Introduction
Transoceanic trade and transport of goods on cargo ships have for centuries been a common pathway by which exotic plant and animal species have entered new habitats (Elton Reference Elton2000; Hulme et al. Reference Hulme, Roy, Cunha and Larsson2009; Roques et al. Reference Roques, Rabitsch, Rasplus, Lopez-Vamonde, Nentwig and Kenis2009). Not all exotics, however, are destined to establish or become severe pests. Indeed, many fail to thrive in novel habitats due to a lack of suitable hosts, inclement climate, and strong competition or exploitation by natives (Liebhold and Tobin Reference Liebhold and Tobin2008), or may simply blend inconspicuously into the local flora (Martin and Valentine Reference Martin and Valentine2011). Of course, there are also many well-known examples of exotic species that have become serious pests, including many herbivorous insects that inflict damage on crops and forests that can exceed even that of native pests (Pimentel Reference Pimentel1986; Brockerhoff et al. Reference Brockerhoff, Liebhold and Jactel2006; Holmes et al. Reference Holmes, Aukema, Von Holle, Liebhold and Sills2009, Reference Holmes, Liebhold, Kovacs and Von Holle2010; Faccoli and Gatto Reference Faccoli and Gatto2016).
A key feature that influences success in managing exotic pests relates to how likely and rapidly it can spread from its point of introduction. In general, species with low mobility are more likely to be amenable to either eradication or containment strategies (Liebhold and Tobin Reference Liebhold and Tobin2008) or brought under control by introductions of natural enemies from their native habitat (Fagan et al. Reference Fagan, Lewis and van den Driessche2002). In contrast, species that have an inherently high dispersal capacity may quickly escape containment zones, spreading at rates that overwhelm monitoring or control efforts (Liebhold and Tobin Reference Liebhold and Tobin2008) or that allow them to evade pressure from introduced biological control agents (Fagan et al. Reference Fagan, Lewis and van den Driessche2002). There are also other life history features of some species that can facilitate spread, allowing individuals during certain life stages to hitch rides with unwitting agents (Brockerhoff et al. Reference Brockerhoff, Liebhold and Jactel2006; Lee and Chown Reference Lee and Chown2009). Invasive species such as gypsy moth (Lymantria dispar (Linnaeus); Lepidoptera: Erebidae), for example, may be carried as “hitchhikers” on vehicles, owing to its tendency to lay egg masses indiscriminately on a variety of substrates. Similarly, species with cryptic life stages in bark or wood may be readily moved in the transport of logs and firewood (Holway and Suarez Reference Holway and Suarez1999; Allen and Humble Reference Allen and Humble2002; Haack et al. Reference Haack, Petrice and Wiedenhoeft2010). Understanding the processes and pathways through which exotics are dispersed is a key first step in developing regulatory strategies to limit their impact and spread. Moreover, knowledge of an insect’s life history may help determine the risk of its human-assisted dispersal.
Here, we discuss field studies conducted to determine the potential for human-assisted range expansion of the beech leaf-mining weevil, Orchestes fagi (Linnaeus) (Coleoptera: Curculionidae: Curculioninae: Rhamphini) (formerly known as Rhynchaenus fagi). This weevil is native to Europe where it has been reported as an occasional eruptive pest of European beech (Fagus sylvatica Linnaeus; Fagaceae). In 2011, O. fagi was discovered on American beech, Fagus grandifolia Ehrhart (Fagaceae), in Halifax, Nova Scotia, Canada, although anecdotal reports of heavy defoliation on beech suggest it may have been in the area since as far back as 2007 (Sweeney et al. Reference Sweeney, Anderson, Webster and Neville2012; John Simmons, personal communication). The damage associated with beech weevil, while fairly mild in its native European range, was particularly striking in the Halifax area, with the majority of leaves bearing the scorched and shriveled appearance characteristic of O. fagi feeding. In 2013, more than 95% of leaves on many beech in Halifax contained larval mines (J.S., unpublished data). Surveys of more than 100 sites in eastern Canada in 2012 and 2013 found O. fagi established in six counties of Nova Scotia, concentrated mainly within 50 km of Halifax, and in Cape Breton (about 300 km from Halifax), with many negative survey finds in between (Canadian Food Inspection Agency 2013). Damage associated with this weevil is a major concern for homeowners in Nova Scotia, where beech is common in yards and parks – if O. fagi were to spread beyond Nova Scotia, there are concerns over what impact it might have on beech throughout eastern Canada. Although dispersal of O. fagi adults is common, it appears to be relatively localised (i.e., <100 m; Bale Reference Bale1981). The discontinuous distribution of the beech leaf-mining weevil suggests it has either been moved by human assistance within Nova Scotia or there has been more than one introduction from Europe (Sweeney et al. Reference Sweeney, Anderson, Webster and Neville2012).
