Introduction
Since the middle of the 20th century, temperatures in the Northern Hemisphere have increased at a faster rate in winter than in summer (Balling et al. Reference Balling, Michaels and Knappenberger1998). For ectotherms, such as herbivorous insects, a significant rise in the daily minimum temperature is likely to favour winter survival, abundance, or range expansion, as reported for several important forest pests (Cooke and Roland Reference Cooke and Roland2003 – forest tent caterpillar, Malacosoma disstria (Hübner); Lepidoptera: Lasiocampidae; Carroll et al. Reference Carroll, Taylor, Régnière, Safranyik, Shore, Brooks and Stone2004 – mountain pine beetle, Dendroctonus ponderosae (Hopkins); Coleoptera: Curculionidae; Battisti et al. Reference Battisti, Stastny, Netherer, Robinet, Schopf and Roques2005 – Thaumetopoea pityocampa (Denis and Schiffermüller); Lepidoptera: Thaumetopoeidae; Jepsen et al. Reference Jepsen, Hagen, Ims and Yoccoz2008 – winter moth, Operophtera brumata (Linnaeus); Lepidoptera: Geometridae; and Epirrita autumnata (Borkhausen); Lepidoptera: Geometridae), including other terrestrial animals (reviewed by Williams et al. Reference Williams, Hugh, Henry and Sinclair2015). However, in some cases, insect survival and abundance may simply increase for other reasons, or their range may expand in response to unusually warm years and then retract in years with prolonged periods of cold weather (Battisti et al. Reference Battisti, Stastny, Buffo and Larsson2006; Bale Reference Bale, Denlinger and Lee2010). Although the frequency of warm winters is likely to increase under global warming (Intergovernmental Panel on Climate Change 2013), the occurrence of extreme cold weather cannot be totally dismissed (Kodra et al. Reference Kodra, Steinhaeuser and Ganguly2011). Hence it remains important to assess the cold hardiness strategies of insects before arguing that winters have warmed up sufficiently to sustain permanent range expansion or residency of unexpected native invaders (Bale and Hayward Reference Bale and Hayward2010; Crozier Reference Crozier2014).
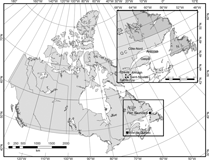
In 2012, the Laurentian Mountains, Québec, Canada, located about 70 km north of Ville de Québec (Fig. 1), experienced a spectacular, unprecedented outbreak of Lambdina fiscellaria (Guenée) (Lepidoptera: Geometridae) (Arsenault et al. Reference Arsenault, Hébert, Tousignant, Berthiaume and Bauce2015). This species is one of the most serious defoliators of eastern boreal forests in Canada. To our knowledge, this was the first outbreak to occur outside of Côte-Nord, the Gaspé Peninsula, and Anticosti Island (Québec, Canada), or the island of Newfoundland (Newfoundland and Labrador, Canada) (Fig. 1). In these areas, characterised by maritime climates, many L. fiscellaria outbreaks have been reported in the past (reviewed by Hébert and Jobin Reference Hébert and Jobin2001).

Fig. 1. Places of origin of the Québec and Newfoundland populations (Ville de Québec and Port Saunders, ▪) and location of the other experimental field sites (Armagh, Épaule, Sainte-Foy, and Saint-Nicolas, •). Other regions identified on the map have been infested previously by Lambdina fiscellaria (Côte-Nord, Gaspé, and Anticosti).
A highly polyphagous insect, L. fiscellaria attacks a wide variety of coniferous and deciduous trees, but it prefers Abies balsamea (Linneaus) Miller (Pinaceae). Abies balsamea, along with Picea glauca (Moench) Voss (Pinaceae), Picea mariana (Miller) Britton, Sterns, and Poggenburg (Pinaceae), and Betula papyrifera Marshall (Betulaceae), dominates the whole area of the Laurentian Mountains (Leblanc and Bélanger Reference Leblanc and Bélanger2000; Delisle-Boulianne et al. Reference Delisle-Boulianne, Boucher, Bélanger and Brière2011). Mature A. balsamea trees can be killed after a single year of severe L. fiscellaria outbreak (Jobin and Desaulnier Reference Jobin and Desaulnier1981). Such outbreaks generally last two to three years in Québec, and longer in Newfoundland (Otvos et al. Reference Otvos, Clarke and Durling1979). Lambdina fiscellaria females generally oviposit from mid-August to early October (Delisle et al. Reference Delisle, West and Bowers1998). Eggs are laid mostly on tree trunks and branches where they quickly enter an obligatory diapause that lasts about three months (Delisle et al. Reference Delisle, Royer, Bernier-Cardou, Bauce and Labrecque2009). Postdiapausing eggs resume their embryonic development as soon as the air warms up in March/April, and they generally hatch from mid-May to late June (Delisle et al. Reference Delisle, Royer, Bernier-Cardou, Bauce and Labrecque2009). Thus, L. fiscellaria eggs may experience very cold air temperatures.
