Introduction
Since its first assessment report, the Intergovernmental Panel on Climate Change (IPCC) has predicted that under current rates of change, climate warming and habitat disturbances would have significant biological impacts on plants and animals (IPCC 1990). In spite of greater environmental awareness from governments, the level of anthropogenic activities and rate of climate change have continued to increase (IPCC 2007) and ecosystems have changed in response to these new environmental conditions (Thuiller Reference Thuiller2007). Although there is a growing body of literature linking climatic changes to alterations of insect diversity, phenology, and distribution range in temperate zones (Parmesan Reference Parmesan1996, Reference Parmesan2006; Parmesan et al. Reference Parmesan, Ryrholm, Stefanescus, Hill, Thomas and Descimon1999; Hickling et al. Reference Hickling, Roy, Hill, Fox and Thomas2006; Menéndez et al. Reference Menéndez, González Megias, Hill, Braschler, Willis and Cillingham2006; White and Kerr Reference White and Kerr2007; Westwood and Blair Reference Westwood and Blair2010), the impacts of climate changes on northern insect communities are relatively poorly documented. Emerging trends, however, are alarming; rates of northern range margin shifts for Finnish butterflies have been unprecedentedly high (Pöyry et al. Reference Pöyry, Luoto, Heikkinen, Kuussaari and Saarinen2009) and the median date of a variety of phenological changes including arthropod emergence peaks has advanced on average by 14.5 days per decade in northern Greenland between 1996 and 2005 (Høye et al. Reference Høye, Post, Meltofte, Schmidt and Forchhammer2007). In the northern Nearctic Region, dramatic shifts in community composition since the 1990s have been linked to recent climate warming for chironomids (Axford et al. Reference Axford, Briner, Cooke, Francis, Michelutti and Miller2009) and microgastrine wasps (Fernandez-Triana et al. Reference Fernandez-Triana, Smith, Boudreault, Goulet, Hebert and Smith2011).
The monitoring of Arctic insect communities has had a short and sporadic history, especially in the Nearctic Region. Consequently, material housed in museum collections represents an invaluable historical resource to document changes in biodiversity (Shaffer et al. Reference Shaffer, Fisher and Davidson1998; White and Kerr Reference White and Kerr2006; Newbold Reference Newbold2010). The Canadian Northern Insect Survey (1947–1961) was an initiative aiming at the discovery and documentation of Arctic species in the Nearctic Region (see Freeman Reference Freeman1958; Danks Reference Danks1981) and has generated the bulk of northern Nearctic specimens from that period housed in museum collections. Some localities (and taxa) were sampled more heavily than others during the northern survey and Churchill, Manitoba, was sampled repeatedly. Muscidae, one of the most species-rich and abundant families of terrestrial insects in the Nearctic Region (Vockeroth Reference Vockeroth1979; Danks Reference Danks1981), was commonly collected during that period. Specimens were subsequently curated mostly by H.C. Huckett, not only for Churchill, but also for the whole of the northern Nearctic Region, an endeavour that culminated in his 1965 monograph on the Muscidae of Canada, Greenland, and Alaska (Huckett Reference Huckett1965b). The work of Huckett (Reference Huckett1965b) and the large muscid holdings found in museum collections form an extensive set of historical baseline data on the distribution of northern Nearctic Muscidae that can now be compared with the results of modern day surveys.
Muscidae is a cosmopolitan family that spans almost the full spectrum of ecological habitats known for Diptera (Courtney et al. Reference Courtney, Pape, Skevington and Sinclair2009). In Arctic and alpine habitats, the majority are saprophagous or predaceous in immature stages and commonly found in humus, various damp or aquatic habitats, and dung or carrion (Skidmore Reference Skidmore1985; Ferrar Reference Ferrar1987) where they probably play an important role in accelerating the decomposition rate of organic refuse in cold environments (Vockeroth Reference Vockeroth1979; Savage Reference Savage2002). Adults such as those of Thricops Rondani and Drymeia Meigen are anthophilous, feeding on pollen and (or) nectar (Pont Reference Pont1993; Elberling and Olesen Reference Elberling and Olesen1999), whereas members of Coenosiinae such as Spilogona Schnabl, Limnophora Robineau-Desvoidy, and Coenosia Meigen are predaceous but occasionally visit flowers (Skidmore Reference Skidmore1985; Larson et al. Reference Larson, Kevan and Inouye2001).
Close to 3000 historical specimens representing more than a hundred species of Muscidae are available from the Churchill area, located in northern Manitoba along the southern boundary of the Arctic biome at the meeting point of boreal forest, tundra, and the ocean. Since 1929, Churchill and its surrounding area have been modified by military and hydroelectric development, as well as increases in freight transport and ecotourism, which have changed terrestrial and aquatic habitats to various extents (Newton et al. Reference Newton, Fast and Henley2002; Edye-Rowntree et al. Reference Edye-Rowntree, Ayotte, Bazlik, Bilenduke, Brandson and Bussell2006). Regional climate has also changed – the mean annual temperature in Churchill rose by 1.78°C between 1970 and 2007 (Ballantyne Reference Ballantyne2009). Climate change can have the effect of shifting ecological zones; a change of 1°C can move ecological zones by more than 160 km (Thuiller Reference Thuiller2007). In North America, an average isothermal shift of 105 km has been reported for 1900–1994 (Karl et al. Reference Karl, Knight, Easterling and Quayle1996), a shift most likely to have intensified subsequently, with 2000–2009 being the warmest decade on record (Hansen et al. Reference Hansen, Ruedy, Sato and Lo2010). Ballantyne (Reference Ballantyne2009) suggested climate warming as a possible cause for the significant increase in tree and shrub cover and decrease in water and vegetative cover measured in Churchill over the 30 years preceding her study.
