Introduction
The raspberry crown borer (RCB), Pennisetia marginata (Harris) (Lepidoptera: Sesiidae), is native to North America and the only species of this genus in areas north of Mexico (Eichlin and Duckworth Reference Eichlin and Duckworth1988). Throughout its range, larvae of this clearwing moth commonly feed within the crowns and stems of cane fruits in the genus Rubus Linnaeus (Rosaceae). Although the incidence of RCB is often sporadic, in some areas and years it can be a highly destructive pest of cultivated raspberry and blackberry (Lovett Reference Lovett1921; Raine Reference Raine1962; McKern et al. Reference McKern, Johnson and Lewis2007). Eggs are laid individually on host-plant leaves from mid-August into October. Larvae begin hatching in early September and initiate feeding within plant crowns located at the base of canes. Larvae eventually tunnel upwards into the pith of the canes where they pupate and emerge as adults almost 2 years later in British Columbia (BC) (Raine Reference Raine1962). Withering, wilting, and dying cane foliage, sometimes with half-grown fruit still attached, are often the first signs of a RCB infestation. Larval feeding within plant crowns also facilitates entry of pathogens that can produce symptoms similar to cane blight (Lawrence Reference Lawrence1904). In regions where RCB has a 2-year life cycle (Raine Reference Raine1962), feeding by second-year larvae in plant crowns often causes canes to break off when growers tie them up in spring. RCB populations build up slowly in commercial cane berry fields and often go undetected until cane vigour is severely reduced (Raine Reference Raine1962).
Detection of RCB in commercial plantings is challenging because the insect spends most of its life as a larva tunnelling inside the lower parts of canes (Lawrence Reference Lawrence1904; Raine Reference Raine1962). Adult crown borers are thought to emerge and fly between July and September, but this timing is difficult to confirm from field observations and likely varies across regions and seasons (Eichlin and Duckworth Reference Eichlin and Duckworth1988). Without precise knowledge of adult flight phenology and egg laying, growers throughout North America often protect their crops with preventative applications of insecticides. These sprays are referred to as drenches when applied using large volumes of water. In BC, Canada, for example, growers within the Fraser Valley routinely drench raspberry and blackberry crops with diazinon between October and March (British Columbia Ministry of Agriculture and Lands 2009). Growers apply these organophosphate sprays without knowing whether RCB is active in their fields or not. In this region, routine applications of organophosphates put the Fraser Valley aquifer at unnecessary risk of contamination. Diazinon and other organophosphates are being replaced by newer, more expensive, life-stage specific insecticides, but these require accurate information about insect phenology to be most effective.
Insect sex pheromones are useful for developing monitoring systems that provide growers with an early warning of an insect's presence and phenology (McNeil Reference McNeil1991). These systems allow for more accurate targeting of insecticide sprays when and where needed. Berry growers in BC are familiar with pheromone-based monitoring systems used to determine seasonal flight phenology and relative population levels of the obliquebanded leafroller, Choristoneura rosaceana Harris (Lepidoptera: Tortricidae), and currant borer, Synanthedon tipuliformis Clerck (Lepidoptera: Sesiidae) (British Columbia Ministry of Agriculture and Lands 2009). Efforts to develop a similar monitoring system for P. marginata have failed because none of the readily available commercial sex attractants identified in other sesiid moths have proved attractive (Tracy Hueppelsheuser, British Columbia Ministry of Agriculture and Lands, Abbotsford, BC, Canada, personal communication). Although related species of sesiid moths often share pheromone components (El-Sayed Reference El-Sayed2009), molecular phylogeny studies suggest Pennisetia is a unique and divergent sesiid genus in North America (McKern et al. Reference McKern, Szalanski, Johnson and Dowling2008). Several compounds are postulated to be sex pheromones of Pennisetia species but no sex pheromones have been isolated from virgin females in this genus (El-Sayed Reference El-Sayed2009). We undertook this study to identify the sex pheromone of P. marginata in an effort to aid development of pheromone-based management tools for this pest.
