Introduction
The macadamia nut borer, Gymnandrosoma aurantianum (= Ecdytolopha torticornis) Lima (Lepidoptera: Tortricidae), is the main pest of Macadamia integrifolia Maiden and Betche (Proteaceae) in Central America (Blanco-Metzler Reference Blanco-Metzler1993, Reference Blanco-Metzler1994; López-Guillén et al. Reference López-Guillén, Ruiz, Brown, Cruz-López, Metz and Alma Solís2021). This moth species is controlled with chemical insecticides (Carvalho Reference Carvalho2003; Parra et al. Reference Parra, Bento, Garcia, Yamamoto, Vilela and Leal2004); however, intensive pesticide use generates environmental pollution, human health issues, and resistance of the target insect pests (Elzen and Hardee Reference Elzen and Hardee2003). For this reason, it is necessary to generate environmentally sound strategies for pest management. Sex pheromones may be an alternative for monitoring or control of insect pests (Witzgall et al. Reference Witzgall, Kirsch and Cork2010).
Previous studies of the chemical ecology of G. aurantianum report that female sex pheromone gland extracts of Costa Rican populations contain four compounds. These compounds have been identified as dodecyl acetate, (E)-8-dodecenyl acetate, (Z)-8-dodecenyl acetate, and (E)-8-dodecenol (Chamberlain et al. Reference Chamberlain, Beevor, Cork and Hall2003). Field studies have reported that traps baited with the major component, (E)-8-dodecenyl acetate, caught males in Costa Rica (Chamberlain et al. Reference Chamberlain, Beevor, Cork and Hall2003) and Guatemala (Navas-Franco Reference Navas-Franco2019). The role of secondary compounds in male attraction is not yet known.
Generally, lepidopteran pheromones consist of a mixture of two or more compounds (Arn et al. Reference Arn, Tóth and Priesner1992). In some species, only the major compound is crucial for attracting males, whereas in other species, males respond only to the mixture of compounds (Cardé et al. Reference Cardé, Baker and Roelofs1975; Linn et al. Reference Linn, Campbell and Roelofs1986, Reference Linn, Campbell and Roelofs1987; Chen et al. Reference Chen, Zhu, Tian, Zhang, Guo and Liu2018). In addition, the geographic distribution of some moth species influences the ratio of pheromone components emitted by females and male response (El-Sayed et al. Reference El-Sayed, Delisle, De Lury, Gut, Judd and Legrand2003; Groot et al. Reference Groot, Marr, Schöfl, Lorenz, Svatos and Heckel2008; Chen et al. Reference Chen, Zhu, Tian, Zhang, Guo and Liu2018; Cruz-Esteban et al. Reference Cruz-Esteban, Rojas, Sánchez-Guillén, Cruz-López and Malo2018). These variations can affect pheromone catches from one region to another. For this reason, their identification is essential to establishing a system of monitoring or control. We think that the G. aurantianum sex pheromone of the Guatemalan population differs from that of the Costa Rican and Brazilian populations and that the addition of one or more minor components to the major component of the pheromone might improve male attraction. The objective of this study was to examine the composition of the G. aurantianum sex pheromone in a population from a macadamia plantation in Guatemala and to determine the effect of adding secondary components to the major component of the pheromone on the capture of males of this species.
Materials and methods
Insects
Gymnandrosoma aurantianum larvae were obtained from damaged fruits picked from trees in a 15-year-old macadamia orchard on the Buena Vista Farm, San Pablo, Department of San Marcos, Guatemala (14° 57′ N, 91° 58′ W, 820 m above sea level). The larvae were placed individually in 5-mL glass tubes (6 cm long, 1.5 cm inside diameter) plugged with cotton to permit air to enter. Larvae were fed an artificial diet (Garcia and Parra Reference Garcia and Parra1999) until pupation. Later, pupae were sexed and placed individually into 30-mL plastic cups (3 cm high, 3 cm bottom diameter, 4 cm top diameter) with a perforated cover until adult emergence. Moths were maintained at 23 ± 2 °C and 75 ± 5% relative humidity and with a 12:12-hour light:dark photoperiod. Adults were fed with a cotton ball saturated with a 10% solution of honey water. Voucher specimens were preserved and deposited in the insect collection of the El Colegio de la Frontera Sur, Tapachula, Chiapas, Mexico.
Samples preparation
Sex pheromone glands of G. aurantianum were obtained from two- to five-day-old virgin females during the scotophase (18:00–20:00 hours). Moths were placed in a freezer at about –20 °C for 2 minutes to immobilise them and make manipulation easier. The tip of the abdomen was gently pressed with entomological tweezer to extrude the gland, and this then was excised with entomological scissors. Sex pheromone glands (n = 25) were dissected and placed for 10 minutes into a glass vial (2 mL) with 1 mL of dichloromethane. After that, glands were removed, and the extract was concentrated to an approximate volume of 100 µL under a flow of nitrogen and stored at –20 °C until use.
Chemical analysis
Gland extracts (1 μL) were analysed in a gas chromatograph (CP-3800; Varian, Palo Alto, California, United States of America) coupled to a mass spectrometer (Saturn 2200; Varian). The samples were injected in splitless mode. The compounds were separated using a nonpolar column (DB-5ms; Agilent Technologies, Santa Clara, California, United States of America) or a polar column (DB-Wax, Agilent Technologies), both 30 m long, 0.25 mm in inside diameter, and with 0.25 µm film thickness.
