Introduction
Operational sex ratio, the ratio of sexually active males to sexually active females at a given time and location (Emlen and Oring Reference Emlen and Oring1977), is a key parameter in the evolution of sex differences. Operational sex ratio is known to have a profound effect on the fitness of both sexes and can influence mating behaviour and intensity of sexual selection (Prohl Reference Prohl2002; Head and Brooks Reference Head and Brooks2006), and inter-sexual and intra-sexual competition (Berglund Reference Berglund1994; Kvarnemo et al. Reference Kvarnemo, Forsgren and Magnhagen1995; Grant and Foam Reference Grant and Foam2002; Ros et al. Reference Ros, Zeilstra and Oliveira2003). It is modified by two important factors: adult sex ratio (Ahnesjo et al. Reference Ahnesjo, Kvarnemo and Merilaita2001), which is the ratio of males and females, and potential reproductive rate (Clutton-Brock and Vincent Reference Clutton-Brock and Vincent1991), which is the potential reproduction of offspring per unit time.
The sex of the predominant competitor is based on and similar to the sex in whose favour the operational sex ratio is biased (Kvernemo et al. 1995). It is also known to increase the intensity of competition for mates as well as the rejection rate in the more abundant sex, with increased selectivity in the limiting sex (Weir et al. Reference Weir, Grant and Hutchings2011). In Bicyclus anynana (Butler) (Lepidoptera: Nymphalidae), more male–male competition was observed when there was more specific male-biased sex ratio and decreasing cage volume (Holveck et al. Reference Holveck, Gauthier and Nieberding2015). However, females accept matings more with decreasing cage volume and increasing density.
Male-biased operational sex ratio has several direct and indirect effects on behavioural patterns. Direct benefits include competition for mates, which can occur via scrambles, endurance rivalry, contests, and mate choice (Andersson Reference Andersson1994). Male-biased sex ratio also results in sperm competition since females mate more than once. This is indicative that males can modify the total production of seminal fluid proteins and sperm and can also optimise their ejaculation (Fedorka et al. Reference Fedorka, Winterhalter and Ware2011). In Drosophila melanogaster Meigen (Diptera: Drosophilidae), females mated with male-exposed males mated longer and were more fecund probably due to precopulatory perception of sperm competition (Bretman et al. Reference Bretman, Fricke and Chapman2009). The indirect effects of male-biased operational sex ratio include physical harassment of the female by aggressive males causing reduction in their longevity in Musca domestica Linnaeus (Diptera: Muscidae) (Ragland and Sohal Reference Ragland and Sohal1973) and Hippodamia convergens Guérin-Méneville (Coleoptera: Coccinellidae) (Bayoumy and Michaud Reference Bayoumy and Michaud2014). Extreme male competition may hamper the reproductive fitness of females, such as the number of offspring produced and their development (Holland and Rice Reference Holland and Rice1999; Friberg and Arnqvist Reference Friberg and Arnqvist2003; Soares and Serpa Reference Soares and Serpa2007).