The life stage of O. fagi most likely to be moved inadvertently by human agency is the adult stage due to its small size (2.2–2.8 mm long) and cryptic habit of spending most of its one-year life cycle in diapause on trees or perhaps surrounding ground litter (Nielsen Reference Nielsen1970; Bale Reference Bale1981). Like many areas in Canada, firewood is a common heating fuel in Atlantic Canada, and wood is often harvested and moved from one area to another. Most firewood is thought to be produced and consumed locally (i.e., within 50–100 km) but it is not uncommon for firewood to be transported longer distances by people travelling to cottages or campgrounds (i.e., >100 km). Red spruce, Picea rubens Sargent (Pinaceae), commonly grows in association with American beech (Blum Reference Blum1990) and is an important commercial species for lumber in Nova Scotia (Woodbridge Associates 2011). Although the overwintering habits of O. fagi adults have been studied to a limited extent in Europe (Nielsen Reference Nielsen1970; Bale Reference Bale1981), our preliminary studies suggested different overwintering patterns may occur in Canada.
To better understand the overwintering distribution and abundance of adult O. fagi within its habitat, we carried out field studies in mixed beech forests north of Halifax, Nova Scotia, Canada. Our objective was to estimate the densities of overwintering O. fagi adults in a variety of substrates, including the trunks of trees species commonly cut and transported for firewood (American beech and red maple, Acer rubrum Linnaeus; Sapindaceae) and commercial saw logs (red spruce). Moreover, owing to the nearly ubiquitous occurrence of beech bark disease in Nova Scotia (Houston and O’Brien Reference Houston and O’Brien1983) coupled with the likely preference among adults for cracked and fissured trunks for safe overwintering sites, we predicted that trunks of American beech affected by beech bark disease would be particularly suitable for overwintering O. fagi.
Methods
Life history
Adults of O. fagi break diapause in early spring in close synchrony with beech budburst and feed and lay eggs on the leaf mid-vein almost as soon as the bud cap splits (Bale Reference Bale1984). After egg hatch, the larvae burrow into the leaf and proceed to mine the leaf for three to six instars, and then pupate within the feeding gallery. In Canada, both adult and larval feeding occurs almost exclusively on beech leaves (Moise et al. Reference Moise, Forbes, Morrison, Sweeney, Hillier and Johns2015), although there are reports in Europe of adult feeding on alternative hosts in both the spring and fall (Bale and Luff Reference Bale and Luff1978). Adults eclose from late June through July, and seek overwintering sites in the leaf litter, in foliage of conifers growing in association with beech, under moss, and in crevices and bark scales on tree trunks (Morris Reference Morris1968; Nielsen Reference Nielsen1970; Bale Reference Bale1981).
Study locations
Study plots were located in two areas in Halifax, Nova Scotia, Canada near Hemlock Ravine Park (44.687413, −63.666748) and Sandy Lake (44.734598, −63.673185). In each of these areas, we selected five plots, a minimum of 20 m apart, and within each plot selected one tree of American beech, red maple, and red spruce that were within 5 m of each other. These were the primary tree species dominating these particular areas, and although tree diameter at breast height (dbh) varied somewhat among plots, within each plot, we generally selected trees of similar dbh (i.e., overall, from 8.6 to 22.0 cm dbh, i.e., at 1.3 m height).
