference Evenden, Whitehouse and Sykes2014). Beetles were weighed to the nearest 0.0001 g (Mettler Toledo; XS105, Columbus, Ohio, United States of America) before flight. Age after emergence influences flight capability of the mountain pine beetle (Evenden et al. Reference Evenden, Whitehouse and Sykes2014), and therefore, beetles were flown 5–7 days post emergence. Beetles were prepared for flight by attaching a tether to the pronotum (Evenden et al. Reference Evenden, Whitehouse and Sykes2014). Flight experiments were conducted in a controlled environmental chamber maintained at 24 °C and a 16:8 light:dark hour photoperiod (621 lux during the photophase). The tethered beetles were attached to the mill for 23 hours. The flight assay was initiated four hours after the beginning of the light period. Males and females were flown on alternate days (n = 3–15 beetles per day) to avoid exposure to chemical cues from the opposite sex, which might affect flight behaviour. The software (LabView; National Instruments Corporation, Austin, Texas, United States of America) output included number of revolutions, longest single flight, and flight duration. The flight distance and duration of flown parent beetles subsequently introduced into the two pine hosts were compared using two-sample t-tests (R version 3.1.1 (R Core Development Team 2014)).
A random sample of beetles across the experiment served as control beetles. Control beetles were initially tethered in the same manner as flown beetles, but the tether was then removed and beetles were kept individually in a perforated microcentrifuge tube (2.0 mL), and provided with a slip of paper to settle on. Control beetles were positioned in the same environmental chamber that housed the flight mills during the flight period.
Bolt infestation and offspring rearing
Beetles subjected to the flight treatment were removed from the tether immediately after each flight period. Both flown and control adults were weighed and stored at 5 °C for a day. Control and flown beetles were introduced into separate uninfested lodgepole pine and jack pine bolts (Table 1). Four bolts of each tree species received beetles from each treatment. Each treatment included at least one bolt from three different trees of the same species. Phloem width was measured for each bolt at three different locations. Pairs of male and female beetles were introduced equidistantly (approximately 10 cm apart) around the base of each bolt in microfuge tubes. A female beetle was introduced, followed by a male beetle after the female had entered the bolt. The number of pairs introduced per bolt (6–9 pairs) was based on the calculated surface area of each bolt to control for phloem resource available per breeding pair (Table 1). Beetles flown on the same day were distributed among different bolts during the introduction process. This process was repeated three times between June and August 2012 until four bolts of each tree species were infested with flown or control beetles. A total of 16 bolts were infested. Dead beetles or beetles that did not enter the bolt within 48 hours were replaced with beetles from the same experimental treatment until pair establishment was successful. Infested bolts were kept for three weeks at 24 °C to allow for beetle mating, egg laying, and initial larval development of the offspring. Bolts were then transferred to cold storage (5 °C) for at least one month to provide appropriate conditions for beetle development (Lusebrink et al. Reference Lusebrink, Erbilgin and Evenden2013).
Following a month of cold storage, bolts were handled in the same manner to rear out the offspring beetles as described for the parental generation above. Offspring emergence began in February 2013 and the offspring were counted and separated by sex. Pronotum width and body length of the emergent offspring were measured using an ocular micrometre on a dissecting microscope (6.3X magnification) to the nearest 0.01 mm. Body size of individual beetles was estimated by calculating the area of an ellipsoid (Knud Thompson Formula S ≈ 4π [apbp + apcp + bpcp]1/p) in which a and b are half the pronotum width, c is the half the length of the beetle, and P = 1.6075 (Michon Reference Michon2009; Xu et al. Reference Xu, Cui, Bansal, Hao, Liu, Chen and Petersen2009). Beetles were weighed and stored at −20 °C for subsequent fat extraction. Following emergence, bolts were peeled to determine the number of beetle pairs that successfully established breeding galleries and to measure the length of the parental galleries (Table 1).
Fat extraction and offspring condition
Adult offspring were dried for 24 hours at 60 °C in an oven, and each was weighed to the nearest 0.0001 g. Beetles were then placed in perforated microcentrifuge tubes (0.2 mL) and submerged in petroleum ether (Fisher Chemical, Fair Lawn, New Jersey, United States of America) in a Soxhlet apparatus (45/50 Pyrex; Fisher Scientific, Ottawa, Ontario, Canada). After eight hours of fat extraction, the beetles were dried again at 60 °C for 24 hours and re-weighed. Individual fat content was determined by subtracting the dry weight after fat extraction from the initial dry weight. Offspring condition was calculated using a body condition residual index that controls for body size (Elkin and Reid Reference Elkin and Reid2005) by regressing offspring fat content against its body size. The residuals of the regression were used to create the residual index.