In two previous studies on L. fiscellaria cold hardiness (Rochefort et al. Reference Rochefort, Berthiaume, Hébert, Charest and Bauce2011; Delisle et al. Reference Delisle, Labrecque, Royer, Bernier-Cardou, Bauce and Charest2013), the authors demonstrated that this species exhibits a freeze-avoidance strategy whereby eggs may die at temperatures well above their supercooling point, the temperature at which they spontaneously freeze (Lee Reference Lee, Denlinger and Lee2010). The fact that no L. fiscellaria outbreak had ever been reported prior to 2012 in the Laurentian Mountains (Hébert and Jobin Reference Hébert and Jobin2001) thus raised two questions: can eggs from local populations survive the harsh winter climate encountered in this region? If they do, how long will the outbreak last?
To address these questions, three specific objectives were considered in this study. First, the cold hardiness of L. fiscellaria eggs, obtained from adults collected as pupae in one of the naturally infested sites of the Laurentian Mountains, was investigated by assessing their capacity to (i) supercool, (ii) survive at various combinations of sub-zero temperatures above the supercooling point and exposure durations, or (iii) successfully overwinter in diverse bioclimatic regions of Québec. These three assays were designed to compare the cold hardiness of eggs from the population at the infested site of the Laurentian Mountains (continental climate) with that of eggs from a population originating from the usual habitat of the species (maritime climate) and reared in our laboratory since 2005. The experiment described in (ii) was also designed to produce a prediction equation of the duration of exposure required to kill 50% of L. fiscellaria eggs as a function of temperature for each population, the so-called LTT50 for “lethal temperature and exposure time.”
As an alternative hypothesis to cold winter, the possibility still exists that spring egg parasitism by Telenomus Haliday (Hymenoptera: Scelionidae) (T. coloradensis Crawford, T. droozi Muesebeck, and T. flavotibiae Pelletier) (Pelletier and Piché Reference Pelletier and Piché2003) was the factor responsible for the collapse of L. fiscellaria population in the Laurentian Mountains. This indeed proved to be the case following two previous L. fiscellaria outbreaks in the province of Québec (Bordeleau Reference Bordeleau1997, Reference Bordeleau1998; Chabot et al. Reference Chabot, Bordeleau and Aubin2001; Hébert et al. Reference Hébert, Berthiaume, Dupont and Auger2001). Therefore, as a second objective, we investigated the incidence of Telenomus attacks as a potential cause of egg mortality following the recent L. fiscellaria outbreak in the Laurentian Mountains.
Regardless of whether the collapse of the Laurentian Mountains population was due to a cold winter or spring parasitism, one would expect a strong correspondence between overwintering egg mortality and field population density. Consequently, as a third objective, we compared year-to-year variations (2012–2015) in the density of L. fiscellaria adults from the infested site of the Laurentian Mountains by monitoring male and female flight activity from mid-August to late October, using either light or pheromone traps.
Materials and methods
Insect origin and rearing
In 2005, a laboratory colony of L. fiscellaria was established using eggs obtained from pupae and adults collected in Port Saunders on the island of Newfoundland, Canada (50º38′N, 57º16′W, 90.8 m) (Fig. 1). In 2012, eggs obtained from adults collected as pupae from one of the infested sites of the Laurentian Mountains (47º19′N, 71º12′W, 820 m), near Ville de Québec (46º46′N, 71º16′W, 74.4 m) (Fig. 1), were used to start a new colony. These two colonies, hereafter referred to as the Québec and Newfoundland populations, were held under constant laboratory conditions using the methodology described below.
