Introduction
Since its introduction to North America in 1999, West Nile virus (WNv) has spread rapidly across the continent with dire public health consequences. Between 1999 and 2010, ∼1.8 million people have been infected, causing about 360 000 illnesses, 12 852 reported cases of encephalitis/meningitis and 1308 deaths (Kilpatrick Reference Kilpatrick2011). The virus is now also a recognised public health threat in Mexico, and Central and South America (Komar and Clark Reference Komar and Clark2006).
The WNv Flaviviridae virus has an enzootic life cycle between birds and mosquitoes. Mosquitoes (Diptera: Culicidae) that bite both WNv bird hosts and humans act as bridge vectors that have the capacity to transfer WNv from infected birds to human populations, causing illness. In Canada, this applies to <20 of the more than 80 known species of mosquito (Thielman and Hunter Reference Thielman and Hunter2007). While the role of many of these potential vectors in WNv epidaemiology is currently poorly understood, exceptions are Culex pipiens Linnaeus (Diptera: Culicidae) and Culex tarsalis Coquillett (Diptera: Culicidae); species considered to be potentially highly competent WNv vectors (Belton Reference Belton1983, Reference Belton2006; Turell et al. Reference Turell, Dohm, Sardelis, O'Guinn, Andreadis and Blow2005).
To combat the public health risk of WNv, the most successful proactive programmes employ mosquito larval control to maintain mosquito abundance below thresholds above which transmission to humans most commonly occurs (Reisen and Brault Reference Reisen and Brault2007). Larviciding is the preferred option here as it can achieve more reliable mosquito control than adulticiding (Nielsen et al. Reference Nielsen, Reisen, Armijos, Wheeler, Kelley and Brown2007; Su Reference Su2008). However, successful mosquito control programmes require detailed surveillance of mosquito abundance to operate successfully (Reisen and Brault Reference Reisen and Brault2007). Larval habitats of mosquitoes that require monitoring in such programmes range from natural habitats, such as floodplains, and ponded streams, to artificial habitats, like ditches, ponds, and drainage systems. Artificial habitats that place WNv mosquito vectors in especially close proximity to human populations are of particular concern, since they have the potential to greatly facilitate the spread of the disease. For example, stormwater management systems have been linked to the spread of WNv (Vazquez-Prokopec et al. Reference Vazquez-Prokopec, Vanden Eng, Kelly, Mead, Kolhe and Howgate2010).
In most urban areas, these stormwater systems channel surface water from roadways and other thoroughfares via catch basins (Fig. 1), which are typical breeding sites for mosquitoes. A grate usually covers a catch basin where surface runoff water enters before falling into a sump. Water can then flow out of the catch basin through a pipe that is set at the top of the sump (Fig. 1). While this design allows settlement and retention of unwanted sediment, debris and pollutants, it also provides ideal larval habitat for species of mosquito that prefer stagnant water that is high in organic matter (Harbison et al. Reference Harbison, Metzger and Hu2010). This design of catch basin can also provide optimal water temperatures (WT) for mosquito larval development for longer periods of time compared with most naturally occurring water bodies (Harbison et al. Reference Harbison, Metzger, Walton and Hu2009b).

Fig. 1 A schematic cross-section through a typical sumped catch basin. Such basins commonly provide habitat for mosquito larvae.
Mosquito species that thrive in such conditions are often highly competent WNv vectors, such as C. pipiens in northeastern and northwestern North America, Culex quinquefasciatus Say (Diptera: Culicidae) across the southern half of the continent, and Culex restuans Theobald (Diptera: Culicidae) in central and eastern portions of North America (Thomson Reference Thomson2008; Jackson et al. Reference Jackson, Gow, Evelyn, Meikleham, McMahon and Koga2009; Harbison et al. Reference Harbison, Metzger and Hu2010). As well as increasing the amount of habitat for WNv mosquito vectors within their native ranges, catch basins can also facilitate the invasion of WNv mosquito vectors into new areas (Norris Reference Norris2004; Morris et al. Reference Morris, Lampman, Ballmes, Funes, Halvorsen and Novak2007). For example, in southwestern Canada, the introduction of C. pipiens coincided with the construction of stormwater drainage systems with catch basins (Vinogradova Reference Vinogradova2000).