Statistical analyses
The mean phloem width of the two pine species was compared using a two-sample t-test. The bolt diameter of bolts from both pine (Pinaceae) species was compared using a general linear model. Flight capacity (flight distance and duration) of the parent beetles introduced to the two pine hosts was compared with two sample t-tests. The effect of pine host and adult flight treatment on gallery length was analysed using a general-mixed effect model (Table 2).
Table 2. Summary of the mixed-effect models used for analyses of average gallery length, total, female and male offspring number, offspring body size, and body condition residual index of mountain pine beetles. Each model tests for effects of fixed factors and the interaction between fixed factors with gallery initiation date and average gallery length per bolt specified as the random factors in each case.

We used generalised mixed-effect models (Bates et al. Reference Bates, Maechler, Bolker and Walker2015) with gallery length/gallery initiation date per bolt specified as a random variable and Poisson error distributions to analyse the effect of parental flight treatment, pine species, and the tree that bolts were obtained from on the number of offspring produced per bolt (Table 2). The effect of parental flight treatment and pine species on offspring sex ratio was tested using a χ2 test. Average offspring body size per bolt was compared using a general-mixed effect model (Bates et al. Reference Bates, Maechler, Bolker and Walker2015) with flight treatment, pine species, offspring sex, and tree specified as fixed factors and gallery length/gallery initiation date per bolt specified as a random effect (Table 2). Offspring condition was assessed using the offspring body condition residual index. A general-mixed effects model compared the body-condition residual indices of offspring produced by flown and control beetles in the two species of pine in which gallery length/gallery initiation date per bolt specified as a random factor (Table 2). Model simplification was achieved using analysis of variance comparisons and by comparison of Akaike information criterion values. The models were checked for homogeneity of variance using Levene’s test and for overdispersion using one sample Kolmogorov–Smirnov test in DARMa package (Hartig Reference Hartig2018). Model residuals were checked for normality using the Shapiro–Wilk test. The model fit was checked using pseudo R2 values. A Tukey’s post hoc test was conducted to test the separation of means of each model (R version 3.1.1 (R Core Development Team 2014)).
Voucher specimens of insects used in this study are housed in the E.H. Strickland Museum, Department of Biological Sciences, University of Alberta, Edmonton, Alberta, Canada.
Results
The mean phloem width was similar between jack pine (0.74 ± 0.05 mm) and lodgepole pine (0.81 ± 0.06 mm) bolts used for introduction of the flown and control parent beetles (t 13 = −0.85, P = 0.41), but lodgepole pine bolts had a larger diameter on average (27.9 ± 1.36 cm) compared to jack pine bolts (23.97 ± 1.15 cm) (F 1,12 = 9.41, P = 0.01) (Table 1). Average gallery length did not differ between the two host tree species (F 1,12 = 0.01, P = 0.92) and was not affected by adult flight treatment (F 1,12 = 1.09, P = 0.36) (Table 1).
Preflight weight was similar for beetles subjected to the flight treatment (10.68 ± 0.30 mg) and those used as unflown control beetles (10.34 ± 0.31 mg) (t 155 = 0.67, P = 0.22). The flight capacity of the beetles (n = 77) that were subsequently introduced to the two pine hosts was similar between host species (flight duration: F 1,74 = 0.31, P = 0.58; flight distance: F 1,74 = 0.38, P = 0.54), but females flew longer (F 1,74 = 8.64, P = 0.0044) and further (F 1,74 = 12.22, P = 0.0008) than males. The average (± SE) flight duration of beetles was 3.20 ± 0.36 hours and the average flight distance was 5.61 ± 0.35 km. As expected, flown beetles lost significantly more weight (1.2 ± 0.04 mg) during the bioassay compared to unflown control beetles (0.78 ± 0.003 mg) (t 181 = 58.367, P < 0.0001). A total of 122 beetle pairs were introduced to the bolts. Ninety-two pairs accepted the hosts, but only 80 pairs were successful in establishing galleries (Table 1). The gallery length results should be interpreted with caution, as gallery construction by adult beetles may not have been completed before the bolts were moved to 5 °C.