A few northern monitoring initiatives based on standardized sampling programmes that include at least some arthropods have been developed since 1990 in Europe (Høye et al. Reference Høye, Post, Meltofte, Schmidt and Forchhammer2007; Schmidt et al. Reference Schmidt, Berg and Meltofte2010) and since 2005 in North America (Buddle Reference Buddle2009; Cannings Reference Cannings2009), but no such data set has been determined to species level for muscid flies or any other calyptrate Diptera. Consequently, we know little of the impacts of environmental changes on a dominant taxon of terrestrial northern insects and even less of the influence of commonly used sampling techniques on the fauna collected. For muscid flies, as in most organisms, sampling methods undoubtedly affect not only the abundance of species in a sample but their composition as well (Longino et al. Reference Longino, Coddington and Colwell2002; Ellison et al. Reference Ellison, Record, Arguello and Gotelli2007).
The impacts of climatic changes on insect distribution have been predicted to be most severe along boundaries of the Arctic biome (Danks Reference Danks1981). Therefore, Churchill, with its strategic location, confirmed warming trend, and large number of historical specimens stored in collections, is a logical locality in which to study northern insect assemblages and monitor changes in their community composition over time. We conducted the first survey of the muscids of Churchill using an explicit and repeatable sampling design and used the opportunity to (1) contrast patterns of species richness and composition of muscid flies in Churchill between two time periods (pre-1965 and 2007); and (2) evaluate the contribution of three different methods commonly used to collect Diptera (Malaise and pan trapping, and sweep netting).
Materials and methods
2007 survey
From 19 June to 25 August 2007, adult muscid flies were sampled in the region of Churchill, Manitoba, in an area of approximately 100 km2 ranging from the mouth of the Churchill River and the Hudson Bay coast to the Twin Lakes (TL) area and referred to as “Churchill” throughout this work (∼98°45′N, 94°07′W; Fig. 1). Vegetation in the sampling area was a mosaic consisting mostly of tree communities juxtaposed to tundra patches in a landscape with a high proportion of wetlands, bogs, and fens (Brook Reference Brook2001). In order to sample from a set of representative habitats from the regional landscape, nine permanent and 26 occasional sites (each visited four times or less) were selected (Fig. 1). Permanent sites were chosen on the basis of their accessibility, level of disturbance, and ecological and geographical characteristics, whereas occasional sites were chosen on the basis of citations in published records, distinction from permanent sites, and presence of peculiarities (atypical plant composition and (or) accumulation of organic material such as garbage or grain piles).

Fig. 1 Map of the area of Churchill, Manitoba, and location of the nine permanent sites and 26 temporary sites sampled for Muscidae in 2007. Original background map courtesy of P. Kershaw.
Occasional sites were sampled only by sweep net with a collapsible insect net (handle 43.2 cm, bag diameter 38.1 cm). All permanent sites were sampled continuously and serviced biweekly using a Townes-style Malaise trap (1.83 × 2.44 × 1.83 m) surrounded by four yellow pan traps (“Carry-out” brand 5 cm high with an outer diameter of 15.2 cm) positioned at cardinal compass points, 2 m from the edges of the Malaise trap. Yellow traps were selected because they catch more muscoid specimens than do white traps (Disney et al. Reference Disney, Erzinclioglu, de C. Henshaw, Howse, Unwin and Withers1982) and have been used in a number of studies on higher fly diversity where Muscidae were well represented (Grégoire Taillefer and Wheeler Reference Grégoire Taillefer and Wheeler2010; Savage et al. Reference Savage, Wheeler, Moores and Grégoire Taillefer2011). Collection heads on the Malaise traps were loaded with either Dichlorvos insecticide cubes (Vapona® brand) or ethanol (70% or 95%); killing agents were alternated at each trap service. Pan traps were half filled with 2:1 water: food-grade propylene glycol and three drops of dishwashing soap. At each site visit, 60 net sweeps were taken without interruption around the Malaise trap, keeping a distance of at least 4 m from the trap, and insects were immediately transferred to killing jars loaded with either ethyl acetate or sodium cyanide.
All specimens collected at occasional sites were processed but, as is often the case with Diptera surveys (Marshall et al. Reference Marshall, Anderson, Roughley, Behan-Pelletier and Danks1994), subsampling was necessary at permanent sites due to the large number of samples and specimens collected. For all permanent sites and for every week of the sampling period, material collected on the first biweekly visit (4-day period) was processed for identification, whereas specimens collected on the second visit (3-day period) were kept in storage. Large specimens were pinned dry or from alcohol, whereas small specimens were chemically dried using hexamethyldisilazane (Brown Reference Brown1993) before being pointed. All specimens were deposited in the Bishop's University Insect Collection, Sherbrooke, Québec, Canada (BUIC) and the J.B. Wallis Museum of Entomology Collection, University of Manitoba, Winnipeg, Manitoba, Canada (JBWM).
Historical data
Historical data were assembled through the combination of published records and material housed in collections. Huckett (Reference Huckett1965b) provided the most extensive list of published records for the area and was supplemented with Huckett (Reference Huckett1965a), Webb (1956), and Arntfield (Reference Arntfield1975). Although museum data are of great value to study the distribution of species, their accuracy can be problematic (Graham et al. Reference Graham, Ferrier, Huettman, Moritz and Peterson2004; Newbold Reference Newbold2010). Therefore, to confirm the identity of all published records and investigate dubious or problematic records and localities (as well as curate undetermined specimens and establish the abundance of species found in collections), 28 North American and three European collections were contacted to enquire about holdings of Muscidae from Churchill (Appendix A). A total of 2947 specimens were found in those collections, and, because all but 66 were collected from 1929 to 1964, 2881 specimens (including over 500 undetermined specimens) were retained and pooled together in the pre-1965 data set. Collecting dates ranged from 20 May to 5 September, with 90% of the specimens collected within the sampling period of the 2007 inventory. According to label data and D.M. Wood (personal communication), all specimens in this data set were collected by sweep net.