Methods and materials
Insects
Adult RCBs were reared from 1.5-year-old larvae, which were collected on 20 April 2008 from a commercial raspberry field in Aldergrove, BC (49°2′22.53′′N, 122°27′32.92′′W). Host raspberry cane stubs showing signs of RCB infestation were dissected and second-year larvae (ca. 2 cm long) were removed and transferred to 2-cm long incisions made into the sides and half way along the lengths of freshly cut, second-year wild blackberry canes (30 × 1.5 cm). Once larvae were transferred, each incision was sealed with wax tape, and severed ends of canes were submerged in water in glass test tubes. Transferred larvae continued to feed, almost always tunnelling upward, and adults emerged from pupae protruding from the side of canes (Raine Reference Raine1962). Thirty such canes were kept in an environment chamber set at 22°C under a 16L:8D photoregime. On 15 July 2008, canes were transferred to 11.4-L plastic buckets into which adults emerged. Moths emerging from 15 July to 15 August 2008 were collected within 12 hours of emergence to prevent mating and kept singly in capped 29.5-mL plastic cups (Solo Cup Company, Lake Forest, Illinois, United States of America) until analysis.
Extraction and analyses of pheromone glands
The abdominal tips of 1-day to 2-day-old females with intact pheromone glands were removed with fine forceps and solvent-extracted for 15 minutes using ∼20 μL of HPLC-grade hexane (EMD Chemicals, Gibbstown, New Jersey, United States of America) per gland. Aliquots of one female equivalent of pheromone gland extract were analysed by coupled gas chromatographic–electroantennographic detection (GC-EAD) (Arn et al. Reference Arn, Städler and Rauscher1975; Gries et al. Reference Gries, Khaskin, Gries, Bennett, King and Morewood2002; Judd et al. Reference Judd, Gries, Aurelian and Gries2011) employing a Hewlett Packard 5890A gas chromatograph (Agilent Technologies Canada Inc., Mississauga, Ontario, Canada) equipped with a flame ionization detector (FID) and one of three GC columns (Table 1). Helium was used as a carrier gas (1 mL/minute flow rate) for the GC with temperature programmes as reported in Table 1. EAD-active components were identified by comparing their retention indices with those of authentic standards on three GC columns (Van Den Dool and Kratz Reference Van Den Dool and Kratz1963; Table 1).
Table 1 Retention indices for candidate pheromone components A, B and, C eliciting antennal responses in Figures 1 and 2, and of geometrical isomers of synthetic 2,13- and 3,13-octadecadienol, -octadecadienyl acetate, and -octadecadienal on three gas chromatography (GC) columns. The retention indices of A, B, and C matched those of (E,Z)-3,13-18:Ald, (E,Z)-3,13-18:OH, and (E,Z)-2,13-18:Ald on all three GC columns, respectively.

Note: (1) cis-double bonds at C2 and C3
of 2,13- or 3,13-octadecadienals, respectively, rearrange during
chromatography, and thus these isomers are not reported here; (2) the
stationary phase of a Zebron-5 and DB-5 column is identical; (3) the
temperature of injection port and flame ionisation detector was 250°C;
(4) the temperature programmes on GC columns were as
follows:

Collection and analysis of pheromone effluvia
For several consecutive days, 1-day to 2-day-old virgin females were held in a vertically positioned, cylindrical, Pyrex glass chamber (10 cm ID × 6 cm length) provisioned with a water-soaked cotton wick. This chamber contained three to eight females at any particular time and dead females were removed daily. A water aspirator drew humidified, charcoal-filtered air at a rate of 1 L/minute through the chamber and an attached glass column (14 × 1.3 cm OD) filled with 150 mg of Porapak Q (50–80 mesh, Waters Associates Inc., Milford, Massachusetts, United States of America). In control aerations the chamber contained no females. A total of 576 female hour equivalents (FHEs) or 24-day equivalents of pheromone emission (1 FHE=amount of pheromone emitted by one female during 1 hour) were captured on Porapak Q. Trapped volatiles were eluted from Porapak Q with 2 mL of redistilled pentane. The eluted material was concentrated under a stream of nitrogen such that 2-μL aliquots contained 10 FHE of pheromone emission, which was then analysed by GC-EAD on a DB-5 column (Table 1).
Pheromone identification
Components in pheromone gland extracts that elicited antennal responses were identified by comparing their respective retention indices on three GC columns (Zebron-5, DB-23, and DB-210) with retention indices of synthetic diene alcohols or acetates (Table 1) that were previously identified as sesiid pheromones. Corresponding aldehydes were synthesised (see below). Components in extracts or effluvia that elicited antennal responses, and their corresponding synthetic standards, were also subjected to GC-mass spectrometry (MS) employing a Saturn 2000 Ion Trap GC-MS (Varian Instrument, Laurent, Quebec, Canada; now part of Agilent Technologies Inc.) fitted with a DB-5 column.