Each year, around mid-May or late May, postdiapausing eggs stored since the previous fall in an outdoor insectary at the Laurentian Forestry Centre in Ville de Québec (Fig. 1) were brought back to the laboratory for incubation and rearing under 16:8 light:dark photoperiod, 15 °C ± 1 °C, and 65% ± 5% relative humidity. Larvae were kept in groups of 100 individuals per plastic box (38 × 27.8 × 14.6 cm); they were reared on current-year and one-year-old shoots of A. balsamea that were renewed at least once a week. Under these laboratory conditions, adults were expected to emerge and reproduce from mid-August to late September/early October, as they would in the field (Delisle et al. Reference Delisle, West and Bowers1998).
For each population, eggs were obtained from > 100 mated females that were kept at 15 °C (16:8 light:dark photoperiod) in individual 150 cm3 plastic cages (Intrapac, Plattsburg, New York, United States of America) (two females per cage), and provided with a polyurethane foam disc for oviposition. Each disc contained approximately 50 eggs. Fifteen days after being laid at 15 °C, fertile eggs turned dark brown, indicating that they had successfully entered diapause (Delisle et al. Reference Delisle, Royer, Bernier-Cardou, Bauce and Labrecque2009). In mid-September, when temperatures normally began to decline (< 15 °C) (see Fig. 2 in Delisle et al. Reference Delisle, Royer, Bernier-Cardou, Bauce and Labrecque2009), all oviposition discs were stored in the outdoor insectary for the winter. Eggs produced in the fall of 2012 and 2013 were used for the field and laboratory assays described below.

Fig. 2. Relative frequency distribution (bars) of the supercooling points of eggs of Lambdina fiscellaria, measured in mid-February, early April, early May, and mid-May, and cumulative distribution (•) of supercooling points. Data obtained from the Québec and Newfoundland populations have been combined.
Experiment 1: supercooling point
The supercooling point of L. fiscellaria eggs from both geographical regions (Québec and Newfoundland) acclimatised to outdoor insectary conditions since the fall of 2012 was measured in mid-February, early April, early May, and mid-May of 2013, using the procedure previously described by Delisle et al. (Reference Delisle, Labrecque, Royer, Bernier-Cardou, Bauce and Charest2013). Fertile eggs were individually attached with an adhesive tape to a type T (copper/constantin) thermocouple (Physitemp Instruments, Chicago, Illinois, United States of America) placed in a multi-layer polystyrene box (20 mm thick), and transferred to a cryo-fridge (SSC750ABA; Thermo Scientific Revco, Asheville, North Carolina, United States of America) for supercooling point determination. In the freezer, temperature was recorded with a type T digital thermocouple. It decreased from 20 °C to −40 °C at a rate of 1 °C per minute, and then from −40 °C to −50 °C at a rate of 0.1 °C per minute for the next 100 minutes. According to Lee (Reference Lee, Denlinger and Lee2010), the supercooling point is the lowest temperature reached before latent heat is released upon freezing.
In this experiment, an equal number of eggs from each population were simultaneously cooled: 32 eggs per population were tested in mid-February (winter) and 25 eggs per population, per date were tested in early April, early May, and mid-May (spring). Overall, 214 eggs were tested for supercooling point determination. After cooling, all eggs were warmed up to 15 °C at a rate of 1°C per minute and incubated under standard conditions at 15 °C, 65% relative humidity, and 16:8 light:dark photoperiod. Eggs were observed daily until the end of the hatching period. Under such temperature conditions, L. fiscellaria eggs required 24–25 days to hatch in February, and 13–14 days in May (Delisle et al. Reference Delisle, Royer, Bernier-Cardou, Bauce and Labrecque2009).
In the initial normal mixed analysis of variance model for supercooling point, the mean supercooling point was assumed to depend on the fixed effects of season (winter or spring), date (month) within spring, population within season, and date by population interaction within season, but no intercept (McCulloch et al. Reference McCulloch, Searle and Neuhaus2008). This formulation provided a more direct assessment of the population and date effects within season. Five orthogonal contrasts among means per population, season, and date were submitted to the Holm-simulated multiple test procedure (Westfall et al. Reference Westfall, Tobias and Wolfinger2011): Québec versus Newfoundland in February, Québec versus Newfoundland in spring (April, early May, and mid-May combined), spring versus February, May (early and mid-May combined) versus April, and early May versus mid-May. In addition, the linear form of the initial model included random effects for day of experimentation (day) within date and season, freezer within day, date and season, population and day combination within date and season, and population and freezer combination within day, date, and season. Random effects and the residual error were each assumed to follow a normal distribution with mean zero and a source-specific constant variance component. The random part of the initial model was reduced by removing random effects with a negligible variance component based on Akaike’s information criterion (Agresti Reference Agresti2013).