In this paper, we investigate the impact of management techniques and targeted applications of larvicide on larval populations of potential WNv mosquito vector species in catch basins. To do this, we examine the influence that larvicide treatment has on larval presence in catch basins, while simultaneously exploring several other parameters (seasonal variation in larval abundance, vegetation type of immediately surrounding area, WT and weather). To do this we use seven years of sampling data from catch basins from our Integrated Mosquito Management Plan of Action (IMMPACT®) programme in the Lower Mainland and on Vancouver Island, British Columbia, Canada.
Materials and methods
Catch basin study area and sampling
Data were analysed from 10 407 sampling events from 2000 unique catch basins over a seven-year period (2004–2010) in Metro Vancouver, Vancouver Island, the Sunshine Coast, and along the Sea-to-Sky Highway in southwestern British Columbia, Canada (Fig. 2). For each municipality or district that was sampled within these areas, catch basins were selected to achieve a relatively even distribution, taking into account accessibility and safety considerations.

Fig. 2 Study area showing locations of catch basins monitored in the Lower Mainland and Vancouver Island, British Columbia, Canada from 2004 to 2010.
The primary objective of this sampling was to monitor the abundance of mosquito larvae in catch basins throughout the mosquito breeding season between late May and late September each year. The total number of sampling events per year ranged from 215 in 2009 to 3078 in 2008 (Fig. 3), with an average of 2.7 sampling events per catch basin each year (ranged from 2.2 in 2004 to 3.2 in 2005). Eighty eight percent (n = 9150) of sampling events occurred prior to any larvicide treatment within any given year (termed “pre-treatment” samples from herein). Indeed, no larvicide treatment of catch basins took place in some years (2004, 2005, 2009, Fig. 3). In other years when funding was available, however, some districts and municipalities treated all of their wet catch basins with a single application of larvicide (2006, 2007, 2008, 2010, Fig. 3) when threshold numbers of WNv vector larvae were exceeded (more than 10% of wet catch basins contained 10 or more late instar larvae). These wide-scale treatment efforts involved every wet catch basin within a district or municipality receiving a water soluble pouch containing 10 g of the bacterial larvicide VectoLex® WSP, Valent Biosciences, Libertyville Illinois, United States of America (active ingredient Bacillus sphaericus) during July or August. Treatment took place, whenever possible, after the annual cleaning of catch basins to avoid removal of larvicide by cleaning machines. The 12% (n = 1257) of sampling events that occurred after such treatment (termed “post-treatment” samples from herein) evaluated the efficacy of bacterial larviciding campaigns.

Fig. 3 The mean number of mosquito larvae per catch basin sample according to year (2004–2010). Error bars show standard errors. The percentage of samples occurring post-treatment is in brackets above bars. The total number of sampling events is below each year.
Mosquito larvae and pupae were sampled from each catch basin using an aquarium net attached to a long wooden pole (Fig. 1). To standardise sample collection, this was placed down a catch basin's grate, and moved in a figure eight pattern before removal. The net was then flushed with clean water into a white basin to maximise visibility of captured mosquitoes. The larvae and pupae were then transferred in water to Ziploc® bags (SC Johnson, Racine, Wisconsin, United States of America) and transported to the laboratory in a cooler. Other parameters that were also collected at time of sampling included: collection date, WT, and vegetation type of the immediately surrounding area. Weather station archives for Vancouver from Environment Canada were used to verify prevailing weather conditions recorded at time of sampling by field technicians. The predominant vegetation type of the street area immediately surrounding each catch basin (<10 m) was classified into one of three categories: if the catch basin likely received leaves, the classification was tree-lined; if grass clippings probably predominated, then it was grass; and, if neither of these were the case, then it was pavement.
Larval sample processing
From 2004 to 2006, all larvae and pupae collected were identified to the lowest possible taxonomic level, given the condition of the specimen, using the keys outlined in Belton (Reference Belton1983) and Wood et al. (Reference Wood, Dang and Ellis1979). Larval specimens were categorised as either “early” (1st or 2nd) or “late” (3rd or 4th) instars. Early instars were reared into late instars for reliable identification in containers with ground up fish flakes. Late instars were preserved for identification in 75% ethanol. Pupae were placed in emergence cages and their subsequent emerged adults identified (and included in the larval count).