We would like to emphasize here the contribution of the late J.G. Chillcott, who collected nearly half the specimens used in this data set in 1952 as part of the Canadian Northern Insect Survey, and the key role of the Canadian National Collection of Insects, Arachnids and Nematodes in Ottawa (CNC) where 2469 specimens of Muscidae from Churchill collected before 1965 are housed. We especially appreciate the assistance of D.M. Wood with specimens of Spilogona in that collection and the remarkable curation work of J.R. Vockeroth on the Nearctic muscids in the CNC, as well as his indispensable chapter on Muscidae coauthored with H.C. Huckett in the second volume of the Manual of Nearctic Diptera (Huckett and Vockeroth Reference Huckett and Vockeroth1987).
Taxonomic identification
Specimens from both primary data sets were identified using the following identification keys: Arntfield (Reference Arntfield1975), Collin (Reference Collin1930), Hennig (Reference Hennig1955–1964), Huckett (Reference Huckett1932, Reference Huckett1934a, Reference Huckett1934b, Reference Huckett1936, Reference Huckett1954, Reference Huckett1965b), Huckett and Vockeroth (Reference Huckett and Vockeroth1987), Malloch (Reference Malloch1918, Reference Malloch1919, Reference Malloch1920, Reference Malloch1923), Michelsen (Reference Michelsen2006), Savage (Reference Savage2003), Snow (Reference Snow1891), and Snyder (Reference Snyder1949a, Reference Snyder1949b, Reference Snyder1954). Taxonomic nomenclature follows the catalogues, revisions, and type studies of Huckett (Reference Huckett1965a), Pont (Reference Pont1984, Reference Pont1986, Reference Pont2011), and Michelsen (Reference Michelsen2006). Species identities were verified through comparison with determined material housed in the CNC, the BUIC, and in the United States of America the American Museum of Natural History, New York, New York (AMNH) and the National Museum of Natural History, Smithsonian Institution, Washington, District of Columbia (USNM).
Identification of female muscid flies can occasionally be problematic. Therefore, in order to allow for the inclusion of female specimens in all analyses requiring abundance data, six pairs of species were merged into the following complexes: Coenosia tarsata Huckett/Coenosia verralli Collin, Limnophora sp. 2/Limnophora rotundata Collin, Phaonia consobrina (Zetterstedt)/Phaonia rugia (Walker), Schoenomyza dorsalis Loew/Schoenomyza litorella (Fallén), Spilogona atrisquamula Hennig/Spilogona pusilla Huckett, and Thricops septentrionalis (Stein)/Thricops spiniger (Stein).
Data analyses
The composition and abundance of taxa captured at a site can be influenced by various factors such as sampling effort, collecting techniques, weather, natural, or anthropogenic changes in habitat or microhabitats sampled, yearly variations in abundance, and timing of survey (Marshall et al. Reference Marshall, Anderson, Roughley, Behan-Pelletier and Danks1994; Shaffer et al. Reference Shaffer, Fisher and Davidson1998; Longino et al. Reference Longino, Coddington and Colwell2002). In our study, sites selected for the 2007 survey included all those listed in the literature, collection dates between surveys overlapped extensively, and sweep netting was used in both time periods. Although we received assurance from collection managers that no disposal of Churchill specimens took place from their Muscidae holdings, it is possible that a series of conspecific specimens were purged by previous collectors interested primarily in species richness, thereby skewing the apparent abundance of species found in collections. It is, however, unrealistic to assume that none of the assumptions related to repeatability will be violated between surveys conducted many decades apart, and care should be taken during survey planning and data analysis to minimize potential biases. Therefore, results from comparative studies of faunal assemblages from different time periods should always be interpreted in the light of those potential biases.
To assess the completeness of the 2007 survey (all material pooled) and the pre-1965 assemblages, we constructed individual-based rarefaction curves to determine whether they had reached an asymptote. We also compared the observed species richness of each data set to two nonparametric estimators of (minimum) total richness: the Chao 1 index (Chao Reference Chao1984) and the abundance-based coverage estimator (ACE; Chao and Lee Reference Chao and Lee1992). These estimators, which rely mostly on information from rare species, were especially relevant for the pre-1965 assemblage because potential biases in museum specimen abundance could have affected the shape of the rarefaction curve.
Individual-based rarefactions standardized to the least abundant sample, the ACE and the Chao 1 index, were used to compare (1) species richness between the nine permanent sites; (2) species richness between Malaise trap, pan trap, and sweep net samples collected at the permanent sites; and (3) species richness between the pre-1965 assemblage and all material collected by sweep net in 2007. The computation of 95% asymmetrical confidence intervals (CIs) for the Chao 1 index (Colwell Reference Colwell2009) allowed for the comparison of richness estimates between assemblages.
Because calculations of all species richness estimators involved abundance data, all 12 species with problematic females were pooled into six species complexes and the six species with no abundance data in pre-1965 were excluded from calculations and comparisons involving this data set. All rarefactions (based on 1000 permutations and with species richness as a diversity index) were performed using Ecosim version 7.72 (Gotelli and Entsminger Reference Gotelli and Entsminger2010); ACE and Chao 1 were calculated in EstimateS version 8.2 (Colwell Reference Colwell2009).