For the nonpolar column, the oven temperature was programmed at 50 °C for 2 minutes, then 15 °C/minute to 280 °C, and then held at this temperature for 10 minutes. The injector port temperature was held at 250 °C. The samples were ionised by electronic impact at 70 eV. Helium was used as the carrier gas (1 mL/minute). For the polar column, the oven temperature was programmed at 50 °C for 2 minutes, then 15 °C/minute to 200 °C, and then held at this temperature for 10 minutes. The injector port temperature was held at 250 °C. The samples were ionised by electronic impact at 70 eV. Helium was used as the carrier gas (1 mL/minute).
Compounds were preliminarily identified using Saturn GC/MS Workstation software (Agilent Technologies), comparing the mass spectra of each peak with results from the National Institute of Standards and Technology (NIST, version 2.0) mass spectra library. Identification was confirmed by comparison of retention times and mass spectra with those of synthetic standards on both the polar and nonpolar columns. Synthetic (E)-8-dodecenyl acetate, (Z)-8-dodecenyl acetate, and (E)-8-dodecenol were purchased from Alfa Chemistry (Ronkonkoma, New York, United States of America), and dodecyl acetate and tetradecyl acetate were purchased from Sigma-Aldrich (Toluca, Mexico). The isometric purity of (E)-8-dodecenyl acetate and (Z)-8-dodecenyl acetate was greater than 98%. It was determined by gas chromatography–flame ionisation detector. All standard solutions were prepared with high-performance liquid chromatography–grade dichloromethane (Sigma-Aldrich).
The relative amount of each compound was expressed as percent peak area relative to the total peak area of the pheromone compounds (normalised to the major compound = 100), according to the results on the polar column. Quantification of the compounds was obtained with a calibration curve, using three known concentrations of synthetic compounds. Five replications were carried out for each compound.
Electrophysiological analysis
Male antennal responses of G. aurantianum to the synthetic compounds identified in the pheromone gland extracts were determined by electroantennography. The males were placed in a freezer at about –20 °C for 2 minutes, which temporarily immobilised them for manipulation. Each moth’s head was cut off, and the reference electrode was inserted into its base. The distal end of the antenna was inserted at the tip of the glass capillary placed inside the recording electrode. The capillaries were filled with Ringer solution (sodium chloride 0.35 g, calcium chloride 0.21 g, potassium chloride 0.35 g, and sodium hydrogen carbonate 0.2 g dissolved in 1 L of distilled water; Malo et al. Reference Malo, Renou and Guerrero2000).
A standard aliquot (1 µL) of the compound (solution prepared with high-performance liquid chromatography–grade dichloromethane at a concentration of 1 µg/µL) was loaded on a piece of filter paper (5 × 10 mm; Whatman No.1; Whatman International, Maidstone, United Kingdom) and exposed to air for 20 seconds on a glass Petri dish to allow the solvent to evaporate, and then introduced into a glass Pasteur pipette or sample cartridge. A new cartridge was prepared for each antenna replicate. A cartridge with a piece of filter paper loaded with 1 μL of dichloromethane was used as a control, which was applied at the beginning and end of each test.
A current of humidified pure air (0.7 L/minute) was constantly passed onto the antenna through a 10-mm diameter L-shaped glass tube. To present a stimulus, the Pasteur pipette tip was inserted into a hole located in the upper middle part of a glass tube, and an air flow (0.5 L/minute) was passed through it by actuating a foot pedal connected to a stimulus controller (Syntech CS-05; Syntech, Buchenbach, Germany). The duration of stimulus was one second. The compounds were randomly selected and applied at an interval of 30 seconds on the same antenna. The signals generated by the antenna were acquired with the signal acquisition interface (Syntech IDAC-2; Syntech, Buchenbach, Germany) and recorded and processed using Syntech EAG, version 2.7 software.
Fifteen antennae of two- to five-day-old males of G. aurantianum were used to evaluate the compounds. Bioassays were performed during the scotophase (18:00–20:00 hours), and each antenna was considered a replicate. The response variable was the amplitude (mV) of the electroantennographic peak elicited by the evaluated compounds.
(E)-8-dodecenyl acetate release rate
The G. aurantianum pheromone release rate from red rubber septa (Wheaton Science Products, Millville, New Jersey, United States of America) was monitored for five weeks under field conditions. Of the (E)-8-dodecenyl acetate solutions, 100 µL (1 mg) was pipetted into dispenser grooves and completely absorbed. Septa were loaded 30 minutes before testing. They were placed in an exhaust hood for 15 minutes and then transferred to capped individual holding vials. A loaded septum was placed inside a glass vial (7 mL), which was then sealed with aluminium foil. Volatiles were sampled for 1 hour with a polydimethylsiloxane/divinylbenzene fibre (film thickness 65 µm; Supelco, Toluca, Mexico) by solid phase micro-extraction. After the sampling period, the fibre was removed from the vial and inserted into a gas chromatography injector and desorbed for 1 minute for analysis by gas chromatography–mass spectrometry.
The solid phase micro-extraction samples were analysed using a gas chromatograph (CP-3800) coupled to a mass spectrometer (Saturn 2200) fitted with an inert nonpolar column of 5% phenylmethyl polysiloxane (VF-5ms, Agilent Technologies) that was 30 m long, 0.25 mm in inside diameter, and with 0.25 µm film thickness. The samples were injected using the splitless mode. The carrier gas was helium (1 mL/minute). The oven temperature was set at 50 °C for 2 minutes, then at 15 °C/minute to 280 °C, and then held at this temperature for 10 minutes. The injector port temperature was held at 200 °C. The samples were ionised by electronic impact at 70 eV.