Statistical analysis
The extremely long-tailed zero-heavy distribution of the larval counts presented difficulty for standard analysis. To overcome this, we considered a simpler binary presence/absence response variable. A mixed effects logistic regression model was then fit to describe the relationship between larval presence and larvicide treatment while controlling for the other parameters that were collected (seasonal variation in larval abundance, vegetation type of immediately surrounding area, WT, and weather), as well as variability across sites and municipalities. Seasonal variation in larval abundance was included in the model using a cubic function of day of the year, d, (d/100 + d 2/104 + d 3/106). This generalised linear mixed model for a binary response not only addressed the zero-heavy non-normal long-tailed distribution of the larval counts, but also dealt with repeated measures, and the unbalanced nature of the data (pre-treatment versus post-treatment samples, Bolker et al. Reference Bolker, Brooks, Clark, Geange, Poulsen and Stevens2009). The following model formulation relates the log-odds of larval presence (y) to the set of covariates considered, namely larvicide treatment (T), seasonal variation in larval abundance (D 1 = (d)/(102), D 2 = (d 2)/(104), D 3 = (d 3)/(106); where d is day of year), surroundings (X 1 = 1 if “paved”, 0 otherwise; X 2 = 1 if “treeline”, 0 otherwise; X 1 = X 2 = 0, if “grass”), WT and weather (W 1 = 1 if “hard rain”, 0 otherwise; W 2 = 1 if “light rain”, 0 otherwise; W 3 = 1 if “overcast”, 0 otherwise; W 4 = 1 if “partly cloudy”, 0 otherwise; W 1 = W 2 = W 3 = W 4 = 0 if “sunny”):
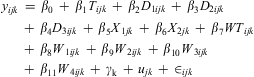 $${{{y}_{ijk}}\, = \,{{{\rm{\beta }}}_{\rm{0}}}\, + \,{{{\rm{\beta }}}_{\rm{1}}}{{T}_{ijk}}\, + \,{{{\rm{\beta }}}_{\rm{2}}}{{D}_{{\rm{1}}ijk}}\, + \,{{{\rm{\beta }}}_{\rm{3}}}{{D}_{{\rm{2}}ijk}}\, \cr + \,{{{\rm{\beta }}}_{\rm{4}}}{{D}_{{\rm{3}}ijk}}\, + \,{{{\rm{\beta }}}_{\rm{5}}}{{X}_{{\rm{1}}jk}}\, + \,{{{\rm{\beta }}}_{\rm{6}}}{{X}_{{\rm{2}}jk}}\, + \, {{{\rm{\beta }}}_{\rm{7}}}W{{T}_{ijk}}\, \cr + \,{{{\rm{\beta }}}_{\rm{8}}}{{W}_{{\rm{1}}ijk}}\, + \,{{{\rm{\beta }}}_{\rm{9}}}{{W}_{{\rm{2}}ijk}}\, + \,{{{\rm{\beta }}}_{{\rm{10}}}}{{W}_{{\rm{3}}ijk}}\, \cr + \,{{{\rm{\beta }}}_{{\rm{11}}}}{{W}_{{\rm{4}}ijk}}\, + \,{{{\rm{\gamma }}}_{\rm{k}}}\, + \,{{u}_{jk}}\, + \,{{ \in }_{ijk}}}$$
$${{{y}_{ijk}}\, = \,{{{\rm{\beta }}}_{\rm{0}}}\, + \,{{{\rm{\beta }}}_{\rm{1}}}{{T}_{ijk}}\, + \,{{{\rm{\beta }}}_{\rm{2}}}{{D}_{{\rm{1}}ijk}}\, + \,{{{\rm{\beta }}}_{\rm{3}}}{{D}_{{\rm{2}}ijk}}\, \cr + \,{{{\rm{\beta }}}_{\rm{4}}}{{D}_{{\rm{3}}ijk}}\, + \,{{{\rm{\beta }}}_{\rm{5}}}{{X}_{{\rm{1}}jk}}\, + \,{{{\rm{\beta }}}_{\rm{6}}}{{X}_{{\rm{2}}jk}}\, + \, {{{\rm{\beta }}}_{\rm{7}}}W{{T}_{ijk}}\, \cr + \,{{{\rm{\beta }}}_{\rm{8}}}{{W}_{{\rm{1}}ijk}}\, + \,{{{\rm{\beta }}}_{\rm{9}}}{{W}_{{\rm{2}}ijk}}\, + \,{{{\rm{\beta }}}_{{\rm{10}}}}{{W}_{{\rm{3}}ijk}}\, \cr + \,{{{\rm{\beta }}}_{{\rm{11}}}}{{W}_{{\rm{4}}ijk}}\, + \,{{{\rm{\gamma }}}_{\rm{k}}}\, + \,{{u}_{jk}}\, + \,{{ \in }_{ijk}}}$$
In this notation, the subscripts identify that yijk is the ith observation made at the jth site within the kth municipality. The fixed effects of the model are larvicide treatment, seasonal variation in larval abundance, vegetation type of immediately surrounding area, and WT. Since quantifying the particularities of any given site or municipality was not the focus in this broader perspective study, these variables are included as normally distributed, random effects and modeled such that:
This type of multi-level model is common in ecology when multiple responses are measured per individual (in this case individual catch basin) and per region (in this case municipality; Bolker et al. Reference Bolker, Brooks, Clark, Geange, Poulsen and Stevens2009).