Pairwise similarity in species composition was assessed for the three collecting methods used at the permanent sites using the classic Jaccard index for presence–absence data (Jaccard Reference Jaccard1901) and the Chao's Jaccard similarity index for replicated incidence-based data (corrected for undersampling; Chao et al. Reference Chao, Chazdon, Colwell and Shen2005; Colwell Reference Colwell2009). Both indices range from 0 to 1, with higher values indicating greater similarity between assemblages. We chose the corrected Chao's Jaccard similarity index partly because the classic Jaccard index underestimates the similarity between species-rich assemblages (Chao et al. Reference Chao, Chazdon, Colwell and Shen2005; Ellison et al. Reference Ellison, Record, Arguello and Gotelli2007; Gotelli et al. Reference Gotelli, Ellison, Dunn, Nathan and Sanders2011) but mostly because replicated incidence data at the nine sites could be used as a measure of abundance rather than raw specimen numbers. This was an important factor because the three different collecting methods differed greatly in the number of specimens they accumulated. Furthermore, the computation of a CI for Chao's Jaccard similarity index allows for testing of the null hypothesis that composition between two assemblages is no greater than what would be expected by chance (Ellison et al. Reference Ellison, Record, Arguello and Gotelli2007; Colwell Reference Colwell2009). Pairwise Jaccard and corrected Chao's Jaccard incidence-based indices were calculated in EstimateS version 8.2 (Colwell Reference Colwell2009).
Similarity in species composition between the pre-1965 assemblage and all material collected by sweep net in 2007 (permanent and occasional sites combined) was assessed using the same indices as above, but calculations for the Chao's Jaccard similarity index were done on abundance rather than frequency data (Chao et al. Reference Chao, Chazdon, Colwell and Shen2005) because there were no replicates for these data sets.
The presence or absence of nonoverlapping species between our historical and 2007 assemblages may either be the result of a failure to detect or an actual change in the distribution of species. Although a statistical comparison of species richness and composition similarity between the contemporary and historical assemblages could only be conducted for material collected by sweep net, the number of historical species recovered in the modern survey increased notably when all specimens collected in 2007 were pooled together regardless of the collecting technique. We therefore decided to investigate for repeated trends in factors possibly involved in the presence or absence of nonoverlapping taxa not only for material collected by sweep net, but also for all material from both time periods. All species unique to a time period were assessed in terms of collecting dates, collecting techniques, abundance in the samples, and individual distribution ranges.
Although not enough data were available on the distribution range and ecological requirements of species in our study to model their past and present distribution range adequately, we could still determine where Churchill lies in relation to their known Nearctic distribution range. Temperature is a major determinant of insect distribution (Hill et al. Reference Hill, Thomas and Huntley1999; Bale Reference Bale2002), and the range limits of insect species are expected to follow certain isothermal shifts (de Groot et al. Reference de Groot, Ketner and Ovaa1995). Because latitude is not a good indicator of temperature across a large region such as the northern Nearctic, we first calculated the 1965 July isothermal line passing through Churchill on the basis of average monthly temperature (11°C) and then calculated the 2007 July 11°C isothermal line to establish whether there has been a discernable northward displacement of the isotherm in the Churchill area between the two time periods (Fig. 2). The distance between the two isothermal lines is about 300 km, and it is possible that the presence or absence of some nonoverlapping species between the time periods might have been influenced by isothermal changes in the region. Therefore, we attempted to identify repeated patterns in the distribution of nonoverlapping species known from at least three other Nearctic localities by evaluating the position of all distribution points relative to the 1965 July isothermal line (instead of latitude) to determine whether they were located north or south of Churchill and then categorized Churchill as either (a) a locality within the known range, (b) the northernmost locality, or (c) the southernmost locality.

Fig. 2 Distribution of Lophosceles minimus Malloch, Helina spinosa (Walker), and Coenosia mollicula (Fallén) in Canada and Alaska (United States of America). Solid line: 11°C (1965): July isotherm passing through Churchill, Manitoba; stippled line: 11°C (2007): July isotherm passing north of Churchill, Manitoba.
The pre-1965 Nearctic distribution records for nonoverlapping species known from at least three other localities other than Churchill were compiled from label information of material housed in the CNC and supplemented with data from Malloch (Reference Malloch1918, Reference Malloch1919, Reference Malloch1920, Reference Malloch1923), Collin (Reference Collin1930), Huckett (Reference Huckett1932, Reference Huckett1934a, Reference Huckett1934b, Reference Huckett1936, Reference Huckett1954, Reference Huckett1965b), and Snyder (Reference Snyder1949a, Reference Snyder1949b, Reference Snyder1954). Geographic coordinates for all localities were obtained from the Biological Survey of Canada (2009), Google Earth (Google 2010), and D. Sikes, University of Alaska, United States of America (personal communication), and mapped in ArcMap (Environmental Systems Resource Institute 2009). The July 11°C isothermal lines based on average monthly temperature for 1965 were created in ArcGIS with Inverse Distance Weighted (IDW), a method of data interpolation that, in the context of isotherm creation, estimates the temperatures at locations where no measured values are available (Anderson Reference Anderson2010). Temperature data of approximately 2100 Canadian localities (Environment Canada 2010) and 19 Alaskan locations (Alaska Climate Research Center 2008) were used. The July isotherm was selected because it represents summer heat availability, which may have an impact on insect distributions (Bale et al. Reference Bale, Masters, Hodkinson, Awmack, Bezemer and Brown2002).