Sources of candidate pheromone components
All candidate pheromone components except (3E,13Z)-octadecadienal [(3E,13Z)-18:Ald)] and (2E,13Z)-octadecadienal [(2E,13Z)-18:Ald] were purchased from Pherobank (Wageningen, The Netherlands). (3E,13Z)-18:Ald was synthesised from (3E,13Z)-octadecadienol [(3E,13Z)-18:OH] (100 mg, 0.375 mmol) with pyridinium chlorochromate (121 mg, 0.566 mmol) in the presence of anhydrous K2CO3 (78 mg, 0.565 mmol) in 3 mL of dichloromethane (Islam et al. Reference Islam, Yamamoto, Sugie, Naka, Tabata and Arita2007). After stirring the reaction mixture for 30 minutes at room temperature, 10 mL of hexane was added, and stirring continued for an additional 5 minutes. Silica filtration of the mixture and purification by flash column chromatography with a hexane/ether mixture (97/3) as an eluant afforded the desired aldehyde at 70% yield. (3E,13E)-18:Ald, (2E,13Z)-18:Ald, and (2E,13E)-18:Ald were synthesised following an analogous procedure.
Field experiments
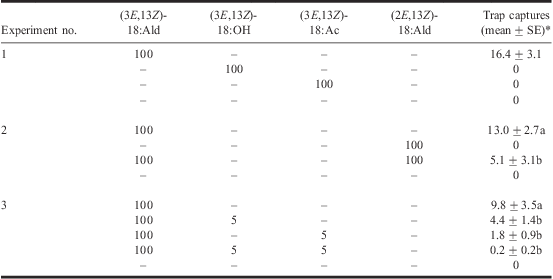
Three field trapping experiments were conducted in a 3.6-ha commercial raspberry planting (cultivar Malahat) with 2-m high canes and a 0.9 × 2.5 m plant × row spacing, in Aldergrove, BC (South Alder Farms Ltd.). All experiments employed a randomised complete block design with 8–10 replicates each. White, sticky, wing traps (Wingtrap-II, Contech Enterprises, Delta, BC, Canada) were hung from the upper trellis wire at a height of 1.3 m within the plant canopy. Traps were deployed at 10-m intervals in linear arrays (blocks) within planted rows and blocks of traps were spaced 20 m apart. Each trap was baited with a grey halobutyl rubber septum (West Pharmaceutical Services, Lionville, Pennsylvania, United States of America) impregnated with candidate pheromone components dissolved in 100 μL of HPLC-grade hexane. In experiment 1 (1–7 September 2008), we tested the attractiveness of the two synthetic candidate pheromone components that elicited the strongest EAD responses and their structural analogue all at 100 μg loads and compared with a solvent control, as follows: (1) (3E,13Z)-18:Ald; (2) (3E,13Z)-18:OH; (3) (3E,13Z)-octadecadienyl acetate [(3E,13Z)-18:Ac]; and (4) an unbaited solvent control. In experiment 2 (8–15 September 2008), we examined the importance of the double bonds at C3 and C13 in the pheromone component (3E,13Z)-18:Ald by testing four treatments (each component at 100 μg): (1) (3E,13Z)-18:Ald; (2) (2E,13Z)-18:Ald; (3) (3E,13Z)-18:Ald plus (2E,13Z)-18:Ald; and (4) an unbaited solvent control. In experiment 3 (18–25 August 2009), we tested whether other EAD-active pheromone gland components affect the attractiveness of the pheromone component (3E,13Z)-18:Ald. Specifically, we tested five treatments: (1) 100 μg (3E,13Z)-18:Ald; (2) 100 μg (3E,13Z)-18:Ald plus 5 μg (3E,13Z)-18:OH; (3) 100 μg (3E,13Z)-18:Ald plus 5 μg (3E,13Z)-18:Ac; (4) 100 μg (3E,13Z)-18:Ald plus 5 μg (3E,13Z)-18:OH plus 5 μg (3E,13Z)-18:Ac; and (5) an unbaited solvent control.