Sample collection
All samples were collected on 15–17 April 2013 before budburst and before weevils had emerged from hibernation. Selected trees (i.e., beech, maple, and spruce) were felled and a 34 cm long section was cut from the basal, middle, and upper sections of the trunk. Each trunk section was labelled and placed in a separate 26 L plastic pail (Ropak©, Springhill, Nova Scotia, Canada) and sealed. For spruce, we also collected two mid-crown branches, which were placed in sealed plastic bags for transport. Beneath the beech trees in each plot, we collected two duff samples from above the compacted soil (35×35 cm) and placed the material in a large, sealed plastic bag. Once the duff was removed, we next collected the soil in the same area to a depth of 5 cm and placed it in sealable 26L plastic pails. In separate areas beneath our beech sample trees, we also collected two 20×20 cm moss samples and placed these in plastic bags.
To provide additional estimates of weevil density on branches, a branch beating survey was conducted on 22–23 October, 2013 to dislodge overwintering weevils from branches of red spruce, beech, and red maple; branches on beech and maple were bare as most of the leaves had already dropped. In each plot, we selected branches that were accessible for beating (i.e., 1–3 m in height) and placed a 1×1 m sheet beneath the branch. Each branch was subjected to 25 vigorous blows from a 150×2.5 cm wooden dowel. These samples were collected using the same protocol as that described by Nielsen (Reference Nielsen1970) to provide a direct comparison with his data from Denmark.
Rearing
All samples were transported to a Level 2 Arthropod (PP2A) containment facility in Fredericton, New Brunswick, Canada and stored at +5 °C before incubation at 21 °C. Individual bolts were placed in emergence cages consisting of a 26 L Ropak© pail with an inverted funnel attached over a hole in the bottom. A mason jar was attached to the funnel using a screw top ring to allow the jar to be easily removed and checked for emerging weevils. Soil samples were treated similarly except that a wide-screen mesh was placed over the mouth of the funnel to limit soil dropping into the jar. The duff and moss samples were individually placed in small Styrofoam boxes (25×25×20 cm) with translucent white screw-top plastic bottles attached to the sides for weevils to emerge into. After four weeks of incubation, each bolt was removed and washed by submerging it in a weak (0.105%) sodium hypochlorite (bleach) solution. This treatment helped to dislodge the remaining weevils from the trunk, after which they floated to the top of solution for collection. Spruce branch samples were washed in the same bleach solution. Adult weevils were preserved in 70% ethanol. For each sample, we recorded the number of weevils that emerged (both naturally and after our bleach wash). Across all samples, we standardised weevil densities per m2 of surface area. For the trunk bolts, bark surface area was estimated by averaging the stem diameter (D) (measured at the top and bottom of each bolt) and using the formula for a cylinder, i.e., π D×L, where L=length of the bolt. Surface areas for branches were estimated using the length and width of branches divided by 2 (i.e., the surface area of a triangle). Surface area for the duff, soil, and moss was calculated based on the dimensions of the material collected (i.e., 35×35 cm).
Bark roughness and percent moss cover
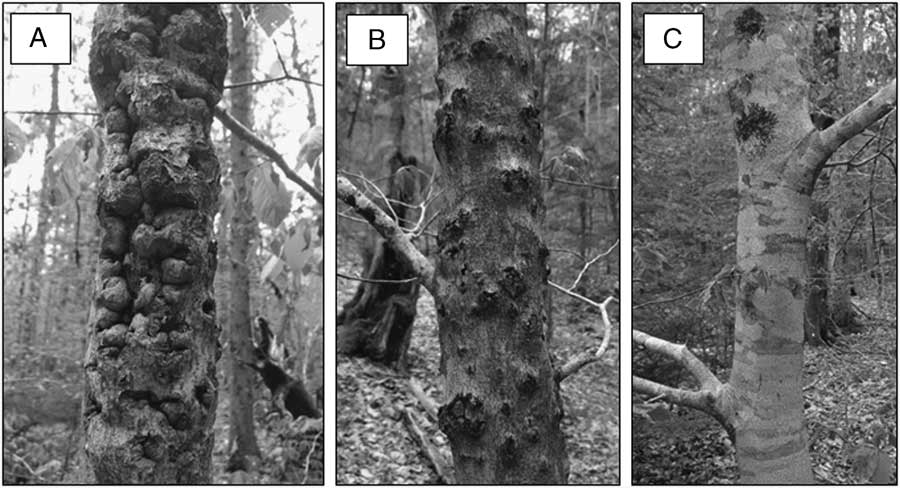
We estimated relative bark roughness on all of the trunk bolt samples as low, moderate, or high, based on a subjective visual assessment of the proportion of bark surface area with cracks, crevices, or niches in which an O. fagi adult could potentially overwinter: low is <25%; moderate is 26–50%; and high is >50% (Fig. 1). Deeper and longer cracks were given higher values than shallow bark scales; however, raised bumps were not considered in our estimates as they do not provide obvious overwintering sites for the weevil. Each bolt was examined by four different observers, and the bark roughness category assigned by consensus. We are confident that our roughness categories provide an accurate measure of relative bark roughness within each tree species but caution the reader that differences among species in bark characteristics make it more difficult to directly compare roughness between species. For example, beech bark is very smooth unless it has been cankered by beech bark disease, whereas red spruce is naturally scaly, with more scales and crevices near the base of the tree than in the mid and upper bole. Percentage of the bark covered by moss was visually estimated to the nearest 5%, although we assigned a value of 1% where trace amounts were observed.