Results
2007 survey
A total of 9817 specimens were collected through the standardized protocol at the nine permanent sites and 478 by sweep net at the occasional sites. Together, these 10,294 specimens represented 155 species (142 named species and 13 morphospecies). Species number dropped to 149 (including 13 singletons and 30 doubletons) when species with indistinguishable females were pooled (Table 1, Appendix B). The assemblage was dominated by 51 species (34%) and 2863 specimens (28%) of Spilogona. The rarefaction curve for this assemblage did not reach an asymptote (Fig. 3), a result consistent with the ACE and Chao 1 index, which, respectively, estimated that 84% and 83% of overall species richness was collected in the 2007 survey (Table 1).
Table 1 Total number of specimens (n), observed species richness (S obs), unique species (S unique), rarefied species richness (S rarefied; richness ± SD), and ACE and Chao 1 estimates of species richness with 95% CIs of Muscidae found in pre-1965 museum material (pre-1965), collected in 2007 (2007 all), collected in 2007 at the permanent sites by each sampling device (2007 Malaise, 2007 pan, 2007 sweep (p)), and collected in 2007 by sweep netting at permanent and temporary sites (2007 sweep (all)) in Churchill, Manitoba.
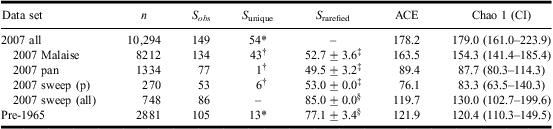
Note: Species numbers reflect the merging of 12 species with indistinguishable females into six complexes.
*Species unique by temporal assemblage.
†Species unique by collecting method for 2007.
‡Species richness rarefied at 270 individuals.
§Species richness rarefied at 740 individuals due to exclusion from calculations of species with no abundance data in the pre-1965 data set.
CI, confidence interval; ACE, abundance-based coverage estimator.

Fig. 3 Individual-based rarefaction curve of species richness (mean ± 1 SD) of all Muscidae collected in Churchill, Manitoba, in 2007. Species numbers reflect the merging of 12 species with indistinguishable females into six complexes.
Observed species richness at the permanent sites ranged from 40 at TGB to 89 at Ramsay Creek (RC); rarefied species richness standardized at 236 individuals also ranked RC as the most species-rich but ranked TL as the least (Fig. 4, Table 2). When sites were ranked in increasing order of rarefied species richness, the standard deviation of estimated species richness of all sites overlapped at least with those of the previous and following ranks, with no site being significantly different from all others (Table 2). ACE and Chao 1 estimates of species richness ranked sites slightly differently from rarefied estimates and from one another (but both ranked Goose Creek Road as most species rich), but there was overlap in CIs of Chao 1 estimates between all of them (Table 2).

Fig. 4 Individual-based rarefaction curves of species richness (mean ± 1 SD) of Muscidae collected in 2007 from nine permanent sites in Churchill, Manitoba. Species numbers reflect the merging of 12 species with indistinguishable females into six complexes. Inset indicates rarefied species richness (±1 SD) for each site standardized at 236 individuals.
Table 2 Total number of specimens (n), observed species richness (S obs), rarefied species richness (S rarefied; richness ± SD, rarefied at 236 individuals), ACE and Chao 1 estimates of species richness with 95% asymmetrical CIs of Muscidae species collected in 2007 at nine permanent sites (abbreviations in parentheses) in Churchill, Manitoba.

Note: Species numbers reflect the merging of 12 species with indistinguishable females into six complexes.
ACE, abundance-based coverage estimator; CI, confidence interval.
Species richness and composition by sampling method
Close to 84% of all specimens from permanent sites were collected by Malaise trap, and these traps collected almost twice as many species as the other two (Table 1). Species richness rarefied at 1334 individuals was significantly higher for Malaise (86.8 ± 3.8%) than pan traps (77.0%); however, when species richness was rarefied at 270 individuals, there was no significant difference between estimated species richness for the three collecting methods (Fig. 5, Table 1). This result, however, utilized species richness curves rarefied near their bases. Results for the ACE and Chao 1 estimates of species richness ranked the Malaise trap and sweep net assemblages as the most and least species rich, respectively, with no overlap in CIs of the Chao 1 index between Malaise trap and the other two methods (Table 1).

Fig. 5 Individual-based rarefaction curves of species richness (mean ± 1 SD) of Muscidae found in pre-1965 museum material (pre-1965) collected in 2007 at the permanent sites by each sampling device (2007 Malaise, 2007 pan, 2007 sweep (p)), and collected in 2007 by sweep netting at permanent and temporary sites (2007 sweep (all)) in Churchill, Manitoba. Species numbers reflect the merging of 12 species with indistinguishable females into six complexes. X-axis and 2007 Malaise rarefaction curve are broken to improve visual display of rarefaction curves with fewer individuals.
Although each collecting method captured unique species, Malaise traps yielded more than six times as many unique species as did the other two techniques combined (Table 1). Both indices of pairwise similarity in species composition indicated the greatest similarity between Malaise and pan traps (which shared 75 of the 77 species collected by pan trap). This was the only pairing with a CI for the Chao's Jaccard incidence-based similarity index >1, indicating that these assemblages are not more different in species composition than could be expected by chance alone (Table 3).
Table 3 Pairwise comparison of Muscidae assemblages collected by Malaise trap, pan trap, and sweep netting at nine permanent sites at Churchill, Manitoba, in 2007 with shared species numbers (S shared), the Classic Jaccard similarity index (Jaccard) and Chao's Jaccard similarity index for replicated incidence-based data (ChaoJaccard) with 95% CIs.

Note: Species numbers reflect the merging of 12 species with indistinguishable females into six complexes.
CI, confidence interval.