For the first solid phase micro-extraction fibre analysis, the peak area of the pheromone component was considered as 100%. The same lure was analysed weekly for a month to measure the loss of the compound over time. Four repetitions were carried out for this experiment. After each analysis, the septa were placed in a delta-type handmade trap (20 cm × 10 cm, transparent colour) made from 3-L recycled plastic bottles. The traps were hung with a steel wire in the macadamia tree branches at a height of 4 m above ground, with 50 m between each trap.
Field test
Compounds identified in the sex pheromone gland were evaluated in two trapping experiments in a 15-year-old macadamia orchard at Buena Vista farm, municipality of San Pablo, Department of San Marcos, Guatemala (14° 57´ N, 91° 59´ W, 820 m above sea level).
Pheromone solutions were pipetted into red rubber septa and completely absorbed. They were placed in an exhaust hood for 15 minutes and then transferred to capped individual holding vials and stored at –20 °C before use. Rubber septa loaded with the pheromone were placed in a delta-type handmade trap (20 cm × 10 cm, transparent colour) made from 3-L plastic bottles. A thin layer of a nondrying Tangle-Trap sticky (The Tanglefoot Company, Grand Rapids, Michigan, United States of America) was placed on a plastic plate at the bottom of each trap to capture G. aurantianum males.
In each experiment, treatments were replicated four times in randomised blocks with 40-m spacing between traps and traps placed 4 m above the ground. Every seven days, we recorded the number of males captured. The sticky plastic plates were replaced with new sticky plates, and the position of treatments was rotated within each block. Lures were replaced every four weeks.
Evaluation of single compounds and blends
We tested 13 different lure treatments (five single compounds, four binary mixtures, three tertiary mixtures, and the five-component mixture), as well as a trap with a solvent blank lure that served as control, from 3 May to 21 June 2021. One milligram of single compounds or blends in ratios determined by chemical analysis (Table 1) was loaded in rubber septa.
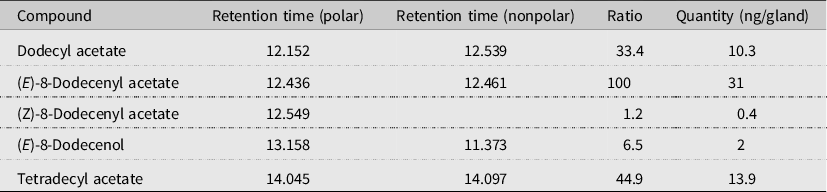
Table 1. Compounds identified in sex pheromone glands of Gymnandrosoma aurantianum virgin females from Guatemala.
Evaluation of doses
Four doses (0.5, 1, 1.5, and 2 mg) of (E)-8-dodecenyl acetate and a trap with a solvent blank lure as control were evaluated from 10 August to 27 October 2021.
Statistical analysis
Data were analysed with R statistical software, version 4.2.0 (R Core Team 2022). The electroantennographic responses (mV) of G. aurantianum males to the compounds were analysed using one-way analysis of variance. For the field experiments, the numbers of moths captured per trap per week were analysed with a generalised linear model with negative binomial response. Both models satisfied the assumptions of normality and homoscedasticity. The means were compared using Tukey’s honestly significant difference test (α = 0.05). Figures were generated from the original data, without fitting.
Results
Chemical analysis
Five compounds from gland extracts of G. aurantianum virgin females were identified as possible components of the sex pheromone: (E)-8-dodecenyl acetate was the major compound, followed by tetradecyl acetate, dodecyl acetate, and two compounds in small amounts, (E)-8-dodecenol and (Z)-8-dodecenyl acetate (Fig. 1), in a ratio of 100:44.9:33.4:6.5:1.2, respectively (Table 1). Similar results were obtained on nonpolar column, except for (Z)-8-dodecenyl acetate, which was not identified because it coelutes with (E)-8-dodecenyl acetate.

Fig. 1. Gland extract analysis of Gymnandrosoma aurantianum virgin females on A, nonpolar column and B, polar column. Compound 1: (E)-8-Dodecenyl acetate, 2: dodecyl acetate, 3: tetradecyl acetate, 4: (E)-8-Dodecenol, 5: (Z)-8-Dodecenyl acetate, C: contaminant.
Electrophysiological analysis
The antennal responses of G. aurantianum males to the synthetic compounds differed significantly (F = 14.34; df = 5,84; P < 0.001). Only the major component (E)-8-dodecenyl acetate and the five-component mixture elicited a significant antennal response (0.66 and 0.68 mV, respectively). The male response to the secondary components, dodecyl acetate, (E)-8-dodecenol, and (Z)-8-dodecenyl acetate, was similar, whereas tetradecyl acetate did not elicit a significant electroantennographic response (Fig. 2).

Fig. 2. Mean (± standard error) antennal responses of Gymnandrosoma aurantianum males from Guatemala to synthetic compounds. Means identified with the same letter are not significantly different (Tukey’s honestly significant difference: α = 0.05).