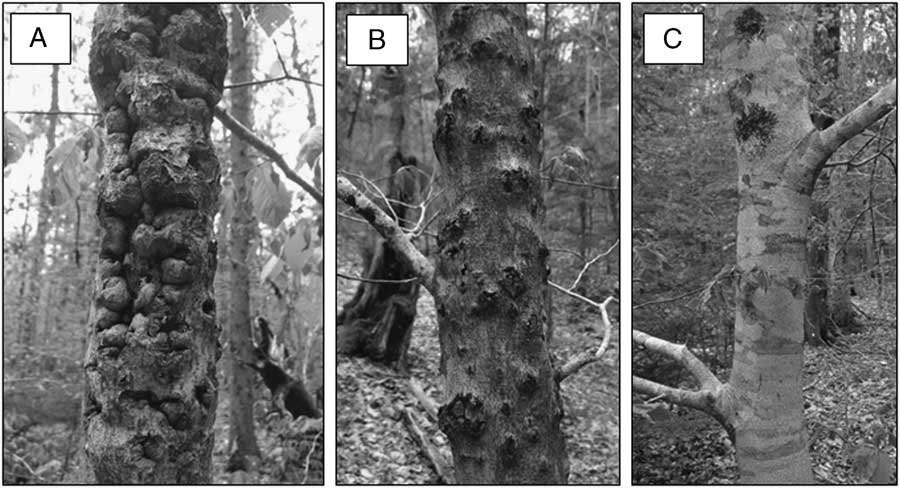
Fig. 1 Examples of American beech bolts with bark roughness categorised as: (A) high, (B) moderate, and (C) low.
Statistical analyses
To determine the effects of site and overwintering substrate on O. fagi density, we fit the data to a generalised linear model (proc GENMOD dist=negbin link=log; SAS Institute 1999). A subsequent analysis was carried out after removing the non-tree stem data (i.e., branches, duff, moss, soil) to assess the effects of site, tree species, and height on the bole on O. fagi density (proc GENMOD dist=negbin link=log; SAS Institute 1999). Linear regression was used to assess the relationship between the surface area of the stem covered by moss and O. fagi density (proc REG; SAS Institute 1999). To determine the effects of relative bark roughness on O. fagi density, we used a generalised linear model for roughness (proc GENMOD dist=negbin link=log; SAS Institute 1999); however, because of the inherent differences in bark structure among the trees studied, we analysed the effects of roughness independently for each of the three tree species and pooled the data from the two sites to increase replication across the three roughness categories (i.e., not all trees and plots had all roughness categories represented).