Not only variations in operational sex ratio, but also other factors like body size, growth rate, and age, are known to modulate the reproductive output of individuals as suggested by life history theory (Stearns Reference Stearns1992). Increasing age is a major factor constraining reproductive success in insects (Fox Reference Fox1993; Bonduriansky and Brassil Reference Bonduriansky and Brassil2002). Ageing is known to (i) reduce offspring survivorship (Hercus and Hoffmann Reference Hercus and Hoffmann2000; Kern et al. Reference Kern, Ackermann, Stearns and Kawecki2001), (ii) decrease longevity of offspring (Priest et al. Reference Priest, Mackowiak and Promislow2002), (iii) diminish fertility (Kindlmann et al. Reference Kindlmann, Dixon and Dostálková2001; Heinze and Schrempf Reference Heinze and Schrempf2012), and (iv) decrease sperm production and viability (Johnson and Gemmell Reference Johnson and Gemmell2012). This deterioration in reproductive performance with increasing age has been observed in the burying beetle, Nicrophorus vespilloides Herbst (Coleoptera: Silphidae) (Cotter et al. Reference Cotter, Ward and Kilner2010) and in Callosobruchus maculatus (Fabricius) (Coleoptera: Chrysomelidae) (Fox Reference Fox1993). The good gene hypothesis predicts that females should prefer older males over the young males (Trivers Reference Trivers and Campbell1972; Halliday Reference Halliday, Krebs and Davies1978, Reference Halliday and Bateson1983; Manning Reference Manning1985; Kirkpatrick Reference Kirkpatrick1987; Andersson Reference Andersson1994; Kokko and Lindstrom Reference Kokko and Lindstrom1996) since older males signal better genes for survival. In Anastrepha ludens (Loew) (Diptera: Tephritidae), older and more experienced males invest more time in copulation than the younger males (Perez-Staples et al. Reference Perez-Staples, Martinez-Hernandez and Aluja2010), and female remating behaviour is determined by male age (Abraham et al. Reference Abraham, Contreres-Navaro and Perez-Staples2016). The literature also shows that in some insects, middle-aged males are preferred as mates (Beck and Powell Reference Beck and Powell2000; Pandey and Omkar Reference Pandey and Omkar2013). In B. anynana, females prefer to mate with middle-aged males over the young males, and this preference is based on specific pheromone component, hexadecanal whose quantity is age-specific (Nieberding et al. Reference Nieberding, Fischer, Saastomoinen, Allen, Wallin, Adenstrom and Brakefield2012). In Coccinellidae, females lay eggs in an age-specific pattern that assumes triangular shape (Dixon and Agarwala Reference Dixon and Agarwala2002), and increases in female and male age are known to affect fecundity and egg viability, respectively. In Ephestia kuehniella Zeller (Lepidoptera: Pyralidae), younger females showed a higher reproductive rate than older aged females (Xu and Wang Reference Xu and Wang2009). Old males of Menochilus sexmaculatus (Fabricius) (Coleoptera: Coccinellidae) mated and reproduced poorly relative to the young and middle-aged males (Singh and Omkar Reference Singh and Omkar2009).
Offspring of younger males have a longer life span than the older males (Hansen and Price Reference Hansen and Price1995; Price and Hansen Reference Price and Hansen1998). However, in Hippodamia convergens, old-aged females produced offspring of higher fitness (Vargas et al. Reference Vargas, Michaud and Nechols2012) than younger females.
Menochilus sexmaculatus, commonly known as the zigzag beetle, was selected for experimentation due to its abundance in local fields, high reproductive output, and wide prey range (Agarwala and Yasuda Reference Agarwala and Yasuda2000). The presence of basic information about precopulatory (Dubey et al. Reference Dubey, Omkar and Mishra2016a, Reference Dubey, Omkar and Mishra2016b) and postcopulatory mate choice behaviour (Chaudhary et al. Reference Chaudhary, Mishra and Omkar2015) further provides a good foundation to build upon. The number of competitors (Chaudhary et al. Reference Chaudhary, Mishra and Omkar2017) and age of mates (Omkar et al. Reference Omkar, Pervez, Mishra, Srivastava, Singh and Gupta2005) are known to affect the mating behaviour, reproductive performance (Mishra and Omkar Reference Mishra and Omkar2004; Pervez et al. Reference Pervez, Omkar and Richmond2004; Srivastava and Omkar Reference Srivastava and Omkar2004), and mate preference (Dubey et al. Reference Dubey, Omkar and Mishra2016a, Reference Dubey, Omkar and Mishra2016b). However, the effects of age in different sex ratio treatments need to be explored. Thus, the rationale of the present experiment is to study whether the presence of competitors affects the performance of males of different ages. We hypothesised that the presence of competitors will enhance mating parameters (time of commencement of mating, copulation duration), reproductive attributes (number of eggs in the first clutch and their viability), and total development duration of offspring irrespective of paternal age. However, in terms of age, older males should perform worse than the young and middle-aged males across sex ratio treatments.