(E)-8-dodecenyl acetate release rate
The amount of (E)-8-dodecenyl acetate decreased with exposure time of the compound-loaded rubber septa to field conditions. The regression analysis indicated that the septum had released 30, 50, 70, 75, and 80% of the pheromone by the end of the first, second, third, fourth, and fifth week, respectively (y = –15.847x + 104.73). After the fourth week, only 25% of the concentration remained (0.25 mg) in the septum, and it was necessary to renew the pheromone to preserve its attractiveness (Fig. 3).
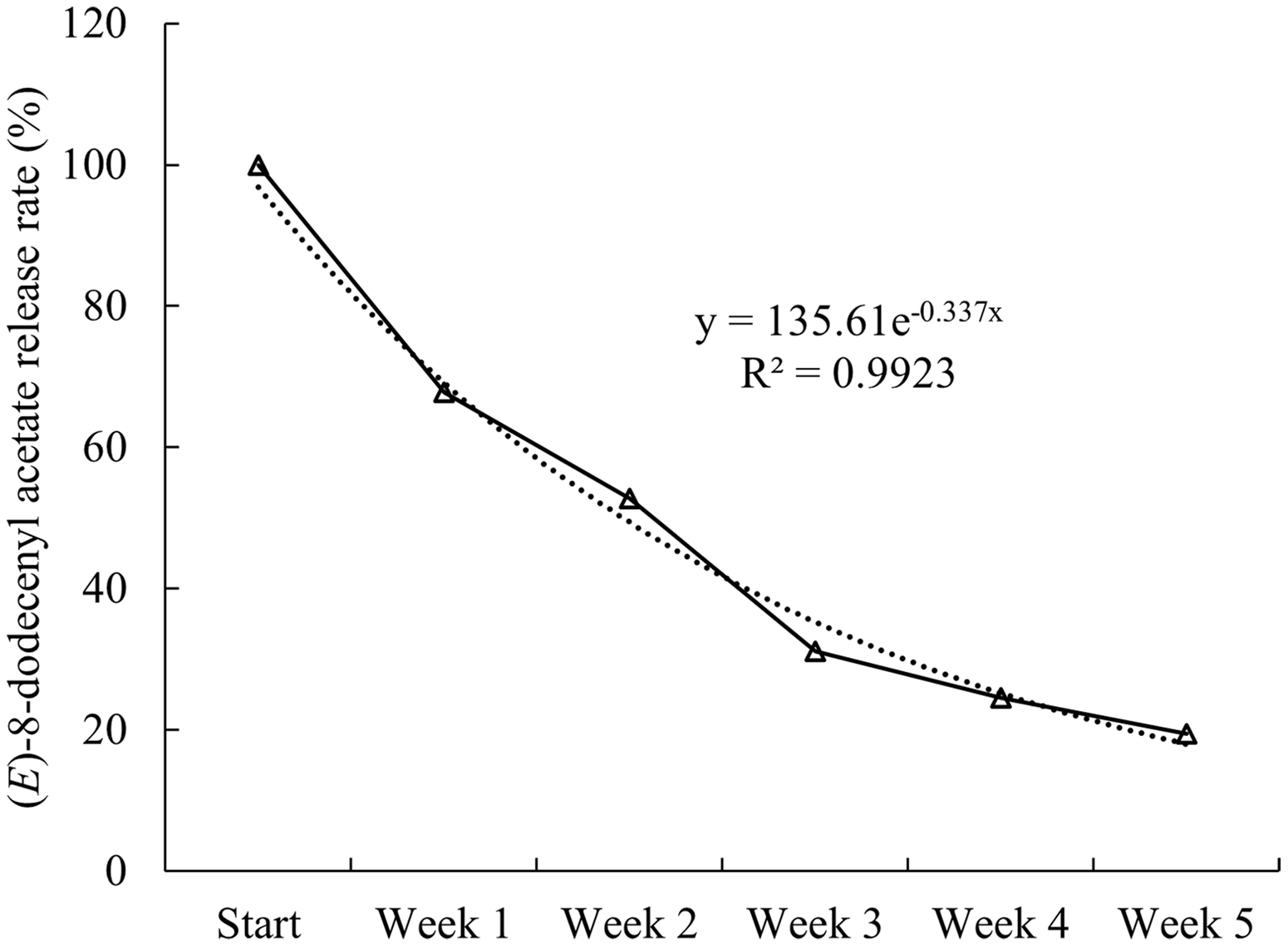
Fig. 3. Regression analysis of the (E)-8-dodecenyl acetate release rate under field conditions.
Field tests
Evaluation of single compounds and blends
Capture of G. aurantianum males differed significantly among treatments (X 2 = 196.98; df = 13; P < 0.001). When tested individually, the secondary components – dodecyl acetate, tetradecyl acetate, (E)-8-dodecenol, and (Z)-8-dodecenyl acetate – had mean catches no different than those of the unbaited traps. Only traps baited with (E)-8-dodecenyl acetate alone or in combination with either (E)-8-dodecenol or dodecyl acetate captured significantly more G. aurantianum males than unbaited traps did. The addition of secondary compounds to the major component of the pheromone did not significantly improve attraction (Fig. 4).