Pre-1965 data
Before our study, 105 species (and 2881 museum specimens) were known from Churchill (Appendix B). Subsequent identification of previously unidentified material and reassessment of determined specimens yielded 110 species (Appendix B). Ninety-nine historical species records were confirmed in collection material, six historical species records could not be linked to specimens, and collection specimens yielded 11 species not listed in the literature (Appendix B). The combination of taxa listed in the literature and those found in collections amounted to 116 species. Consideration of species complexes and exclusion of the six species lacking abundance data reduced the taxon count to 105, 20 of which were found as singletons and 13 as doubletons (Table 1, Appendix B). Spilogona was dominant, represented by 38 species (36%) and 869 specimens (44%). The rarefaction curve for the pre-1965 assemblage did not reach an asymptote, a result consistent with the ACE and Chao 1 index, which, respectively, estimated that 86% and 87% of overall species richness was collected.
Michelsen (Reference Michelsen2006) suggested that material listed as Limnophora nigripes Robineau-Desvoidy by Huckett (Reference Huckett1965b) was most probably Limnophora rotundata. Based on the examination of Churchill specimens from 2007, Michelsen (personal communication) confirmed the presence in our material of L. rotundata as well as a new species similar but distinct from L. nigripes (Limnophora sp. 2). Based on the examination of historical specimens, we conclude that material from Churchill determined as L. nigripes by Huckett (Reference Huckett1965b) was misidentified and belongs instead to this new undescribed taxon.
New records
Of the 155 muscid species collected in the Churchill area in 2007, 54 are new regional records, resulting in a new total of 170 species known for the area. For Manitoba, 31 of these records were unique to the 2007 collections, nine were found in material from both time periods, and two were found in unsorted pre-1965 museum specimens but not again in 2007 (Appendix B). Helina humilis (Stein) and Spilogona griseola (Collin) are new records for Canada and the Nearctic Region, respectively. Although we are confident that none of our unidentified morphospecies are currently known from the Nearctic, further examination will be necessary to determine their status as either new species or new records.
2007 vs. pre-1965 assemblages
Because all historical material was collected by sweep net, we contrasted estimated species richness and species composition of the pre-1965 assemblage with that of material collected by sweep net at permanent and temporary sites in 2007. Rarefied species richness standardized at 740 specimens was lower for pre-1965 (77.1 ± 3.4) than for 2007 sweep (85.0 ± 0.0). This trend was also observed with Chao 1 estimated species richness but, although there was no overlap in standard deviation of rarefied species richness for these data sets, the CIs of the Chao 1 estimates overlapped extensively (Table 1, Fig. 5). ACE-estimated species richness was slightly higher for pre-1965 than for 2007 sweep (Table 1). When species numbers were adjusted by merging of species with indistinguishable females into complexes and exclusion of species not represented by museum specimens, 2007 sweep (all) and pre-1965 samples shared 67 species. Pairwise similarity for the pre-1965 and the 2007 sweep assemblages was high (Classic Jaccard = 0.540; Chao Jaccard abundance-based estimate = 0.960) and the CI (0.864–1.056) of the Chao's Jaccard similarity index encompassed 1, indicating that these assemblages are not more different in species composition than could be expected by chance alone.
When all species found in 2007 samples, historical collections, and (or) in historical records were taken into account, the 2007 sweep and pre-1965 assemblages shared 75 species (i.e. 65% of the 116 historical taxa were collected again in 2007 by sweep net). When all material from the 2007 survey was combined, 87% (101 of 116) of historical species were recovered in the 2007 survey.
Nonoverlapping species
When temporal assemblages were compared, 54 species were unique to 2007 all and 15 to pre-1965 (Table 1). Of the 54 new records, 36 (67%) were collected by Malaise traps (eight also collected by pan traps) but not by sweep net (Table 4). In addition, more than half of the new 2007 records were rare in samples and collected as either singletons (18) or doubletons (10; Table 4). Two of the 15 species unique to the pre-1965 data set could not be located in collection material, but collecting dates for the remaining 13 species (seven as singletons, three as doubletons) fell within the date range of the 2007 survey (Table 4).
Table 4 List of nonoverlapping species of Muscidae between all species collected in 2007 and all species found in pre-1965 museum material and (or) listed in the literature for Churchill, Manitoba.

Note: Specimen abundance listed by collecting device (M, P, S) and position of Churchill relative to the Nearctic distribution range of taxa known from at least three other Nearctic localities.
M, Malaise trap; P, pan trap; S, sweep netting.
There were sufficient data for 32 of the new 2007 records to enable assessment of their distribution ranges for the two collection periods. Churchill (in 2007) was categorized as a locality within the known distribution range for 17 species and as the northernmost locality for 15 species (Table 4, Fig. 2). For species only found in pre-1965, Churchill (in 1965) was categorized as within the known distribution range for nine species, as the northernmost locality for three species and as the southernmost locality for two species (Table 4, Fig. 2).
Discussion
Our 2007 survey of muscid flies from Churchill yielded 155 species. The bulk of the material was collected at the nine permanent sites, some of which yielded more specimens and species than others. Estimates of species richness did not single out a specific site as significantly more species rich than the others, but some of the sites with relatively low abundance and observed species richness (such as TL and Twin Golf Balls) were exposed to winds, whereas the site with the highest observed species richness (RC) was protected by trees and shrubs. In addition, RC and Farnworth Lake, the two sites with highest rarefied species richness, appeared more heterogeneous in composition than most others, with freshwater and terrestrial habitats located near the traps.