Results
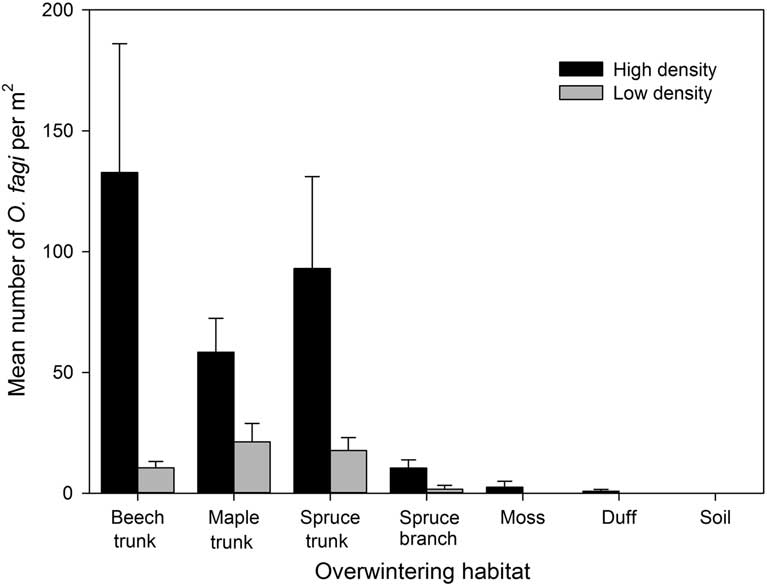
A total of 863 weevils were collected in this study. Sex ratios were slightly female biased with 54% and 61% female at the two sites. The total density of overwintering O. fagi differed significantly between sites (χ2=16.29, df=1, P<0.001) and were generally highest on tree trunks, with only a small number found on branches or in duff, moss, or soil (χ2=103.91, df=6, P<0.001) (Fig. 2). There was a significant interaction between site and overwintering substrate (χ2=31.79, df=6, P<0.001). Based on our additional branch beating surveys in the same plots, no O. fagi were found overwintering on either beech or maple branches, although a total of 29 weevils (14 males and 15 females) were dislodged from spruce branches.
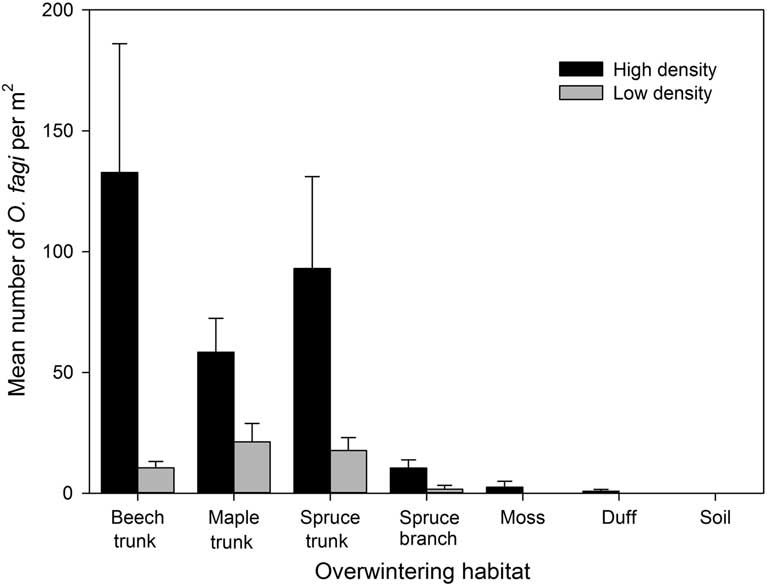
Fig. 2 Mean (±SE) number of Orchestes fagi adults per m2 overwintering on the main trunk of beech, maple, and spruce, on spruce branches, and in the duff, moss, and soil in sites with relatively high and low densities of O. fagi. Weevil densities differed significantly between sites and among the different substrates (generalised linear model, χ2>16.29, P<0.001).
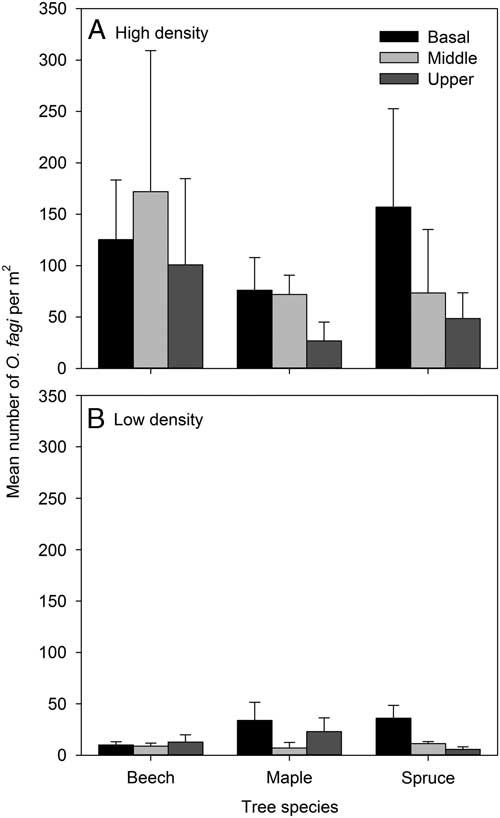
Analyses of weevil distribution on the tree stems alone showed there was a significant difference in O. fagi density between sites (χ2=27.21, df=1, P<0.001) but no main effect nor significant interaction with either tree species or height on the bole (χ2⩽4.17, df=2–4, P⩾0.124) (Fig. 3). There was no significant relationship between O. fagi density and percent moss cover on tree trunks (F 1,88=0.01, P=0.932, r 2 <0.001).