Diel periodicity of pheromone response
In 2010 we conducted an experiment to determine the diurnal periodicity of male pheromone responses in relation to temperature. On each of three test days, 10 sticky wing traps were baited with 100 μg of (3E,13Z)-18:Ald loaded on grey septa and deployed as before at 10-m intervals within a single row of raspberries transecting an infested field. Moth catches and temperature were recorded hourly from 07:00 to 20:00 hours Pacific Daylight Time (PDT) on 30 August, 3 September, and 13 September, in Aldergrove, BC. Temperatures were measured on site with a thermometer (Fisherbrand Red-Spirit No-Roll Laboratory Thermometer with range: −20°C to +110°C) hung at mid-canopy height within the same row of plants as the traps. The thermometer was shielded from direct sunlight at all times. Moth catches were counted and removed from all traps each hour. On each test day all traps were deployed with new lures and sticky bottoms in a different raspberry field on the same farm.
Statistical analyses
In experiments 1–3, trap-catch data were subjected to a two-way randomised block analysis of variance (ANOVA) and mean catches for each treatment were separated using the Student–Newman–Keuls’ multiple comparison procedure with the experiment-wise error rate set at α = 0.05 (Zar Reference Zar1984). All analyses were performed using Sigmastat® 3.0.1 (SYSTAT Software Inc., San Jose, California, United States of America).
Results and discussion
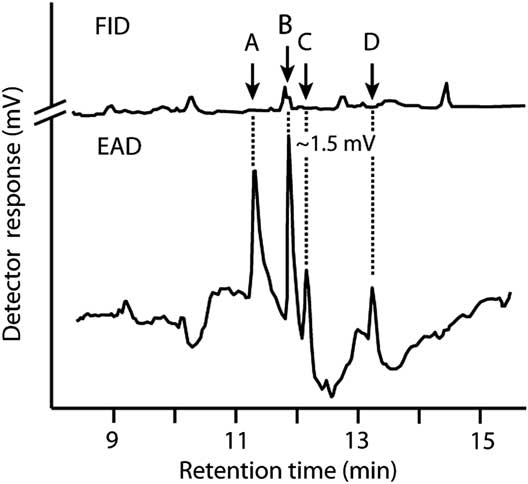
Four components (A, B, C, and D in Fig. 1) elicited responses from male antennae in GC-EAD analyses of pheromone gland extracts from females. Component B consistently elicited the strongest response and was hypothesised to be (3E,13Z)-18:OH based on its relative retention index (corresponding with an antennal response) on Zebron-5, DB-23, and DB-210 columns, which matched the index of authentic (3E,13Z)-18:OH on these different columns (Table 1), supporting the structural assignment. Component A had retention indices, and inter-column differentials of retention indices, that supported an aldehyde rather than an alcohol or acetate functionality. Accordingly, component A was hypothesised to be (3E,13Z)-18:Ald. This aldehyde is heat labile (Islam et al. Reference Islam, Yamamoto, Sugie, Naka, Tabata and Arita2007), and readily rearranges to (2E,13Z)-18:Ald, which elutes later due to the conjugated 2E double bond. Component C was therefore hypothesised to be (2E,13Z)-18:Ald. The assignments of A and C were supported by identical GC retention times of synthetic (3E,13Z)-18:Ald and (2E,13Z)-18:Ald, respectively. When the former was injected in the GC, it yielded (2E,13Z)-18:Ald as an abundant rearrangement product, as supported by GC-MS of synthetic standards. Synthetic (3E,13Z)-18:Ald, (3E,13Z)-18:OH, and (2E,13Z)-18:Ald had retention indices on each of three GC columns that were identical to those appearing as EAD-active components A, B, and C on the same columns, respectively; all three synthetic components elicited strong responses from male antennae. These results strongly supported structural assignments even though all components remained below detection threshold of the mass spectrometer. Compound D was tentatively identified as (3E,13Z)-18:Ac.