Materials and methods
Maintenance of laboratory stock
Adults of M. sexmaculatus (voucher specimens deposited in the National Zoological Collections, Zoological Survey of India, Gangetic Plains Regional Centre, Patna, India; accession number: 3142) were collected (50 beetles) from the agricultural fields of Lucknow, India (26°50′N, 80°54′E). These were paired in plastic Petri dishes (9.0 × 2.0 cm) and fed on an ad libitum supply of bean aphid, Aphis craccivora Koch (Hemiptera: Aphididae) (infested on cowpea, Vigna unguiculata (Linnaeus) Walpers (Fabaceae) reared in glasshouses at 25 ± 2 °C, 65 ± 5% relative humidity). The Petri dishes containing mating pairs were placed in Biochemical Oxygen Demand incubators (YSI-440; Yorco Super Deluxe, New Delhi, India) at 27 ± 1 °C; 65 ± 5% relative humidity; 14:10 light:dark hours, and inspected twice daily (1000 and 1500 hours) for oviposition. This was done to acclimatise the M. sexmaculatus to laboratory conditions. The eggs laid were separated daily and held in plastic Petri dishes (size as above) until hatching. Each hatched larva was reared individually in a Petri dish (size as above) until adult emergence to avoid larval cannibalism.
Experimental stock
Males and females from the above basal stock were allowed to mate under laboratory conditions (same as above). After natural dislodging, females were kept separately in a plastic Petri dish (size same as above). Eggs were separated daily and larvae were reared to adult. Emerged adults were further maintained in isolated well-fed conditions until they reached the appropriate ages for experimentation: 10–15-day olds for the young age treatment (Y), 30–40-day olds for middle age treatment (M), and 50–60-day olds for the old age treatment (O) (following Singh and Omkar Reference Singh and Omkar2009).
Experiment
Following the methodology of Carrillo et al. (Reference Carrillo, Danielson-Francois, Siemann and Meffert2012), we set up three different treatments simulating different operational sex ratios: no (1♀:1♂), moderate (1♀:2♂), and extreme (1♀:5♂) competition. Except for the first treatment, which also acted as a control, the other two sex ratios were male-biased. As per Singh and Omkar (Reference Singh and Omkar2009), middle-aged females are better performers than young and old-aged females; therefore, we took middle-aged females as the focal individuals. Males based on treatment were young, middle, or old aged. Males of similar age were placed in Petri dishes (14 × 2.0 cm), into which females were introduced. As soon as mating commenced, all males except the male that started mating with female were removed from the Petri dish to avoid interference with the mating pair. In cases where mating did not occur within 30 minutes, the replicate was discarded. Each treatment of operational sex ratio and age was replicated 15 times. Mating was observed until the pair separated itself. Mating parameters, that is, time to commence mating and copulation duration, were recorded for each replicate.
Mated females were isolated and observed until the first clutch of eggs was laid. The eggs were counted and kept separately in Petri dishes (9.0 × 2.0 cm) until hatching. Each hatched larva was reared individually in a Petri dish (size as above) until adult emergence. Total developmental duration of offspring, that is, from incubation period to adult emergence was observed for each replicate.
Statistical analysis
Data on dependent factors (mating parameters, number of eggs in the first clutch, egg viability, and total developmental duration) were normally distributed (Kolmogorov–Smirnoff test) with a homogeneous variation. Furthermore, the aforementioned data (dependent factors) were analysed by the general linear model with sex ratio and male age as independent factors. All the analyses were followed by the comparison of means using post hoc Tukey’s honest significance test at 5%. The correlation between the copulation duration and reproductive parameters was subjected to regression analysis (Figs. 4–5). All analyses were performed using MINITAB-16 statistical software (Minitab, State College, Pennsylvania, United States of America).
Results
Time to commence mating and copulation duration were significantly influenced by sex ratio and male age (Table 1). The interactions between these factors were also significant (Table 1). Mating commences within 1.7–2.2 minutes in the extreme competition treatment, followed by modest (3.0–4.6 minutes) and no competition (4.6–5.2 minutes) treatments (Fig. 1A). Older males showed significantly delayed commencement of mating (5.2–2.2 minutes) in comparison to middle-aged (3.0–1.3 minutes) and young males (4.6–1.7 minutes) irrespective of treatments (Fig. 1A). Males in extreme competition treatment (1:5) mated for 127.0–145.4 minutes as compared to the other treatments (Fig. 1B). However, old males showed a longer mating duration in all sex-ratio treatments (Fig. 1B).