Results
In total, 92 633 mosquito larvae were collected from 10 407 sampling events from 2000 wet catch basins from 2004 to 2010. The majority (70.0%, n = 7276) of sampling events yielded zero larvae. The remaining non-zero counts (30.0%, n = 3131) exhibited a skewed distribution, with a mean of 29.5, a median of 9, and a maximum of 2328 larvae.
These data indicated that catch basins were consistently dominated by C. pipiens (89%, 71 597 of the 80 668 mosquito larvae that were identified to species). The remaining 11% of the larvae were made up exclusively of Culiseta incidens Thomson (Diptera: Culicidae), another species that potentially transmits WNv (Belton Reference Belton2006). As a result of this uniformity in species composition, all larvae collected after 2006 were counted, but identification was limited to random identification checks for confirmation only.
The vast majority of larvae (98.4%, n = 91 181) were collected from pre-treatment catch basin samples; only 1.59% of larvae (n = 1452) were found in post-treatment samples. Indeed, parameter estimates from the model suggest that larvicide treatment has the effect of reducing the odds of larvae presence by a factor of ∼7.23 (β1 [treatment] = −1.98, P-value ≤0.001; Appendix 1). To explore the treatment effect further, we also ran the model omitting the years during which there was no treatment (2004, 2005, and 2009) from the data. Parameter estimates remained similar. Most importantly, the latter showed treatment has the effect of reducing the odds of larval presence by 8.15 (= 1/0.123). The treatment effect, therefore, is not simply due to annual mosquito fluctuations confounded with treatment. This detrimental impact of larvicide treatment on larval presence is reflected in the trend of reduced weekly mean number of larvae per post-treatment catch basin compared with pre-treatment ones over the mosquito breeding season (Fig. 4). Its effect is also illustrated in the trend of lower proportion of samples with larvae present among post-treatment samples compared with pre-treatment ones, regardless of WT (Fig. 5) or vegetation type of immediately surrounding area (Fig. 6). It may also, in part, account for the wide annual variation in mean number of larvae per catch basin sample; the years when substantial larvicide treatment of catch basins occurred (more than 10% of sampled catch basins) had lower mean values than those years when it did not (Fig. 3).

Fig. 4 The mean number of mosquito larvae per sample from pre-treatment (blue) and post-treatment (orange) catch basins each week (2004–2010).

Fig. 5 The proportion of pre-treatment (blue) and post-treatment (orange) catch basins containing mosquito larvae according to mean catch basin WT (2004–2010). Error bars show score confidence intervals.

Fig. 6 The proportion of pre-treatment (blue) and post-treatment (orange) catch basins containing mosquito larvae according to vegetation type of immediately surrounding area to catch basins (2004–2010). Error bars show score confidence intervals.
There was marked seasonal variation in the weekly mean number of larvae per catch basin: few larvae were observed until mid-June each year, with numbers usually peaking in early August before declining rapidly in September (Fig. 4). Linked to this seasonal variation in larval abundance is the positive correlation between WT and larval presence, which was supported by model parameter estimates (β7 [WT] = 0.14, P-value ≤0.001, Fig. 5, Appendix 1).
The model also indicates that larvae were less likely to occur in catch basins associated with paved areas (63% of observations) than in those beside grassy areas (13%) (β5 [paved] = −0.40, P-value ≤0.001), with catch basins in tree-lined areas (24%) providing the highest chance of larval presence (β6 [treeline] = 0.24, P-value = 0.019, Fig. 6, Appendix 1).