Although our survey was based on a single collecting season and the Churchill landscape has been moderately but continuously altered by human activity since 1965 (Newton et al. Reference Newton, Fast and Henley2002; Edye-Rowntree et al. Reference Edye-Rowntree, Ayotte, Bazlik, Bilenduke, Brandson and Bussell2006), our results indicate no major differences in estimated species richness and composition of Muscidae between the pre-1965 and 2007 assemblages collected by sweep net in Churchill. When all material collected by different methods in 2007 was pooled, 87% of pre-1965 species were recovered in the 2007 survey. These results contrast with trends reported for microgastrine wasps (Hymenoptera: Braconidae) of Churchill by Fernandez-Triana et al. (Reference Fernandez-Triana, Smith, Boudreault, Goulet, Hebert and Smith2011) who reported close to a 30% increase in estimated species richness in 2005–2007 vs. 1940–1950 assemblages and the recovery of only 53% of historical species. Microgastrines are parasitoids of Lepidoptera, and such specialists may be more sensitive to recent environmental changes in the Churchill area than are ecologically diverse muscid flies. However, Fernandez-Triana et al. (Reference Fernandez-Triana, Smith, Boudreault, Goulet, Hebert and Smith2011) did not correct for differences in collecting methods between surveys, a factor that we found to have an important impact on species richness and composition of muscid flies assemblages collected at the permanent sites.
Estimates of species richness at the permanent sites indicated that Malaise traps generally yielded more species than did pan traps or sweep nets. Species composition was significantly different between sweep nets and the other two techniques, but not between Malaise traps and pan traps. This was not surprising because all but two species collected by pan traps were also collected by Malaise traps (Table 3). In higher Diptera, including Muscidae, Malaise traps have been reported to yield more specimens and a wider array of taxa than do other collecting methods (Marshall et al. Reference Marshall, Anderson, Roughley, Behan-Pelletier and Danks1994; Savage et al. Reference Savage, Wheeler, Moores and Grégoire Taillefer2011). In comparison with samples collected by pan and Malaise traps, however, we note that muscid species composition and gender in sweep net samples are invariably biased because of specific behaviours such as hovering, swarming, or attraction to decomposing organic matter or other transient resources that predispose some species to easy sampling by sweep net.
We therefore conclude that Malaise traps and standardized sweep netting are sufficient for surveys of muscid flies in northern habitats, but recommend at least 120 standardized sweeps (instead of the 60 we conducted in 2007) to increase specimen numbers collected by this method. Pan trapping can be labour intensive because of the need for frequent servicing and large amounts of preserving fluids and may therefore be impractical in remote northern localities with limited road access. However, in regions affected by strong winds or when research objectives require high numbers of replicated samples, pan traps may have to be used instead of Malaise traps to complement sweep netting.
Malaise traps played an important role in the recovery of many pre-1965 species missed by sweep netting in 2007 and in the large number of new species records for Churchill in 2007. Although we were only able to compare species richness and composition of assemblages between time periods for sweep net samples, we assessed which factors (in addition to collecting method) might have influenced the presence of nonoverlapping species between time periods for the complete pre-1965 and 2007 assemblages. These observations are speculative, but we consider it relevant to include them here because they address the specific ecological requirements of individual species (rather than treating them as random effects), and should therefore be taken into account in the design and interpretation of future studies of northern muscid flies diversity.
In our 2007 survey, 36 of the 54 new records for the Churchill area were not collected by sweep net. Their absence from the pre-1965 assemblage is possibly related to collecting technique (failure to detect) rather than an actual distribution change. Notably, all six species of Mydaea Robineau-Desvoidy collected in 2007 (110 specimens, including four new records) were relatively abundant in samples (ranging from 3 to 42 individuals) but none of the specimens were captured by sweep netting.
Among new records collected only by Malaise traps were two haematophagous species, the stable fly, Stomoxys calcitrans L. and moose fly Haematobia alcis (Snow). The presence of S. calcitrans is not surprising, because the species is widely distributed and polyphagous, has great dispersal capacities, and may breed in the dung of a wide range of vertebrates (Greenberg Reference Greenberg1971). However, overwintering temperature requirements (Jones and Kunz Reference Jones and Kunz1997) suggest that S. calcitrans is probably an adventive species in Churchill. Haematobia alcis, however, is rarely collected, feeds exclusively on moose (Alces alces (L.)), and requires fresh moose dung for breeding. Although the single specimen collected from Churchill in 2007 currently represents the northernmost confirmed locality for H. alcis, moose range extends north into Nunavut (Feldhamer et al. Reference Feldhamer, Thompson and Chapman2003) and H. alcis may also be present there.
Some of the new 2007 records caught by sweep netting are species known to be associated with or attracted to specific substrates. Hydrotaea aenescens (Wiedemann), the synanthropic “dump fly,” was collected by sweep netting at the municipal garbage depot. This species was not recorded from the garbage sampled in Churchill by Webb (Reference Webb1956). However, Hydrotaea aenescens might not be able to overwinter in the wild in Churchill (Hogsette et al. Reference Hogsette, Farkas and Coler2002), and the storage of garbage (including recycling and compost) in a specialized building since 2004 (Eliasson Reference Eliasson2004) may have provided warmer conditions favourable to the establishment of this species. The dump fly was also collected, along with Hydrotaea cristata Malloch, on rotting grain piles at the occasional sites. Although grain has been shipped through Churchill since 1931 (Penner Reference Penner2002), to the best of our knowledge this substrate had not been sampled for muscids before the 2007 survey. Hydrotaea cristata is known from localities further north than Churchill (Table 4), and this apparent sampling deficiency may explain the absence of this species from the pre-1965 assemblage.