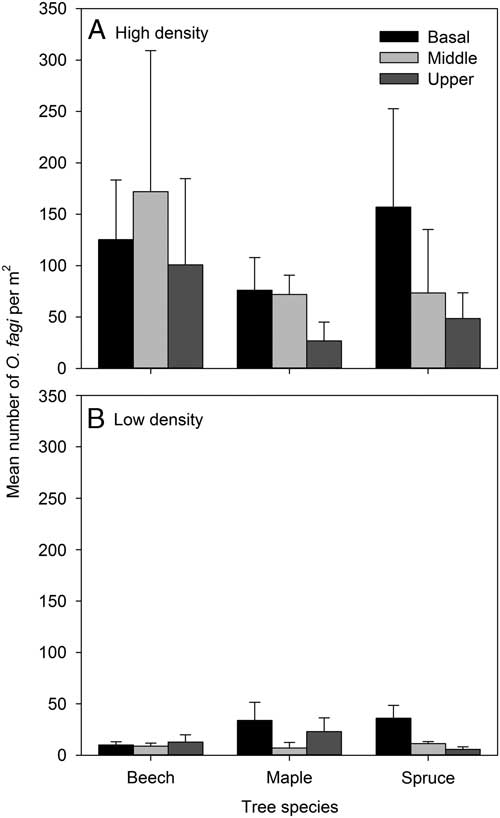
Fig. 3 Mean (±SE) number of Orchestes fagi adults per m2 overwintering on basal, middle, or upper sections of the main trunk of beech, maple, and spruce in in sites with relatively high and low densities of O. fagi. Weevil densities differed significantly between sites (χ2=27.21, df=1, P<0.001) but not among basal, middle, and upper sections of the trunk.
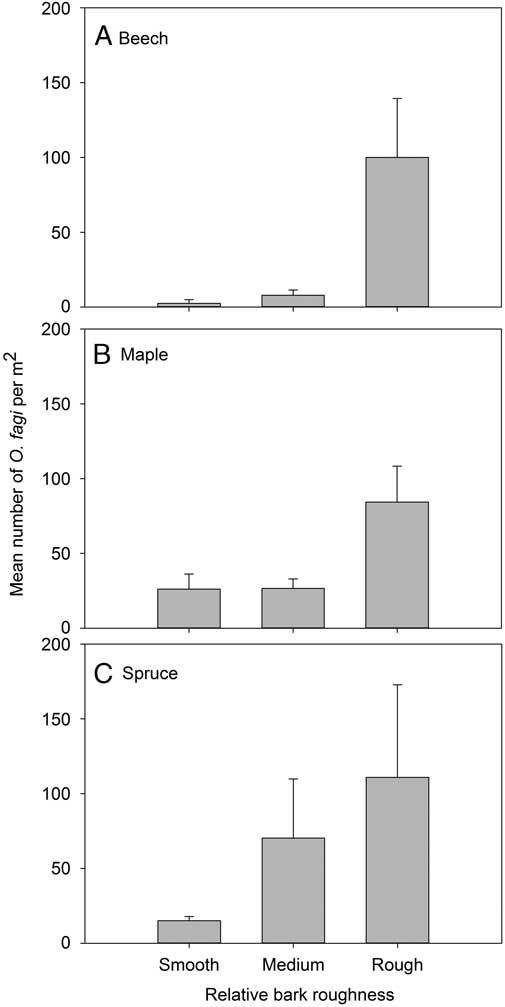
Bark roughness had a significant effect on O. fagi density for beech (χ2=13.48, df=2, P=0.001) and spruce (χ2=10.87, df=2, P=0.004), but not maple (χ2=4.28, df=2, P=0.118) (Fig. 4).