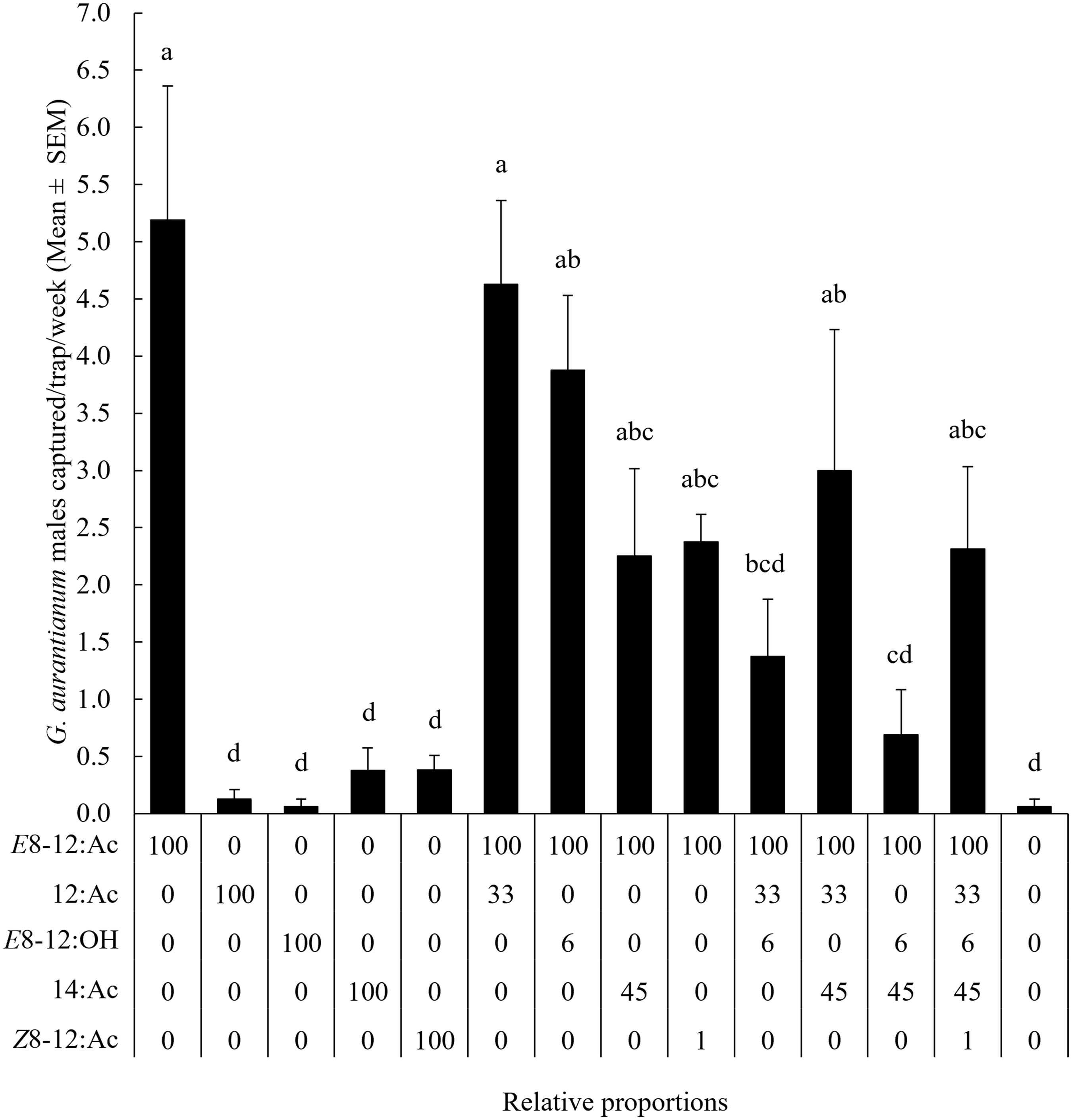
Fig. 4. Mean (± standard error) capture of Gymnandrosoma aurantianum males in Guatemala in adhesive traps baited with 1 mg of the synthetic compounds, single and blended. Means identified with the same letter are not significantly different (Tukey’s honestly significant difference: α = 0.05). E8–12:Ac, (E)-8-Dodecenyl acetate; 12:Ac, dodecyl acetate; E8–12:OH, (E)-8-dodecenol; 14:Ac, tetradecyl acetate; and Z8–12.Ac, (Z)-8-dodecenyl acetate.
Evaluation of doses
The different doses of (E)-8-dodecenyl acetate evaluated were significantly different (X 2 = 48.67; df = 3; P < 0.001). Treatments with 1 and 2 mg of this compound captured similar numbers of G. aurantianum males, whereas with doses of 1.5 and 0.5 mg, capture decreased significantly (Fig. 5).