Table 1. Mating and reproductive parameters of Menochilus sexmaculatus when females mated with different aged males in different sex ratio treatments.


Figure 1. Mating parameters of Menochilus sexmaculatus (A) Time of commencement of mating, (B) Copulation Duration, when females mated with males of different ages in different sex ratios. Values are mean ± standard error. Sample size n = 15 replicates per treatment. Lower case letters represent the comparison of mean within each sex ratios treatment. Upper case letters in brackets represent comparison of mean across different sex ratios. Similar alphabet indicates lack of significant difference at P < 0.001.
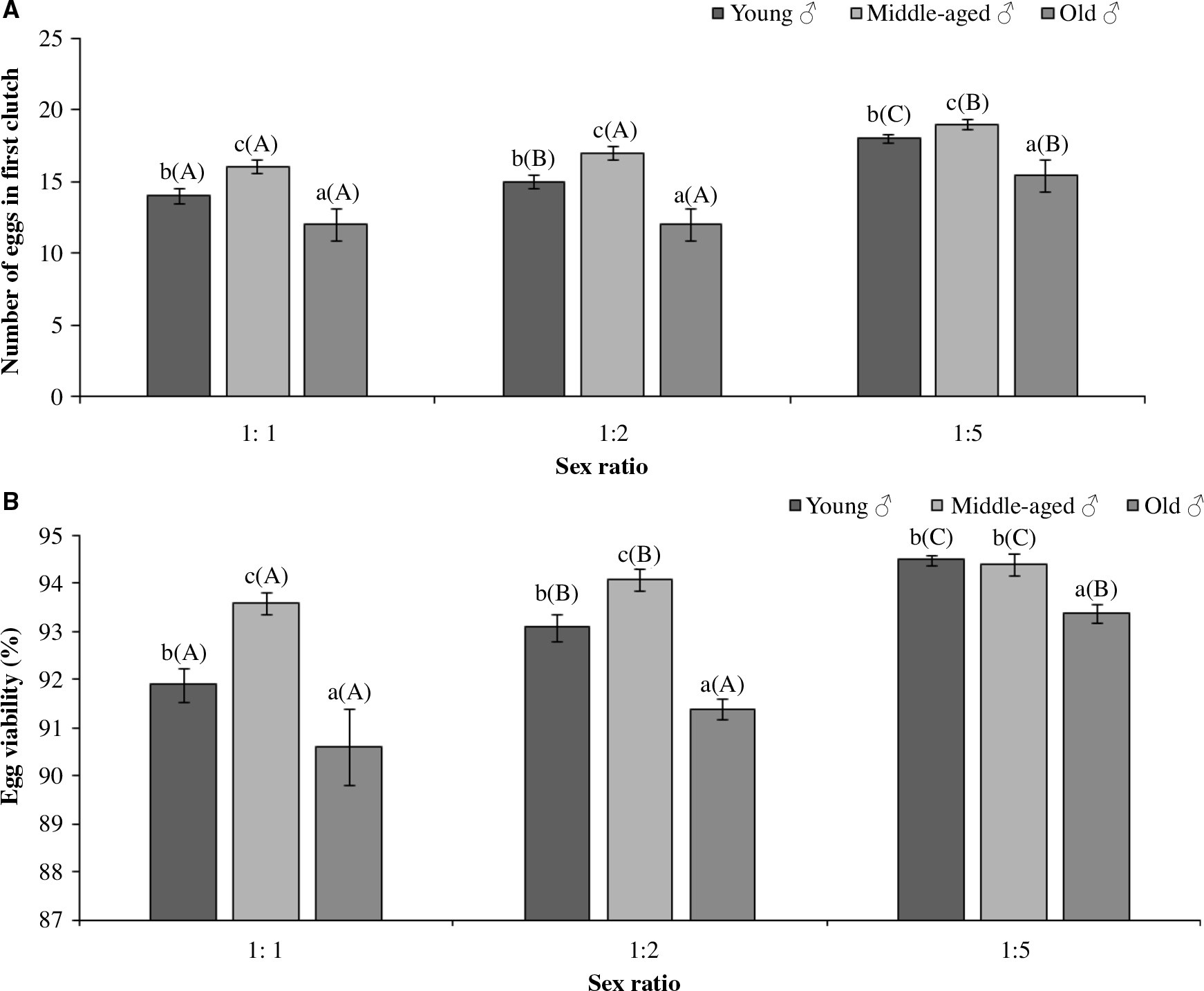
Both the sex ratio and age of males had significant effects on the number of eggs laid in the first clutch and their per cent viability. Results also revealed significant influence of sex ratio and age of males on the number of eggs laid in the first clutch (Table 1). However, the interaction between the two factors was insignificant (Table 1). Comparison of means revealed that in extreme competition treatments involving different aged males, the number of eggs laid in the first clutch was more for the middle-aged males (16–19 eggs) and least for the old-aged males (12.0–15.4 eggs) (Fig. 2A).