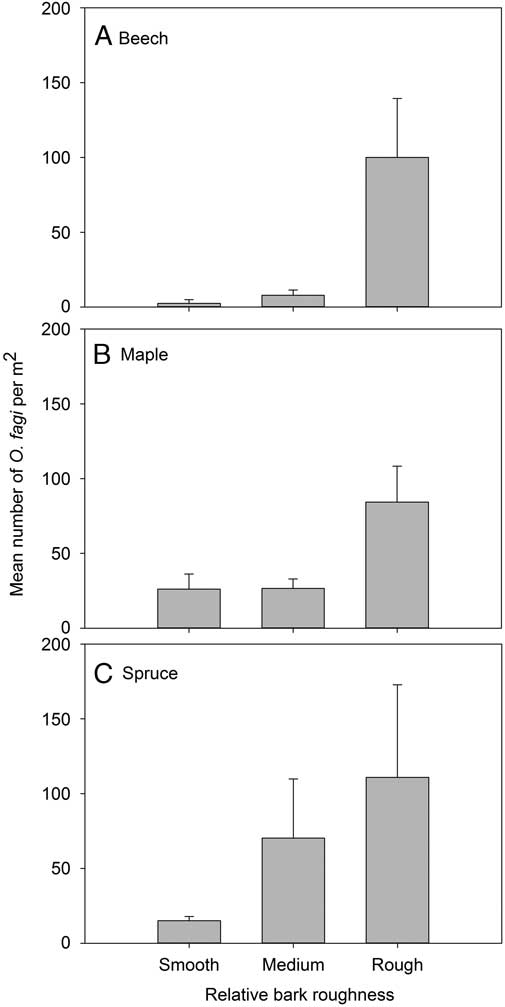
Fig. 4 Mean (±SE) number of Orchestes fagi adults per m2 overwintering on trees in relation to bark roughness on trunks of (A) beech, (B) maple, and (C) spruce. Relative bark roughness estimates were calibrated to each species examined and did not allow us to compare roughness among species.
Discussion
Our study provides strong evidence that mature deciduous and coniferous trees growing near high populations of O. fagi are likely to harbour adults on their stems during the overwintering period in Canada (i.e., from as early as August to the following May, based on counts of O. fagi adults in branch beating samples on American beech in Halifax; J.S., unpublished data). Overwintering adults were detected on all of the tree species examined, with only a weak bias towards beech, the primary host. Relatively few weevils were found on tree branches or in moss, soil, and duff. As a result, fall/winter harvest of mature trees in areas experiencing O. fagi outbreaks and transport of logs or firewood from these areas to areas uninfested with O. fagi has the potential to facilitate the expansion of this invasive beyond its current range. Orchestes fagi is a rare example of a non-wood boring exotic beetle that has a high risk of spread through human agency, due to the long period of its life cycle (eight to nine months) that the small and cryptic adults spend in diapause on the trunks of host and non-host trees.
Recent studies indicate a variety of life history adaptations in O. fagi in Canada that stand in contrast to observations made for populations in Europe (Moise et al. Reference Moise, Forbes, Morrison, Sweeney, Hillier and Johns2015); however, there has been limited work looking at the overwintering distribution of O. fagi on either continent (e.g., Morris Reference Morris1968; Nielsen Reference Nielsen1970; Bale Reference Bale1981). The most extensive work has been conducted by Nielsen (Reference Nielsen1970), who carried out field surveys in Denmark that found O. fagi in a variety of habitats, including moss on stems and stumps, under beech and spruce bark, in leaf litter and soil; however, there was no standardised unit of collection in this study, making it difficult to make inferences about the relative distribution of weevils. Our present study found almost no adults overwintering in the ground surrounding the trees, with most weevils occurring on the trunk. More consistent with Nielsen’s (Reference Nielsen1970) results, we found that some weevils overwinter on spruce branches when available, which may be due to the presence of needles, cones, and other structures that provide favourable protection during overwintering. However, we found no weevils in our branch beating samples of branches of beech or red maple, whereas in Denmark, Nielsen (Reference Nielsen1970) collected averages of seven and 14 overwintering O. fagi per 25 beat samples from branches of Norway spruce (Picea abies (Linnaeus) Karsten; Pinaceae) and European beech, respectively. Although O. fagi densities on trunks did not differ significantly among the basal, mid-level, and upper levels of the bole, there were clearly greater numbers on parts of the trunk with relatively high roughness. Presumably, a rough trunk provides more suitable overwintering sites per surface area than a smooth trunk, i.e., more cracks and crevices in which adult O. fagi are less exposed to adverse weather, desiccation, or predators during their relatively long period in diapause. However, little is known about the effects of predation and winter temperatures on O. fagi adult survival in different substrates in either its invaded or native habitats, so this remains speculative pending further study. Coulson and Bale (Reference Coulson and Bale1996) observed low survival of O. fagi adults exposed for extended periods to constant temperatures at or below −10 °C or +10 °C and speculated mortality was caused by desiccation from evaporative water loss at lower temperatures and exhaustion of food reserves at higher temperatures. They recorded high overwintering survival rates in the field in England (United Kingdom) when O. fagi adults were provided larch cones as substrate but found the cones provided little thermal buffering against external air temperatures.