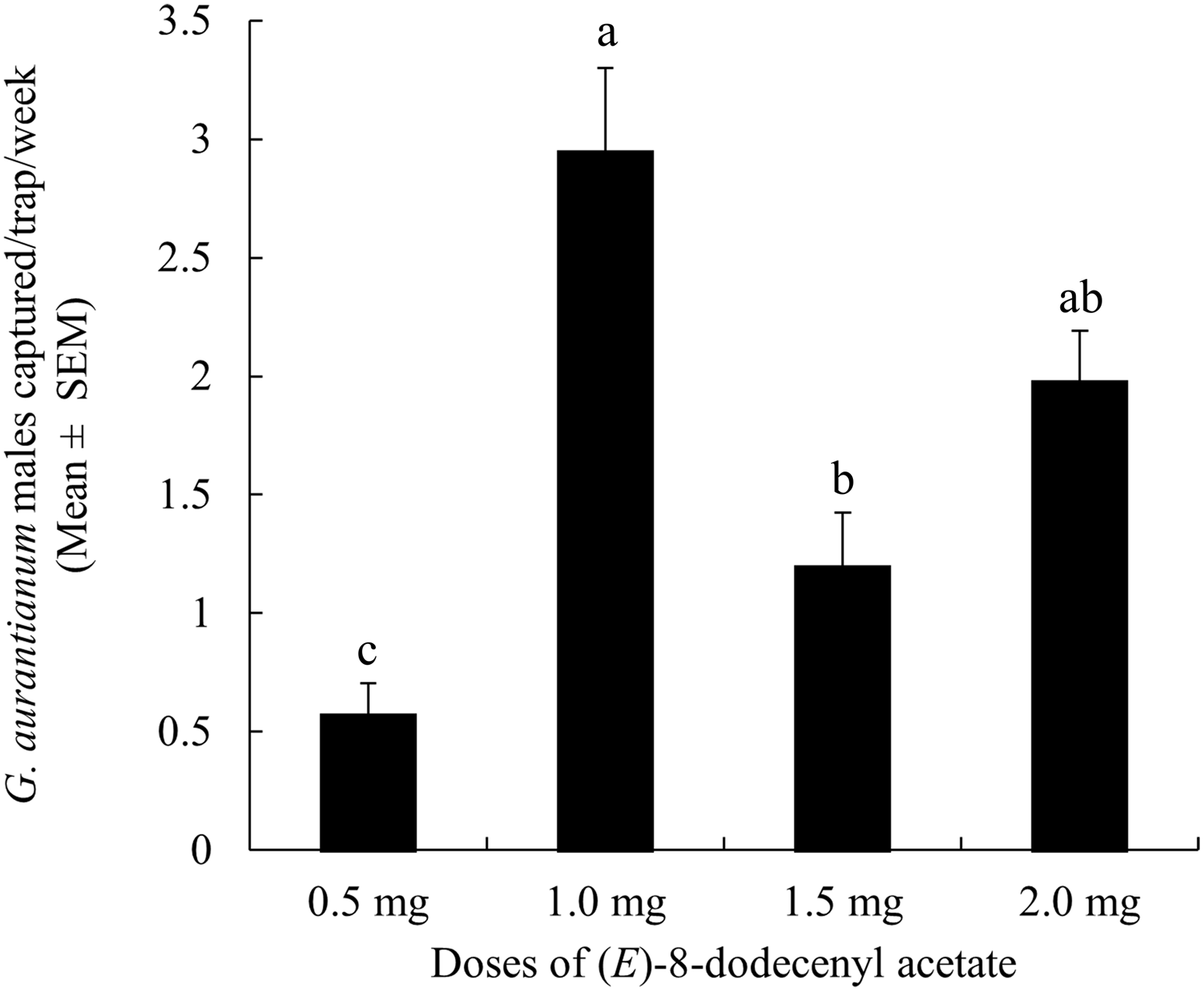
Fig. 5. Mean (± standard error) capture of Gymnandrosoma aurantianum males in adhesive traps baited with four different doses of (E)-8-dodecenyl acetate. Means identified with the same letter are not significantly different (Tukey’s honestly significant difference: α = 0.05).
Discussion
We examined the composition of the G. aurantianum sex pheromone glands from a Guatemalan population to determine whether adding secondary components to the major component of the gland content improved the capture rate of males of this species. In the study, we found that the G. aurantianum sex pheromone gland from Guatemalan populations is composed of five possible components of the sex pheromone: (E)-8-dodecenyl acetate as its major component, followed by tetradecyl acetate, dodecyl acetate, and two compounds in small quantities, (E)-8-dodecenol and (Z)-8-dodecenyl acetate (Table 1). Our results agree with those of Chamberlain et al. (Reference Chamberlain, Beevor, Cork and Hall2003), who reported that (E)-8-dodecenyl acetate is the major component in sex glands of Gymnandrosoma aurantianum (= Ecdytolopha torticornis) from Costa Rican populations, but our results differ in the amount of (Z)-8-dodecenyl acetate. We found this compound in smaller amounts, and we additionally identified tetradecyl acetate.