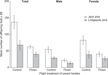
There was an interaction between parental flight treatment and pine species that affected the total number of offspring produced per bolt (χ2 1 = 5.4416, P = 0.0197) (Fig. 1). Control beetles produced more offspring than flown beetles in both pine hosts (Tukey’s post hoc test, P < 0.05), but more offspring emerged from jack than lodgepole pine (χ2 1 = 14.8712, P = 0.001). There was a significant interaction of flight treatment and tree that affected the total number of offspring (χ2 4 = 11.3744, P = 0.0227). In lodgepole pine, flown parents produced fewer offspring than control parents in only one experimental bolt. In jack pine, flown parents produced fewer offspring than control parents in all trees (Tukey’s post hoc test, P < 0.05). An interaction of flight treatment and tree affected the number of female offspring (χ2 4 = 11.5055, P = 0.0422). Flown parents produced fewer female offspring than control beetles in one of the trees in both hosts. More female offspring emerged from jack pine than lodgepole pine (χ2 1 = 14.81, P < 0.0001). An interaction effect between flight treatment and tree influenced the number of male offspring (χ2 2 = 29.7817, P < 0.0001). Flown parents produced fewer male offspring than control parents in two jack pine trees and one lodgepole pine tree (Tukey’s post hoc test, P < 0.05). The sex ratio of offspring of flown adults in lodgepole (1.93:1, female: male) was marginally more female biased than the sex ratio of offspring of control beetles in lodgepole pine (1.3:1) (χ2 1 = 3.44, P = 0.05). The sex ratio did not differ by parental flight treatment (χ2 1 = 1.8, P = 0.17) for offspring reared in jack pine (flown: 1.87: 1; control: 1.5: 1, female:male).

Fig. 1. Average number of offspring produced per bolt produced by flown and control parental mountain pine beetles subsequently introduced to lodgepole pine and jack pine for reproduction. Six to nine pairs of flown and control adult beetles were introduced to individual lodgepole pine (n = 4 per treatment) and jack pine (n = 4 per treatment) bolts. Emergent males, females, and total number of offspring were counted (n = 1405).
Parental flight treatment did not influence offspring body size (χ2 1 = 0.19947, P = 0.1579). The pine species offspring were reared in also did not influence body size (χ2 1 = 0.5348, P = 0.4646). There was no effect of individual tree on the body size of offspring (χ2 1 = 0.4090, P = 0.9817). As would be expected, female offspring were larger than males (χ2 1 = 303.9, P < 0.0001). An interaction between pine species and flight treatment influenced the body condition residual index of offspring (F 1,16 = 5.2787, P = 0.0472). Offspring from flown adults had a slightly lower body condition index compared to the offspring from control parents in lodgepole pine (Tukey’s post hoc test, P < 0.05) (Fig. 2). Control parents produced offspring with a slightly higher body condition index in lodgepole pine than in jack pine. The body condition residual index did not differ between male and female beetles (F 1,16 = 1.2864, P = 0.2745) (Fig. 2).

Fig. 2. Body condition residual indices of male and female mountain pine beetle offspring produced by flown and control parental beetles in lodgepole and jack pine (n = 1384). Six to nine pairs of parental beetles were introduced to lodgepole pine (n = 4 per treatment) and jack pine (n = 4 per treatment) bolts.
Discussion
This study reveals an impact of flight on subsequent reproduction in the mountain pine beetle. Beetles subjected to a flight treatment before inoculation into bolts produced fewer offspring per bolt than control beetles. Individual tree effects, however, influenced the offspring number produced by flown adults. Flown adults produced fewer offspring than control beetles only in one lodgepole pine tree. In jack pine, flown adults produced fewer offspring than control beetles in all three trees. It is not known, however, if offspring produced per female is affected by flight treatment. In previous studies, reduced body condition induced by starvation of mountain pine beetle adults did not impact the number of eggs laid by females (Elkin and Reid Reference Elkin and Reid2005). This suggests that flown beetles are able to compensate, at least in part, for lost energy through feeding in the newly colonised tree (Elkin and Reid Reference Elkin and Reid2005). Female mountain pine beetles can allocate resources to somatic condition or reproductive investment but this allocation process is independent of beetle condition (Elkin and Reid Reference Elkin and Reid2005). In the few studies that directly test for an effect of flight on reproduction in other bark and ambrosia beetles, evidence for trade-offs is equivocal (Biedermann et al. Reference Biedermann, Klepzig and Taborsky2011; Fraser et al. Reference Fraser, Brahy, Mardulyn, Dohet, Mayer and Grégoire2014). Trade-offs between flight and reproduction, however, is evident in other insects (Isaacs and Byrne Reference Isaacs and Byrne1998; Zhang et al. Reference Zhang, Wu, Wyckhuys and George2009; Gibbs and Dyck Reference Gibbs and Dyck2010; Elliott and Evenden Reference Elliott and Evenden2012; Duthie et al. Reference Duthie, Abbott and Nason2014). Future studies on reproductive trade-offs in the mountain pine beetle would benefit from assessment of offspring production per female in live tree hosts (Esch et al. Reference Esch, Langor and Spence2016).