It is worth noting that many of the beech in our study were afflicted with beech bark disease, which resulted in a generally high degree of bark roughness at all heights along the bole on our study trees. Most (~90–95%) of the beech in northeastern North America is infected with this disease (Morin et al. Reference Morin, Liebhold, Tobin, Gottschalk and Luzader2007), which is caused by an introduced European scale insect, Cryptococcus fagisuga Lindlinger (Hemiptera: Eriococcidae), and fungal pathogens, such as the exotic Neonectria faginata (Lohman, Watson, and Ayers) Castlebury and Rossman (Nectriaceae), and the native Neonectria ditissima (Tulasne and Tulasne) Samuels and Rossman (Ehlrich Reference Ehlrich1934). Beech bark disease infects the bark, cambium, and sapwood of infected trees causing lesions, cankers, and deep fissures in the trunk leading to tree mortality, and presumably creating an abundance of overwintering sites for adult O. fagi. Although heavily infected beech is also no longer usable for most wood products, it is still commonly used for firewood.
Beech bark disease has caused significant mortality of American beech in areas where it is present and the residual beech trees that survive in the “aftermath forest” are often weakened and susceptible to infestation by ambrosia beetles and other fungi (McCullough et al. Reference McCullough, Heyd and O’Brien2003). Insect defoliation often increases susceptibility of beech to attack by Armillaria (Fries) Staude (Physalacriaceae) fungi (Tubbs and Houston Reference Tubbs and Houston1990). Further weakening of beech by repeated annual defoliation by O. fagi may have increased the rate of tree mortality, adding to the already significant impact of beech bark disease. Although beech is used mainly as firewood in Atlantic Canada, it also serves a more prominent role in industries in other regions of North America (e.g., Ontario, Canada) as a source of lumber for flooring, furniture, and railway ties (McLaughlin and Griefenhagen Reference McLaughlin and Griefenhagen2012). Recognising the potential impact O. fagi may have and the role humans might play in facilitating its range expansion is an essential first step in developing management strategies.
Human-assisted spread of invasive species is one of the major challenges to commerce and trade in our current era of globalisation (Hulme et al. Reference Hulme, Roy, Cunha and Larsson2009) and our data confirm that O. fagi has a high risk of accelerated spread through human movement of logs and firewood. Evidence that O. fagi has already been moved by human assistance in Nova Scotia is its establishment in both Cape Breton and Halifax, Nova Scotia, (more than 300 km distant from each other) but apparent absence at most sites 30 km distant from Halifax (Sweeney et al. Reference Sweeney, Anderson, Webster and Neville2012). Unlike many exotic species, the risk of range expansion in O. fagi through human agency is not associated only with transport of its primary host plant. All trees, but perhaps particularly mature individuals with rough bark, are likely to harbour overwintering adults and, if harvested and transported, may accelerate the spread of O. fagi through the region and beyond.
Acknowledgements
The authors are grateful to J. Fidgen, E. Moise, Z. Sylvain, D. Pureswaran, Andrew Smith, and several anonymous reviewers for comments on an earlier version of the manuscript. Financial and logistical support was provided by SERG International, Forest Protection Limited, Canadian Forest Service (Natural Resources Canada), and the Invasive Species Council.