In our field tests, when the synthetic compounds were tested individually, (E)-8-dodecenyl acetate, the major sex gland component, was the only compound responsible for G. aurantianum male attraction. Similarly, Ma et al. (Reference Ma, Li, Sun and Wen2014) reported that Euzophera pyriella (Yang) (Lepidoptera: Pyralidae) was attracted only by (Z,E)-9,12-tetradecadienol, the major component of its sex gland, although other compounds were identified as components of its gland content. The (E)-8-dodecenyl acetate is a compound widely found in the family Tortricidae; it also forms part of the pheromone complex of other macadamia borers, such as Cryptophlebia leucotreta (Meyrick) (Lepidoptera: Tortricidae) (Persoons et al. Reference Persoons, Ritter and Nooyen1977) and Cryptophlebia batrachopa (Meyrick) (Hall et al. Reference Hall, Beevor, Cork, Nesbitt, Si and Croix1984). Both of these tortricid species produce (E)-8-dodecenyl acetate and (Z)-8-dodecenyl acetate in different ratios: C. leucotreta produces 90% (E)-8-dodecenyl acetate and 10% (Z)-8-dodecenyl acetate (Newton et al. Reference Newton, Thomas, Mastro and Schwalbe1993), and C. batrachopa produces 0.5% (E)-8-dodecenyl acetate and 99.5% (Z)-8-dodecenyl acetate (Hall et al. Reference Hall, Beevor, Cork, Nesbitt, Si and Croix1984). Witzgall et al. (Reference Witzgall, Sauter, Buser, Rauscher, Arn, Charmillot and Wildbolz1989) identified four compounds in the glands of Grapholita lobarzewskii (Nowicki) females (Lepidoptera: Tortricidae): (E)-8-dodecenyl acetate, (Z)-8-dodecenyl acetate, dodecyl acetate, and tetradecyl acetate. We identified the same compounds in G. aurantianum glands, but in different ratios, and we also found (E)-8-dodecenol. In contrast, in other borers, such as Cydia strobilella (Linnaeus) (Lepidoptera: Tortricidae), Bédard et al. (Reference Bédard, Gries, Gries and Bennett2002) reported that (E)-8-dodecenyl acetate was the only component of these species sex pheromone. We confirmed that tetradecyl acetate was unattractive to G. aurantianum males in field tests, either when presented by itself or when blended with the major component, and did not even elicit antennal response. However, tetradecyl acetate has been reported as being part of the sex pheromone of some tortricids (El-Sayed Reference El-Sayed2022), such as Argyrotaenia pomililiana (Trematerra and Brown) (Lepidoptera: Tortricidae) (Cichón et al. Reference Cichón, Trematerra, Coracini, Fernandez, Bengtsson and Witzgall2004), C. leucotreta (Persoons et al. Reference Persoons, Ritter and Nooyen1977), and Grapholita dimorpha (Komai) (Lepidoptera: Tortricidae) (Murakami et al. Reference Murakami, Sugie, Fukumoto and Mochizuki2005), among others. The high electroantennographic response of G. aurantianum to (E)-8-dodecenyl acetate matches its high level of attraction in field-trapping tests.
The quantity of compounds per gland and their ratios that Chamberlain et al. (Reference Chamberlain, Beevor, Cork and Hall2003) reported also differ from our findings. We found that the females from Guatemala produce more dodecyl acetate and less (Z)-8-dodecenyl acetate than do females from Costa Rica. The differences in the results could be due to the techniques used, but this is unlikely because we followed the same protocol used by Chamberlain et al. (Reference Chamberlain, Beevor, Cork and Hall2003). Instead, there may be different races of the same species, as occurs with Ostrinia nubilalis (Hübner) (Lepidoptera: Crambidae) (Sorenson et al. Reference Sorenson, Kennedy, Van Duyn, Bradley and Walgenbach1992). Other examples of geographic variation in composition of the pheromone and male response have been reported in Adoxophyes spp. (Lepidoptera: Tortricidae) (Yang et al. Reference Yang, Jeon and Boo2005), Choristoneura rosaceana (Harris) (Lepidoptera: Tortricidae) (El-Sayed et al. Reference El-Sayed, Delisle, De Lury, Gut, Judd and Legrand2003), Zeiraphera diniana (Guenée) (Lepidoptera: Tortricidae) (Guerin et al. Reference Guerin, Baltensweiler, Arn and Buser1984), and other economically important moths (Cardé et al. Reference Cardé, Baker and Roelofs1975; Yang et al. Reference Yang, Jung, Han, Boo and Yiem2002; Cardé and Haynes Reference Cardé and Haynes2004; Groot et al. Reference Groot, Marr, Schöfl, Lorenz, Svatos and Heckel2008; Chen et al. Reference Chen, Zhu, Tian, Zhang, Guo and Liu2018; Cruz-Esteban et al. Reference Cruz-Esteban, Rojas, Sánchez-Guillén, Cruz-López and Malo2018). To test this hypothesis, it would be necessary to compare the two populations with the same protocol.
Another important aspect of optimising the efficiency of a pheromone for use in pest monitoring is determining the number of components and the function of the minority compounds. In the case of G. aurantianum, only the major component had been evaluated for capture of males in traps in macadamia orchards in Costa Rica (Chamberlain et al. Reference Chamberlain, Beevor, Cork and Hall2003) and Guatemala (Navas-Franco Reference Navas-Franco2019), whereas in Brazil, the sex pheromone is composed of (E)-8-dodecenyl acetate and (E)-8-dodecenol (Leal et al. Reference Leal, Bento, Murata, Ono, Parra and Vilela2001). The binary mixture attracts G. aurantianum males in citrus plantations (Leal et al. Reference Leal, Bento, Murata, Ono, Parra and Vilela2001) and has been used as a commercial pheromone (Ferocitrus Furão, Fuji Flavor Co., Tokyo, Japan) in field testing to monitor this species in citrus (Carvalho Reference Carvalho2003; Parra et al. Reference Parra, Bento, Garcia, Yamamoto, Vilela and Leal2004; Yamamoto et al. Reference Yamamoto, Molina, Felippe and Nociti2006). However, this binary mixture captured fewer G. aurantianum males than the single compound (E)-8-dodecenyl acetate did in macadamia.