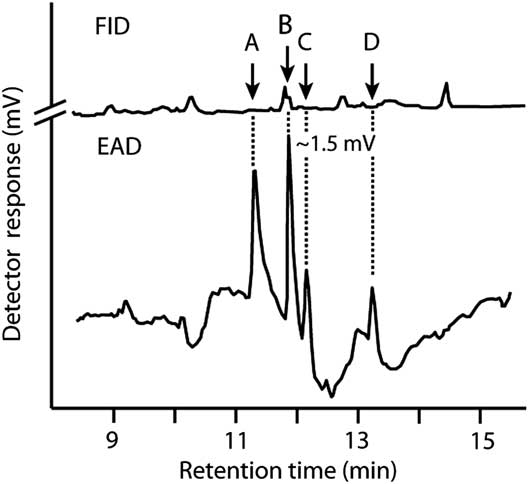
Fig. 1 Representative recording of responses of a gas chromatograph flame ionisation detector (FID) and an electroantennographic detector (EAD: male Pennisetia marginata antenna) to one female equivalent of pheromone gland extract from female Pennisetia marginata. Components that elicited antennal responses were identified as (3E,13Z)-octadecadienal (A), (3E,13Z)-octadecadienol (B), and (2E,13Z)-octadecadienal (C) on a Zebron-5 column (see the section “Materials and methods”). Component D was tentatively identified as (3E,13Z)-octadecadienyl acetate. Chromatography as reported in Table 1.
GC-EAD analyses of effluvia from calling females revealed four components (Fig. 2), including (3E,13Z)-18:Ald (A) and (2E,13Z)-18:Ald (C). (3E,13Z)-18:OH, component B in Figure 1, was not detectable in the effluvia (Fig. 2). Alcohols commonly serve as the biosynthetic precursor to aldehyde pheromones in several Lepidoptera and these are often stored in the pheromone gland but not emitted by calling females (Weatherston and Maclean Reference Weatherston and Maclean1974; Teal et al. Reference Teal, Tumlinson, McLaughlin, Heath and Rush1984). Components E and F in female effluvia remain unidentified (Fig. 2).

Fig. 2 Representative recording of responses of a gas chromatograph flame ionisation detector (FID) and an electroantennographic detector (EAD: male Pennisetia marginata antenna) to 40 female hour equivalents (FHEs) of pheromone emission (1 FHE=amount of pheromone emitted by one female during 1 hour). Components A and C were identified as (3E,13Z)-octadecadienal and (2E,13Z)-octadecadienal, respectively. Components E and F remain unknown. Note that the temperature programme (see Table 1) differed from that of Figure 1.
In field experiment 1, (3E,13Z)-18:Ald was the only candidate pheromone component that attracted male RCBs (Table 2). Although previous studies have reported a few catches of RCB with (3E,13Z)-18:OH (Solomon et al. Reference Solomon, Oliveria, Tumlinson and Doolittle1982; Brown and Snow Reference Brown and Snow1985), we did not capture a single moth in traps baited with this alcohol or the acetate analogue (Table 2). Failure of any lure except (3E,13Z)-18:Ald to attract male moths negated the need for statistical analysis in experiment 1, and clearly indicated that the RCB sex pheromone has an aldehyde functionality, which is rare among sesiid moths (Francke et al. Reference Francke, Karalius, Plass, Lehmann, Dos Santos and Bûda2004). Results of experiment 2 revealed the importance of the double bonds at C3 and C13 in (3E,13Z)-18:Ald because (2E,13Z)-18:Ald proved to be unattractive (Table 2). Furthermore, (2E,13Z)-18:Ald appears to act as a pheromone antagonist because a 1:1 blend of (3E,13Z)-18:Ald and (2E,13Z)-18:Ald was less attractive than (3E,13Z)-18:Ald alone (Table 2).
Table 2 Results of field trapping experiments with candidate sex pheromone components and blends (μg per lure) detected in extracts of pheromone glands and effluvia of female Pennisetia marginata.
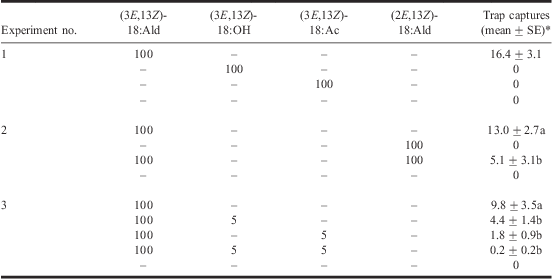
*Note: Treatments with zero moth catches and no variance were excluded from statistical analyses. Within a column and experiment, means for a given treatment followed by different letters are significantly different (Student–Neuman–Keuls’ test, α = 0.05) following significant ANOVA (P ≤ 0.05).