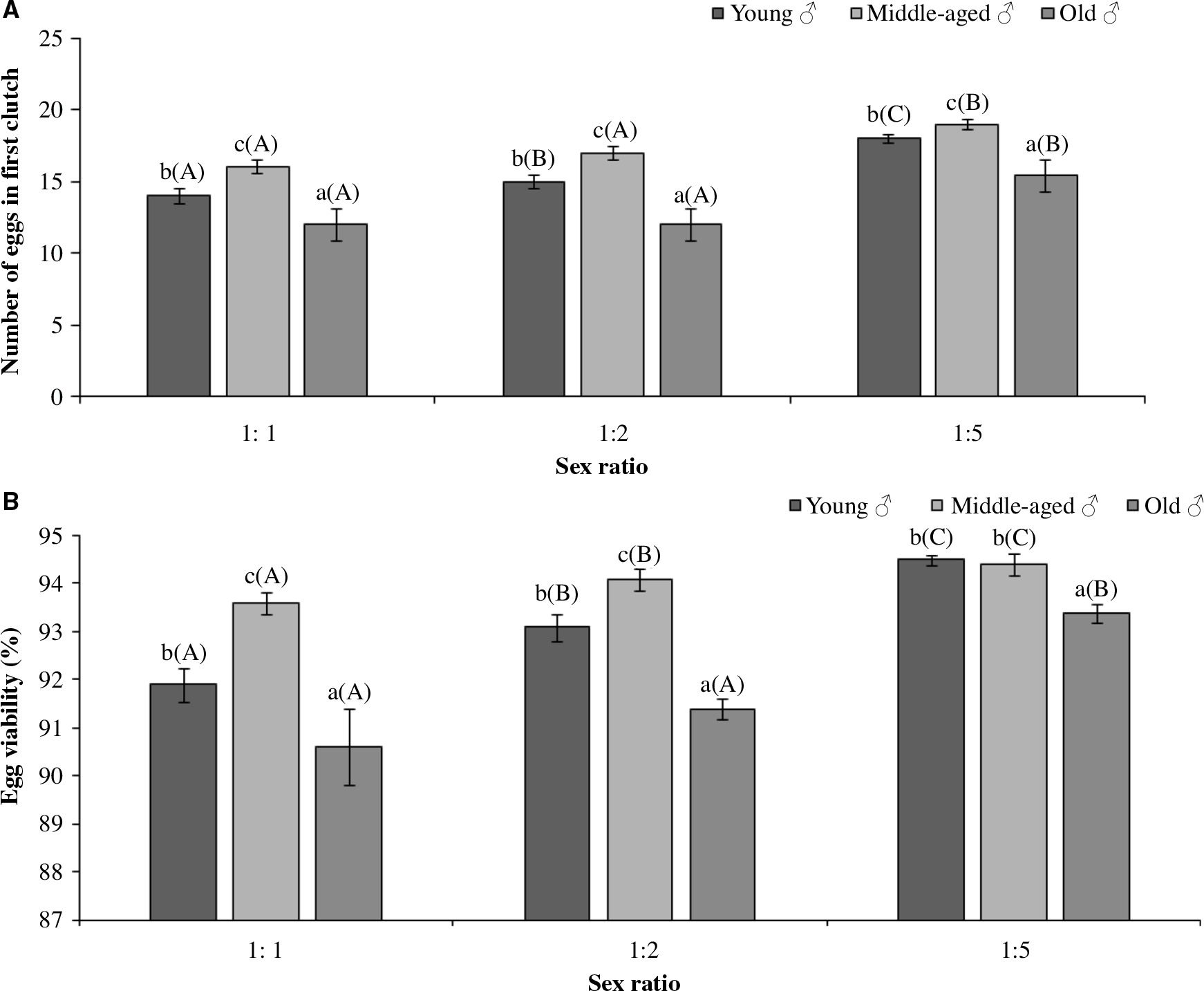
Figure 2. Reproductive parameters of Menochilus sexmaculatus (A) Number of eggs in first clutch, (B) Percent Egg Viability, when females mated with males of different ages in different sex ratios. Values are mean ± standard error. Sample size n = 15 replicates per treatment. Lower case letters represent the comparison of mean within each sex ratios treatment. Upper case letters in brackets represent comparison of mean across different sex ratios. Similar alphabet indicates lack of significant difference at P < 0.001.