The number of offspring produced per bolt was also influenced by the species of bolt that beetles were reared in. More offspring emerged from jack pine than lodgepole pine. Studies to date are highly variable with regard to host effects on reproductive output of mountain pine beetles. Similar numbers of offspring per mated pair of adults emerge from bolts of lodgepole, jack, and red pine (Pinus resinosa Aiton) (Cale et al. Reference Cale, Taft, Najar, Hughes, Sweeney and Erbilgin2015). Although establishment of egg galleries is greater in lodgepole pine than whitebark pine (Pinus albicaulis Engelmann) bolts, both hosts are equally suitable for brood production in terms of offspring number and offspring fat content (Esch et al. Reference Esch, Langor and Spence2016). Naturally infested bolts of limber pine (Pinus flexilis James) produce more larvae with larger body size compared to offspring produced in bolts of lodgepole pine, on a per-bolt basis (Cerezke Reference Cerezke1995). Beetles had higher fecundity and produced more eggs in living stands of limber pine compared to lodgepole pine (Langor Reference Langor1989). Artificially infested lodgepole pine bolts produced fewer offspring with smaller females as compared to four other pine host species (Amman Reference Amman1982). Similarly, beetles inoculated into lodgepole pine bolts in our study produced fewer offspring compared to jack pine. Although brood production increases with the phloem thickness (Amman and Pasek Reference Amman and Pasek1986), phloem width did not vary with host species in the current study.
Mountain pine beetles that emerge from jack pine contain higher fat reserves than those from lodgepole pine (Lusebrink et al. Reference Lusebrink, Erbilgin and Evenden2016). Nutritional quality may differ between the two pine hosts and affect the success of developing brood. Jack pine contains a higher concentration of fatty acids compared to lodgepole pine (Ishangulyyeva et al. Reference Ishangulyyeva, Najar, Curtis and Erbilgin2016). Reproductive success of the ambrosia beetle Pityopthorus lautus Eichhoff (Coleoptera: Curculionidae: Platypodinae) correlates with phloem nitrogen and carbohydrate levels (Kirkendall Reference Kirkendall1983). Host defensive chemistry may also impact brood development, but further studies on mountain pine beetle reproduction in jack pine in nature are required as jack pine monoterpene composition varies geographically with climatic conditions, which may influence host susceptibility (Taft et al. Reference Taft, Najar, Godbout, Bousquet and Erbilgin2015). The trees for each pine species tested in the current study were selected from the same stand, and the differences in host suitability between species revealed here may simply reflect stand and not pine species differences.
Offspring body size was not affected by parental flight treatment. The reduction in fat reserves during flight (Evenden et al. Reference Evenden, Whitehouse and Sykes2014) may be compensated for by feeding in the newly colonised host (Elkin and Reid Reference Elkin and Reid2005). A significant interaction between flight treatment and pine species influenced offspring body condition in this study. Offspring of control mountain pine beetles have better body condition than offspring from flown beetles when reared in lodgepole pine, but flight did not affect the offspring body condition in jack pine. The effect of flight on offspring condition may vary with the host species. Control beetles produced offspring with slightly higher body condition when reared in lodgepole pine than jack pine, suggesting that a maternal effect may render offspring better able to resist the defences produced by lodgepole pine. Lodgepole pine produces higher levels of defensive monoterpenes than does jack pine (Clark et al. Reference Clark, Pitt, Carroll, Lindgren and Huber2014; Lusebrink et al. Reference Lusebrink, Erbilgin and Evenden2016). Beetles in good condition can survive higher concentrations of monoterpenes compared to beetles in poor body condition (Reid and Purcell Reference Reid and Purcell2011; Manning and Reid Reference Manning and Reid2013; Reid et al. Reference Reid, Sekhon and LaFramboise2017). Nutritional quality of the two hosts may also affect offspring condition. Total phloem nitrogen content is higher in lodgepole pine than jack pine (Lusebrink et al. Reference Lusebrink, Erbilgin and Evenden2016), while jack pine contains higher concentrations of fatty acids compared to lodgepole pine (Ishangulyyeva et al. Reference Ishangulyyeva, Najar, Curtis and Erbilgin2016). The body condition residual index should, however, be interpreted with caution because it may not predict energy stores of individual beetles (Kelly et al. Reference Kelly, Tawes and Worthington2014).