In experiment 3, we tested the hypothesis that the unattractive, but EAD-active, (3E,13Z)-18:OH and (3E,3Z)-18:Ac might be synergists of (3E,13Z)-18:Ald when combined in two- or three-component blends. In both cases, the addition of as little as 5% of either of these compounds in binary blends with (3E,13Z)-18:Ald reduced blend attractiveness (Table 2). A ternary blend of all three compounds was also less attractive than (3E,13Z)-18:Ald alone (Table 2). Results of pheromone analyses and field trapping experiments indicate that (3E,13Z)-18:Ald is the major and perhaps only pheromone component in P. marginata. Single-component pheromones are unusual in Lepidoptera, and even Sesia apiformis (Clerck) (Lepidoptera: Sesiidae), the only sesiid moth known to use an aldehyde pheromone component (El-Sayed Reference El-Sayed2009), only responds to (2E,13Z)-18:Ald when combined with (3E,13Z)-18:OH (Francke et al. Reference Francke, Karalius, Plass, Lehmann, Dos Santos and Bûda2004). To the best of our knowledge, P. marginata is the only sesiid species currently known to use (3E,13Z)-18:Ald as a pheromone component, and the only sesiid species known to respond to an aldehyde alone. Additional components may be unnecessary in this communication system because (3E,13Z)-18:Ald appears to be an uncommon pheromone signal (El-Sayed Reference El-Sayed2009).
Among those Pennisetia spp. studied to date, most have been attracted to (3E,13Z)-18:Ac and (3E,13Z)-18:OH alone, or in combinations (Sharp and Eichlin Reference Sharp and Eichlin1979; Solomon et al. Reference Solomon, Oliveria, Tumlinson and Doolittle1982; Brown and Snow Reference Brown and Snow1985; Priesner et al. Reference Priesner, Witzgall and Voerman1986; Szöcs et al. Reference Szöcs, Tóth, Sziráki and Schwarz1989). For example, trapping studies showed that the European raspberry clearwing moth, Pennisetia hylaeiformis (Laspeyres), is maximally attracted to a 1:1 binary blend of (3E,13Z)-18:Ac and (3E,13Z)-18:OH (Priesner et al. Reference Priesner, Witzgall and Voerman1986). These results were supported by electrophysiological studies that found two specialist receptor cells each tuned to one of these components (Priesner et al. Reference Priesner, Witzgall and Voerman1986). Although Szöcs et al. (Reference Szöcs, Tóth, Sziráki and Schwarz1989) reported catches of three specimens of P. hylaeiformis with a binary blend of (3E,13Z)-18:Ac and (3E,13Z)-18:Ald, this result should not be considered evidence of a role for the aldehyde in this pheromone system because P. hylaeiformis is attracted by (3E,13Z)-18:Ac on its own (Priesner et al. Reference Priesner, Witzgall and Voerman1986). The degree to which the pheromones used by P. marginata and P. hylaeiformis appear to differ is intriguing given their close relatedness (Eichlin Reference Eichlin1986). Eichlin and Duckworth (Reference Eichlin and Duckworth1988) stated that the two species share a common host range and bear a superficial resemblance, with minor differences in male genitalia being the only taxonomic difference. Geographic separation of these species provides differences in regional competition that may have caused a divergence of their pheromone blends. The evolution of multi-component pheromones is often driven by sympatric species competing for particular communication channels. Whereas P. hylaeiformis must compete with several sympatric Pennisetia species in Europe (Spatenka et al. Reference Spatenka, Gorbunov, Lastuvka, Tosevski and Arita1999), P. marginata is the only representative of this genus in areas north of Mexico (Eichlin and Duckworth Reference Eichlin and Duckworth1988). We note, however, that the sex-attractant blend used by P. hylaeiformis is the same blend that maximally attracts the strawberry crown moth, Synanthedon bibionipennis (Boisduval) (Lepidoptera: Sesiidae) (Nielsen et al. Reference Nielsen, Purrington, Campbell, Wilmot, Capizzi and Tumlinson1978). Synanthedon bibionipennis is a North American species with which P. hylaeiformis has likely had no contact, but the distribution and seasonal phenology of the former species partially overlaps that of P. marginata (Eichlin and Duckworth Reference Eichlin and Duckworth1988). In the Pacific Northwest, S. bibionipennis sometimes infests Rubus species (Mote et al. Reference Mote, Wilcox and Hill1929; Bruck et al. Reference Bruck, Edwards and Donahue2008). It seems entirely possible that P. marginata makes no use of (3E,13Z)-18:Ac or (3E,13Z)-18:OH in its pheromone communication system (Figs. 1, 2) because of competition for this pheromone channel. However, a common ancestry with P. hylaeiformis may have provided P. marginata with an olfactory system capable of detecting these components, thus providing a means for behavioural discrimination between conspecifics and sympatric competitors like S. bibionipennis. Given that there are two North American records of P. marginata responding to (3E,13Z)-18:OH (Solomon et al. Reference Solomon, Oliveria, Tumlinson and Doolittle1982; Brown and Snow Reference Brown and Snow1985), it would be interesting to examine the pheromone responses of different populations of P. marginata in relation to its overlapping distribution with S. bibionipennis and their respective host plants.