Similarly, percentage egg viability was significantly influenced by both the sex ratio and the age of males (Table 1). The interaction between the above two factors was insignificant (Table 1). Thus, between the different sex ratio trials, treatments with more number of males had 93.4–94.5% viability (Fig. 2B). Furthermore, irrespective of the sex ratio, the lowest percentage egg viability was found when females mated with older males (90.6–93.4%) (Fig. 2B).
Sex ratio did not affect offspring development duration, while the age of males significantly influenced the offspring developmental duration (Table 1). The interaction between the two independent factors was also insignificant (Table 1). There was no significant difference in offspring development across all the trials (Fig. 3). However, across all sex ratios, the longest developmental duration was 14 days when females copulated with old males (Fig. 3).

Figure 3. Offspring developmental duration of Menochilus sexmaculatus when females mated with males of different ages in different sex ratios. Values are mean ± standard error. Sample size n = 15 replicates per treatment. Lower case letters represent the comparison of mean within each sex ratios treatment. Upper case letters in brackets represent comparison of mean across different sex ratios. Similar alphabet indicates lack of significant difference at P < 0.001.
The regression analysis was conducted to see the relationship between the reproductive parameters and copulation duration. The relation of copulation duration with size of the first clutch and per cent viability showed a linear trend, that is, the number of eggs increased with further increase in copulation duration when females mated with young, middle-aged, and old males in each sex ratio treatment (Figs. 4–5).

Figure 4. Relation between copulation duration and number of eggs in first clutch in different sex ratio treatments when females mated with different age males. A, Young age. B, Middle age. C, Old age.

Figure 5. Relation between copulation duration and per cent egg viability in different sex ratio treatments when females mated with different age males. A, Young age. B, Middle age. C, Old age.
Discussion
The present study reveals that sex ratio and male age influence the mating and reproductive behaviour of M. sexmaculatus. In all the sex ratio treatments, an extreme competition environment elevated the performance of the males. Between different aged males, older males underperformed in comparison to young and middle-aged males. This indicates that young and middle-aged males are probably more capable of overcoming the constraints of prevailing intrasexual competition, owing to their better fitness levels in terms of mating and reproductive parameters.
As seen from the figures, males performed better in terms of mating and reproductive parameters under extreme competition (1:5) followed by modest (1:2) and no competition (1:1) under all age treatments. The early commencement of mating in the presence of potential male competitors has also been reported in previous studies (Krupa and Sih Reference Krupa and Sih1993; Jormalainen et al. Reference Jormalainen, Tuomi and Yamamura1994; Dick and Elwood Reference Dick and Elwood1996; Clutton-Brock et al. Reference Clutton-Brock, Rose and Guinness1997). Among age groups, the delay in commencement of mating by old individuals may be attributed to the female assessment of male quality followed by mating avoidance with older males (Bonduriansky and Brassil Reference Bonduriansky and Brassil2002; Jones and Elgar Reference Jones and Elgar2004; Beck and Promislow Reference Beck and Promislow2007; Omkar et al. Reference Omkar, Pandey, Rastogi and Mishra2010). The decreased mating performance of old males has also been reported in other Coccinellidae species (Bista and Omkar Reference Bista and Omkar2015). The copulation duration, as shown in Figure 1B, was higher under extreme competition, which is probably a consequence of the increased possibility of sperm competition due to the male-biased sex ratio (Siva-Jothy Reference Siva-Jothy1987; Siva-Jothy and Tsubaki Reference Siva-Jothy and Tsubaki1989; Parker Reference Parker1993; Chaudhary et al. Reference Chaudhary, Mishra and Omkar2017). This has also been previously observed in Rhagoletis juglandis (Cresson) (Diptera: Tephritidae) (Alonso-Pimentel and Papaj Reference Alonso-Pimentel and Papaj1996) and M. sexmaculatus (Chaudhary et al. Reference Chaudhary, Mishra and Omkar2017).