In the present study, we evaluated the compounds identified in the sex pheromone gland extract. Our results indicate that the number of male moths trapped did not differ between mixtures and (E)-8-dodecenyl acetate alone. Moreover, when the secondary compounds were added to (E)-8-dodecenyl acetate, the catches diminished. We therefore conclude that the secondary components, dodecyl acetate, tetradecyl acetate, (E)-8-dodecenol, and (Z)-8-dodecenyl acetate, are not part of the sex pheromone blend of G. aurantianum female. This coincides with reports on G. lobarzewskii, where the minor components, dodecyl acetate and tetradecyl acetate, which are present in the female gland, did not affect trap catch (Witzgall et al. Reference Witzgall, Sauter, Buser, Rauscher, Arn, Charmillot and Wildbolz1989). Likewise, Newton et al. (Reference Newton, Thomas, Mastro and Schwalbe1993) reported that decyl acetate, dodecyl acetate, tetradecyl acetate, hexadecyl acetate, and octadecyl acetate, individually or blended with 9:1 (E)-8-dodecenol and (Z)-8-dodecenyl acetate, did not lead to significant increases in C. leucotreta catches. This could be because, in some species of lepidopterans, lesser sex pheromone components do not function as attractants and conversely play an important role as interspecific inhibitors that allow reproductive isolation of sympatric, closely related species (Kong et al. Reference Kong, Liu, Wang, Zhang and Zhang2012; Mozuraitis and Buda Reference Mozuraitis and Buda2013). For example, the lesser component (Z)-11-hexadecenol separates the sympatric species Chilo suppressalis (Walker) (Lepidoptera: Pyralidae) and Helicoverpa armígera (Hübner) (Lepidoptera: Noctuidae) (Chen et al. Reference Chen, Zhu, Tian, Zhang, Guo and Liu2018). In the same way, adding (Z)-9-hexadecenyl acetate, a minor component of the pheromone blend of Mythimna unipuncta (Haworth) (Lepidoptera: Noctuidae), reduces the number of male Sesamia nonagrioides (Lefebvre) (Lepidoptera: Noctuidae) captured in field traps baited with compounds of their pheromone (Eizaguirre et al. Reference Eizaguirre, López, Sans, Bosch and Albajes2009). In some cases, it has also been reported that secondary components can increase the attractiveness of a pheromone. For example, Downham et al. (Reference Downham, Hall, Chamberlain, Cork, Farman and Tamò2003) report that in Maruca vitrata (Fabricius) (Lepidoptera: Crambidae), adding (E,E)-10,12-hexadecadienol and (E)-10-hexadecenal to the major component, (E,E)-10,12-hexadecadienal, has this effect.
Besides evaluating the effect of adding secondary components of the pheromone, we also evaluated the doses. Our results revealed that the traps baited with doses greater than 1 mg of (E)-8-dodecenyl acetate captured more G. aurantianum males than did traps baited with lower doses (0.5 mg). We therefore can propose the use of 1 mg of the single compound, (E)-8-dodecenyl acetate, for monitoring this moth. This dose has been used to monitor G. aurantianum populations in Costa Rica (Chamberlain et al. Reference Chamberlain, Beevor, Cork and Hall2003) and Guatemala (Navas-Franco Reference Navas-Franco2019) in macadamia crops. In other species, such as C. leucotreta, the dose of 1 mg has also been used, and captures of males do not differ significantly from captures with higher doses (10 and 50 mg) of the pheromone (Newton et al. Reference Newton, Thomas, Mastro and Schwalbe1993). Moreover, Anshelevich et al. (Reference Anshelevich, Kehat, Dunkelblum and Greenberg1993) indicate that, with 0.2 and 2 mg of the pheromone per dispenser, the response of Cryptoblabes gnidiella (Millière) (Lepidoptera: Pyralidae) males increases, whereas with doses of 10 and 20 mg, capture rates decrease significantly. Similarly, with Cydia pomonella (Linnaeus) (Lepidoptera: Tortricidae), the dose of 0.1 mg captures a similar number of males as the dose of 1 mg does, but higher doses (5 mg) decrease capture in traps (Kehat et al. Reference Kehat, Anshelevich, Dunkelblum, Fraishtat and Greenberg1994), and for E. pyriella, 0.2 µg is sufficient to capture males (Ma et al. Reference Ma, Li, Sun and Wen2014). Therefore, doses higher than 2 mg per dispenser seem to reduce catches of these species. In this respect, Muirhead-Thomson (Reference Muirhead-Thomson1991) suggests it is important that the synthetic attractant has a concentration similar to the natural concentration released by the population’s females because either excess concentrations or very low concentrations elicit abnormal behaviour in males, resulting in low capture rates. It is thus essential to evaluate the amount of pheromone released by G. aurantianum females.
In conclusion, our results indicate that adding the secondary components, tetradecyl acetate, dodecyl acetate, (E)-8-dodecenol, and (Z)-8-dodecenyl acetate, to the major component, (E)-8-dodecenyl acetate, does not significantly improve capture of G. aurantianum males in traps and that the single compound (E)-8-dodecenyl acetate is sufficient to attract G. aurantianum males. One milligram of this compound is recommended for monitoring G. aurantianum because higher doses increase costs, especially if used in large areas of macadamia cultivation.
Acknowledgements
The authors thank the Consejo Nacional de Ciencia y Tecnología (CONACYT) for the scholarship granted to R.A.A. for doctoral studies (CVU: 641600), which allowed the development of this research. They also thank Ingeniero Pedro Zaldaña Morales for allowing us to carry out our experiments at Buena Vista farm (Guatemala, CA) and for all the attention provided.
Competing interests
The authors declare that they have no competing interests.