Diurnal flight activity of male P. marginata, as reflected by pheromone trap catches, occurred throughout the day (09:00–20:00 hours PDT), but had an obvious peak at 16:00 hours on days 1 and 3 (Fig. 3A, 3C). This activity pattern is somewhat different from that of P. hylaeiformis, which exhibited a peak of activity before noon and another in late afternoon (Priesner et al. Reference Priesner, Witzgall and Voerman1986). Part of the within-day increase in catches of RCB with increasing temperatures could be related to an expected increase in the release of pheromone from rubber septa lures as air temperatures increased. However, on the second, and hottest of our three test days, peak captures of P. marginata were shifted 2 hours later into early evening (Fig. 3B) when air temperature was 22°C and below the daily maximum, but similar to temperatures at peak captures on the other days. This suggests that peak pheromone response, and by inference mating, likely occurs in mid to late afternoon and is modified daily by an interaction between light intensity and temperature, as it is in other moths (Castrovillo and Cardé Reference Castrovillo and Cardé1979). We did not monitor captures of P. marginata between 20:00 and 07:00 hours PDT, but the patterns observed suggest that like P. hylaeiformis (Priesner et al. Reference Priesner, Witzgall and Voerman1986), P. marginata is strictly a day-flying moth.

Fig. 3 Mean (± SE) hourly captures of male Pennisetia marginata in 10 sticky wing traps each baited with a grey rubber septum loaded with 100 μg of (3E,13Z)-octadecadienal on 30 August (A), 3 September (B), and 13 September (C) in relation to air temperature within the canopy of three different raspberry fields on the same farm at Aldergrove, BC, in 2010. PDT is Pacific Daylight Time.
We conclude that any development of monitoring or mass-trapping techniques for management of P. marginata in BC, and probably other parts of North America, could use (3E,13Z)-18:Ald. In our experience, however, this will be challenging because (3E,13Z)-18:Ald is difficult to synthesise in large quantity and it appears to lose its activity quite quickly when loaded on rubber septa lures and deployed in the field. We did not attempt to use anti-oxidants or ultra-violet (UV) protectants to stabilise (3E,13Z)-18:Ald on rubber septa because the nature of its instability is not exclusively related to oxidation or UV degradation. Alternatively, it may be worth examining the attraction of formate analogues of this aldehyde pheromone (Todd et al. Reference Todd, Miller, Vetter and Baker1992) and/or the use of pheromone antagonists (Table 2) to manage this pest (Leskey et al. Reference Leskey, Bergh, Walgenbach and Zhang2009).
Acknowledgements
We thank Tracy Hueppelsheuser for raising our awareness that raspberry crown borer was a serious pest, and for helping to locate infested plant material. We thank Mark Gardiner for technical assistance rearing insects, Grigori Khaskin for oxidising (3E,13Z)-octadecadienol, South Alder Farms Ltd. for providing access to their commercial raspberry fields, and Stevo DeMuth for graphical illustrations. This research was supported, in part, by funding provided by Agriculture and Agri-Food Canada to G.J., an Industrial Postgraduate Scholarship from the Natural Sciences and Engineering Research Council of Canada (NSERC) to C.T., with industry co-funding from the BC Raspberry Industry Development Council, the Oregon Raspberry and Blackberry Commission, and the Lower Mainland Horticulture Improvement Association, and by an NSERC-Industrial Research Chair to G.G., with Contech Enterprises Inc. and Global Forest Science as industrial sponsors.