The cumulative effect of larvicide treatment, WT, and vegetation type of immediately surrounding area on larval presence is illustrated in Fig. 7: larvicide treatment consistently reduced the presence of larvae regardless of temperature (which tended to peak with larval presence in mid-summer) and adjacent land use (where larval presence tended to be lowest in paved areas and highest in tree-lined ones).

Fig. 7 Plots illustrating generalised linear mixed model fit. For every calendar day during the observation period (late-June to late-September 2004–2010), the vertical position of circles and diamonds indicate the proportion of larvae positive samples collected for untreated and treated sites, respectively. Further, the circle (diamond) size corresponds to the total number of untreated (treated) samples collected for the given day. The solid lines show probability of occurrence given vegetation type of immediately surrounding area (A: pavement, B: grass, C: trees), mean monthly WT (as observed and listed below months), and given clear weather.
In order to evaluate the goodness of fit of the logistic model to the data, we can visually evaluate how the data compared with the model fit (Fig. 7). We can also consider statistical measures, such as the Cox–Snell and Nagelkerke Pseudo R2s, which provide intuitive measures of the quality with which the model approximates the data (see Cox and Snell Reference Cox and Snell1989; Nagelkerke Reference Nagelkerke1991 for details). When comparing our model to that of the ordinary “intercept only” model, the Cox–Snell Pseudo R2 is 0.22 and the Nagelkerke Pseudo R2 is 0.31. The pseudo R2 values can be interpreted in the same manner as the R2 coefficient of determination used in ordinary linear regression, indicating that the model provides a good approximation to the data and a basis for prediction.
Discussion
With over 90 000 mosquito larvae collected from nearly 10 500 catch basin sampling events, this study demonstrates the capacity of artificial waterbodies to harbour mosquito larvae. Indeed, artificial waterbodies tend to harbour markedly more mosquito larvae than natural wetlands (Su et al. Reference Su, Webb, Meyer and Mulla2003, Jackson et al. Reference Jackson, Gow, Evelyn, Meikleham, McMahon and Koga2009). This may be because temporary, artificial habitats, such as catch basins, often lack predators found in more natural water bodies that help to regulate mosquito populations.
While a range of natural control measures, such as vegetation enhancement, may be effective in many above ground habitats, such as semi-natural wetlands (Jackson et al. Reference Jackson, Gow, Evelyn, Meikleham, McMahon and Koga2009), such methods are impractical for managing mosquito populations in artificial water bodies such as extensive networks of catch basins, leaving targeted larvicide treatment as the only viable course of control available (Metzger Reference Metzger2004). The efficacy that such treatment can have in reducing larval presence in catch basins has been previously documented (Siegel and Novak Reference Siegel and Novak1997, Reference Siegel and Novak1999), and is corroborated by our study; parameter estimates show that larvicide treatment reduced the odds of larvae presence by more than a factor of seven.
However, many jurisdictions have limited resources and may not be able to incorporate a wide-scale programme that encompasses the entire drainage system of a given region, which may include many thousands of catch basins. Our data suggests that in these cases an effective targeted approach may be achievable. For example, by predicting which catch basins are most likely to contain larvae at any given time, the relationships we observed between larval presence and variation in vegetation type, WT, and season could help to increase the efficiency and efficacy of targeted mosquito control operations.
Treatment could be prioritised based on the vegetation type of the immediately surrounding area; according to our findings, catch basins located in paved areas were significantly less likely to harbour larvae compared with those in grassed areas, with those found among trees having the highest chance of larval presence. In warm water with high levels of organic material, algae may prosper, providing ample food for filter feeding mosquito larvae. Lacey et al. (Reference Lacey, Lacey, Peacock and Thiery1988) also observed a decline in larvicidal activity of B. sphaericus in habitats that were highly organically enriched.
While it would be useful to investigate the exact role of different forms of organic input on mosquito production in the future, our results suggest it could be advantageous to focus catch basin cleaning resources in areas dominated by C. pipiens to tree-lined and grass-lined streets prior to the onset of the mosquito breeding season and larvicide treatment; indeed, removing organic matter through catch basin cleaning can decrease the number of mosquito larvae in catch basins by up to 40% (Stockwell et al. Reference Stockwell, Wessell, Reed, Kronenwetter-Koepel, Reed and Turchi2006). These decisions may be area specific, however, as habitat preferences vary between mosquito species. For example, Rey et al. (Reference Rey, O'Meara, O'Connell and Cutwa-Francis2006) found that C. quinquefasciatus and C. nigripalpus Theobald (Diptera: Culicidae) in Florida, United States of America were most common in paved areas.