The sex ratio of the offspring produced by both flown and unflown control beetles was female biased. The offspring of control beetles emerged in 1.3:1 female:male ratio from lodgepole pine which was marginally lower than the 1.93:1 female:male ratio that emerged from lodgepole pine infested with flown beetles. The flight treatment of parents did not influence the sex ratio of offspring reared in jack pine as a 1.5:1 and 1.87:1 female:male ratio emerged from bolts infested by control and flown parents, respectively. The sex ratio of offspring from flown beetles is similar to the sex ratio of emergent mountain pine beetle recorded from naturally infested trees (Reid Reference Reid1958; Safranyik Reference Safranyik1976; Amman Reference Amman1984; Amman and Bartos Reference Amman and Bartos1991; Lachowsky and Reid Reference Lachowsky and Reid2014). The female-biased sex ratio in natural conditions is most likely due to male winter mortality during development (Lachowsky and Reid Reference Lachowsky and Reid2014; James et al. Reference James, Janes, Roe and Cooke2016). Additional mechanisms such as body lipid content may contribute to the sex-ratio bias (Lachowsky and Reid Reference Lachowsky and Reid2014) as cold tolerance depends on lipid content in bark beetles (Lombardero et al. Reference Lombardero, Ayres, Ayres and Reeve2000). Both preflight (Reid and Purcell Reference Reid and Purcell2011; Graf et al. Reference Graf, Reid, Aukema and Lindgren2012) and postflight (Evenden et al. Reference Evenden, Whitehouse and Sykes2014) adult males have lower absolute and relative amounts of fat compared to females. Male larvae may also have less fat, which would make them less tolerant to cold temperatures. The cold conditions that mountain pine beetle offspring were subjected to in the current study (5 °C), however, would not be expected to induce much mortality, which may be why the sex ratios observed in our study were not as strongly female biased as those typically observed in nature. The slight difference in offspring sex ratios produced by control and flown adult beetles in lodgepole pine may be related to body condition of the offspring. Flown beetles with less fat than control beetles (Evenden et al. Reference Evenden, Whitehouse and Sykes2014) may produce smaller offspring (Elkin and Reid Reference Elkin and Reid2005).
Our results indicate that mountain pine beetles have physiological trade-offs between flight and reproduction in terms offspring produced per bolt and offspring condition. This suggests that prolonged adult dispersal may decrease beetle fitness despite the possibility of locating a higher quality host or unrelated mates (Chubaty et al. Reference Chubaty, Roitberg and Li2009). Further studies with more trees from different stands are needed to assess the effects of flight on offspring fitness in different host species. Pioneer female mountain pine beetles that fly long distances in search of suitable hosts produce fewer offspring than nonpioneering females that join the aggregation later in the dispersal period (Latty and Reid Reference Latty and Reid2009). Beetles with a moderate level of energy, however, are more likely to pioneer while beetles with smallest and greatest energy reserves avoid pioneering (Latty and Reid Reference Latty and Reid2010). It is not clear from this study whether the trade-off between flight and reproduction exists on a per capita basis.
This study and others (Erbilgin et al. Reference Erbilgin, Ma, Whitehouse, Shan, Najar and Evenden2014; Lusebrink et al. Reference Lusebrink, Erbilgin and Evenden2016) show that the novel jack pine host is suitable for mountain pine beetle brood production using artificially infested bolts, although offspring condition is better in the native lodgepole pine host. Studies that use naturally infested pine bolts (Cerezke Reference Cerezke1995) and live standing trees (Cullingham et al. Reference Cullingham, Cooke, Dang, Davis, Cooke and Coltman2011) also show that jack pine is a suitable host for brood production. The physiological trade-offs between flight and reproduction appear to vary with host species. Future research will be needed to understand how the effect of energy use during flight on subsequent reproduction can be influenced by environmental factors and affect the host colonisation pattern in the expanding range of the mountain pine beetle in the boreal forest.
Acknowledgements
We thank Devin Letourneau of Alberta Agriculture and Forestry for bolt collection and Caitlin Reich for carrying out the flight bioassay. This research was supported by a grant to Maya Evenden from the Natural Science and Engineering Research Council of Canada (grant number NET GP 434810-12) to the Turning Risk Into Action Network, with contributions from Alberta Agriculture and Forestry, fRI Research, Manitoba Conservation and Water Stewardship, Natural Resources Canada – Canadian Forest Service, Northwest Territories Environment and Natural Resources, Ontario Ministry of Natural Resources and Forestry, Saskatchewan Ministry of Environment, West Fraser, and Weyerhaeuser. Research presented in this manuscript was conducted in accordance with all applicable laws and rules set forth by provincial and federal governments and the University of Alberta, and all necessary permits were held when the research was conducted.