Species represented by singletons or doubletons accounted for more than half of the new records for 2007. The trend was even more pronounced in taxa unique to the pre-1965 assemblage; 12 of the 15 species unique to it were represented by singletons or doubletons. Documenting distribution changes of species rarely encountered in samples requires strict adherence to the requirements for repeatability of surveys (Shaffer et al. Reference Shaffer, Fisher and Davidson1998). Some of these requirements were relaxed in the 2007 study (e.g. sampling effort) and, on the basis of only one or the two specimens, it is premature to interpret the presence/absence of a nonoverlapping species as a distribution change. Such an interpretation for a particular species will require the collection (and/or finding in museums) of further specimens and (ideally) greater knowledge of its ecological requirements.
The position of Churchill relative to the Nearctic distribution range was evaluated for all nonoverlapping taxa with sufficient data points. Although the position of Churchill as the northernmost locality for nearly half of the new records (15 of 32) may suggest a repeated pattern of northern range extensions in the 2007 assemblage, when rare taxa (one or two specimens) and those not collected by sweep netting were removed from the list, only four species still fit that pattern: H. aenescens (see above), Hydrotaea scambus (Zetterstedt), Coenosia mollicula (Fallén), and Phaonia serva (Meigen) (Table 4). The possibility of northern range extensions in these taxa at other low Arctic localities should be investigated, especially as they all differ substantially in adult and larval requirements.
With respect to taxa unique to the pre-1965 data set, each of the three species represented by more than two specimens had a distribution range extending north and south of Churchill in 1965. Of interest is the disproportionate representation of species of Spilogona among the 15 species unique to pre-1965 (73% compared with 35% for the entire pre-1965 assemblage). Unlike most other muscids in our data set, Spilogona and other Limnophorini breed in various damp or aquatic substrates, including boggy tundra pools (Skidmore Reference Skidmore1985). Axford et al. (Reference Axford, Briner, Cooke, Francis, Michelutti and Miller2009) reported the complete disappearance from a lake on Baffin Island (Nunavut, Canada) between 1950 and 2000 of all common chironomids (Diptera: Chironomidae) requiring an optimal water temperature of less than 10°C. Although the changes in the Churchill muscid fauna between 1965 and 2007 are not as clear as that, the dominance of aquatic and semiaquatic taxa among those not collected again in 2007 suggests that a decrease in water cover reported for the Churchill area (Ballantyne Reference Ballantyne2009) may have reduced the availability of breeding habitats for local Limnophorini. Climate change is expected to alter Arctic freshwater ecosystems drastically (Arctic Climate Impact Assessment [ACIA] 2004). Therefore, we recommend that Limnophorini be prioritized in future Diptera surveys in Churchill (and northern localities in general) to monitor the population dynamics of a group closely associated with wet tundra environments, as well as to increase the probability of collecting some of the taxa unique to the pre-1965 data set.
Climate obviously has an impact on northern muscid fly biology; spring emergence of muscid adults monitored over a 10-year period at a high Arctic locality in northern Greenland occurred considerably earlier at the start of the decade than at the end (Høye and Forchhammer Reference Høye and Forchhammer2008). However, adjustments in species richness and composition of biological communities may lag significantly behind climate changes (Menéndez et al. Reference Menéndez, González Megias, Hill, Braschler, Willis and Cillingham2006). In terms of regional species richness and composition, our results indicate that the muscids of Churchill appear to have been largely resilient to climatic and anthropogenic habitat changes. Consequently, the new baseline data presented here will allow us to monitor the resilience of this community in a future where environmental changes are predicted to intensify (IPCC 2007).
Acknowledgements
This paper is dedicated to Dr. R. Roughley who passed away during the early stages of the project. We thank the collection managers and staff who responded to our queries, especially the following members of the Diptera unit at the CNC: S.C. Brooks, J.M. Cumming, J.E. O'Hara, J.H. Skevington, B.J. Sinclair, D.M. Wood, and J.R. Vockeroth. We are grateful to R. Gagne (USNM), N. Wyatt (BMNH), T. Nguyen (AMNH), and R. Mooi (Manitoba Museum) for assistance with specimens; J. Fernández-Triana, R. Westwood, H. Weber, J. Fitzsimmons, L.-A. Fishback, J. Macdonald, N. Holliday, D. Holder, and P. Kershaw for discussion and/or support; and A. Maniam, A. Patenaude, M. Lécuyer, C. May, and M. Pettitt for field and lab assistance. We thank A.C. Pont and V. Michelsen for their taxonomic input. This work was part of an M.Sc. thesis done under the supervision of J. Savage, R. Roughley, and T. Galloway. Laboratory space was provided by Bishop's University, the University of Manitoba, and the Churchill Northern Studies Centre. Financial support was provided by the Natural Sciences and Engineering Research Council (postgraduate scholarship and northern research internship to A. Renaud and research grants to J. Savage and R. Roughley), Fonds de recherche du Québec – Nature et technologies (postgraduate scholarship), the Entomological Society of Manitoba and the University of Manitoba (graduate student travel awards), the Churchill Northern Studies Centre, the Wapusk National Park of Canada, and the Canadian Centre for DNA Barcoding (Guelph).
Appendix A North American and European museums that responded to a request regarding Muscidae specimens from Churchill, Manitoba, collected before 1965.

Appendix B Species and specimen numbers by collecting method for the 2007 survey of Muscidae of Churchill (Manitoba) (M: Malaise trap; P: pan trap; S(p): sweep netting at permanent sites; S(o): sweep netting at occasional sites) and for all species found in pre-1965 museum material and/or listed in the literature for Churchill (Manitoba). Specimen numbers for species with indistinguishable females reported separately for males and pooled for females. Boldface type denotes a new distribution record for Manitoba; N/A denotes a species listed in the literature but for which no specimens were found in collections.

*Specimens discovered in museum collections belonging to taxa not listed in the literature.