In addition to vegetation adjacent to catch basins, mosquito larval abundance in catch basins also varies markedly with WT throughout the mosquito breeding season. The presence of few larvae before mid-June each year is consistent with Spielman (Reference Spielman2001), who found that C. pipiens first occurred when WTs reached ∼15 °C. Given this consistent finding, conducting catch basin sampling before week 25 or 26 seems to be an unnecessary use of resources, at least within this study area in southwestern British Columbia.
Although numbers usually peaked in early August before declining rapidly in September, the peaks in larval numbers shown in Fig. 4 may be revealing successive cohorts of overlapping generations, as has been observed in other studies of C. pipiens (Spielman Reference Spielman2001; Edillo et al. Reference Edillo, Kiszewski, Manjourides, Pagano, Hutchinson and Kyle2009). While more research into these patterns is needed, these peaks may be used to judge the optimal time of larvicide treatments. For example, a prolonged residual effect has been observed when intact mosquito larval cadavers are present for larvicides such as B. sphaericus used in this study, which uses their nutrients to grow and sporulate (Becker et al. Reference Becker, Zgomba, Petric, Beck and Ludwig1995; Uspensky et al. Reference Uspensky, Klein and Braun1998; Lacey Reference Lacey2007).
Long-term management of mosquitoes should involve new structural designs that consider reducing the numbers of catch basins with sumps, and incorporating more filtration measures. Research into different catch basin design and modifications, and their effect on mosquitoes is in progress (Harbison et al. Reference Harbison, Metzger, Allen II and Hu2009a, Reference Harbison, Metzger and Hu2010; Hamer et al. Reference Hamer, Kelly, Focks, Goldberg and Walker2011).
The rapid advance of WNv in recent years across North America warns of potential future health concerns arising from vector association with stormwater management. There are many other potential mosquito vectors of disease around the world that could invade new geographical regions. For example, Aedes japonicus Theobald (Diptera: Culicidae), a bridge vector of WNv and a vector of both dengue and chikungunya viruses, has been introduced separately to Ontario, Canada, and Washington state, United States of America (Thielman and Hunter Reference Thielman and Hunter2007; Irish and Pierce Reference Irish and Pierce2008). Larvae of this species are known to inhabit catch basins (Anderson et al. Reference Anderson, Ferrandino, Dingman, Main, Andreadis and Becnel2011). Aedes albopictus Skuse (Diptera: Culicidae), the Asian Tiger mosquito and a known vector of dengue, has spread across 36 states in the United States of America and much of Central America since its discovery in Tennessee, United States of America in 1983 (Enserink Reference Enserink2008). Outbreaks of other zoonotic mosquito-borne diseases are also on the rise (Jones et al. Reference Jones, Patel, Levy, Storeygard, Balk and Gittleman2008). For example, there have been recent outbreaks of the arbovirus chikungunya in Italy and Australia, also carried by A. albopictus (Reiter et al. Reference Reiter, Fontenille and Paupy2006). Indeed, the incidence of mosquito-borne diseases may increase due to anthropomorphic changes in the distribution and abundance of mosquito vectors brought about, for example, through global trade and travel, the decline of predatory species, degradation of natural habitats and increased urbanisation, and climate change.
By prioritising and targeting control efforts to most efficiently reduce WNv mosquito vector populations, the knowledge gleaned from this study can help to minimise the risk of WNv transmission to humans in Metro Vancouver, and may be extrapolated to other metropolitan areas in North America. A similar research strategy could be applied to emerging threats from other potential mosquito vectors of disease around the world to inform best practices to manage mosquitoes. With more information regarding the characteristics of the artificial habitat preferred by such vector mosquitoes, their populations can be reduced more effectively, and the incidence of mosquito-borne disease minimised.
Acknowledgements
The authors thank the past and present staff of Culex Environmental Ltd., Metro Vancouver, the Sunshine Coast communities, Vancouver Island communities, the Fraser Health Authority, the University of British Columbia, Simon Fraser University, and the Greater Vancouver municipalities who kindly contributed to the combined data presented in this paper. This study was conducted as part of Culex Environmental's Research and Development Program, which is supported by the Canada Revenue Agency Scientific Research and Experimental Development (SR&ED) Program.
Appendix 1 Summary of results for all tested variables in the mixed effects logistic regression model.