The sex ratio not only influenced the time of commencement of mating and copulation duration but also the reproductive parameters. From Figure 2A–B, it is evident that the number of eggs laid in the first clutch, and percentage egg viability was elevated under extreme competition, which could be due to the elevated production of accessory seminal products. Studies across taxa have shown that ejaculatory investment is dependent on male–male interactions and also modifies the levels of sperm competition (Simmons Reference Simmons2001; Pound and Gage Reference Pound and Gage2004; Simmons et al. Reference Simmons, Emlen and Tomkins2007). In Drosophila Fallén species, premating acquaintance with a rival male increased the transfer of seminal protein ovulin and sex peptides to females (Wigby et al. Reference Wigby, Sirot, Linklater, Buehner, Calboli, Bretman and Chapman2009). Among age groups, females mated with older males produced the fewest eggs in the first clutch and had the lowest percentage egg viability. Older males are known to produce poor-quality sperms (Amin et al. Reference Amin, Bussière and Goulson2012) in smaller quantities and have a reduced ability to transfer sperm (Obata Reference Obata1987; Pervez et al. Reference Pervez, Omkar and Richmond2004). The decline in reproductive aptitude with an increase in age has been reported in Dermestes maculatus De Geer (Coleoptera: Dermestidae) (Jones and Elgar Reference Jones and Elgar2004), Exomala orientalis (Waterhouse) (Coleoptera: Scarabaeidae) (Wenninger and Averill Reference Wenninger and Averill2006), and Acanthoscelides obtectus (Say) (Coleoptera: Chrysomelidae) (Maklakov et al. Reference Maklakov, Kremer and Arnqvist2007).
Offspring development duration, however, shows no significant difference across the sex ratio treatments, indicating that offspring fitness is modulated only by parental fitness levels and not the number of competitors that parents are exposed to while searching for the mates. Besides the sex ratio, male age also influenced life attributes in M. sexmaculatus. The offspring sired by old males showed the longest developmental duration, which was possibly due to the aggregation of mutations in older parents (Partridge and Barton Reference Partridge and Barton1993; Pletcher and Curtsinger Reference Pletcher and Curtsinger1998; Moore and Harris Reference Moore and Harris2004).
Although our study shows that extreme competition enhances male performance and reproductive parameters in all age groups and that old males perform worst within each operational sex ratio treatment, setting up experiments in which males of different age consecutively compete with each other under each operational sex ratio condition, might give a better insight into the operational sex ratio male age question.
Thus, it can be concluded that sex ratio and age significantly influenced the mating and reproductive parameters of M. sexmaculatus. Mating commenced early and also occurred for the longer duration under extreme competition. Reproductive parameters were elevated under extreme competition. In terms of age, older males were poor performers regardless of operational sex ratio, which probably proves that age more strongly influences the life attributes of the M. sexmaculatus.
Acknowledgements
S.S. acknowledges Ian Skicko, Research Scholar, Evolutionary Ecology, University of Exeter, Exeter, United Kingdom for improving the language of the paper. S.S. also acknowledges Dr. Rahul Joshi, Scientist, Zoological Survey of India for providing accession number of the experimental species and Basic Scientific Research Fellowship by the University Grant Commission, New Delhi, India (F.No.25-1/2014-15(BSR)/7-109/2007/BSR) dated 25 August 2015.