The following applies to this and other statistical analyses. The denominator degrees of freedom (df) of F-statistics were approximated by the method of Kenward and Roger (Reference Kenward and Roger1997) and may be fractional. Means and other statistics quoted in the text are followed by their confidence limits in parentheses separated by a comma. Unless stated otherwise, mixed models were fit with the GLIMMIX procedure of SAS 9.2 (SAS Institute, Cary, North Carolina, United States of America). Adjusted P-values from multiple tests are identified as P adj.
Experiments 2A and 2B: cold tolerance assays
For laboratory cold tolerance assays, approximately 3000 fertile and healthy eggs (per population, per year) were collected from oviposition discs, carefully mixed within each population, and distributed in batches of 10 eggs per 1 dram glass vial (replicates), which were covered with a small foam plug. In the fall preceding the assays, these eggs were acclimatised in an outdoor insectary.
The aim of experiment 2A was to estimate the combinations of constant sub-zero temperatures above the mean supercooling point and duration of exposure to such temperatures required to kill 50% of the egg population (LTT50). All combinations of six exposure durations (0, 2, 4, 8, 12, and 16 hours) and five constant sub-zero temperatures (−25 °C, −30 °C, −33 °C, −35 °C, and −37 °C, ± 0.05 °C) were tested in mid-February 2013. For each exposure duration and temperature, four replicates of 10-egg vials per population were transferred from the outdoor insectary (−15 °C to −25 °C) to an ultra-low-temperature freezer (Thermo Scientific Revco Ultima Plus, 5308, Marietta, Ohio, United States of America), for a total of 240 vials (two populations × 30 combinations of a temperature and exposure duration × four vials or replicates). The zero-hour duration of exposure to the five sub-zero temperatures was considered as a single control treatment with 20 replicates. Prior to each temperature assay, all control vials were handled exactly like the experimental ones. At the beginning of each temperature assay, control vials were transferred to standard conditions of 16:8 light:dark photoperiod and 15 °C for incubation.
To estimate the LTT50 in mid-April 2014 (experiment 2B), a similar cold tolerance experiment was conducted using eggs from the Québec population exclusively because available eggs from the Newfoundland population were too few. All combinations of five exposure durations (0, 2, 4, 8, and 16 hours) and three sub-zero temperatures (−30 °C, −33 °C, and −35 °C) were tested, using four 10-egg vials per combination, for a total of 60 vials (15 combinations × four vials or replicates) of 10 eggs each. As before, the zero-hour duration of exposure corresponds to a single control treatment with 12 replicates.
After each temperature and exposure duration assay, experimental vials were immediately transferred to standard conditions for incubation as the control vials had been. Throughout the incubation period, newly hatched larvae in each vial were counted daily. After the hatching period was completed, unhatched eggs (recognisable by their dark and undefined content) were also counted, including those that contained a dead pharate larva (first instar). The number of hatched larvae plus eggs that contained a developed dead larva was considered as the number of eggs that developed successfully.
In the cold tolerance experiment of mid-February 2013 (experiment 2A), the proportion of eggs with a developed larva in the control vials suggested that a fraction C of eggs abort naturally before they reach full development, even under favourable environmental conditions. In this circumstance, the (unconditional) probability of development, π, under a particular treatment is equal to (1 – C)θ where θ is the (conditional) probability that an egg develops, given that it is viable. In the initial nonlinear mixed binomial model for the number of developed eggs, logit(1 – C) = log[(1 – C)/C] was assumed to depend linearly on a reference parameter, α0, associated with the Newfoundland population and a differential population effect, αQC, due to the Québec population. When exposure duration was greater than zero, logit(θ) was assumed to depend linearly on a reference parameter, β0, a population effect, βQC, the effects of five quantitative covariates (temperature, exposure duration, their squares, and their product) with parameters βt, βd, βt2, βd2, and βtd, respectively, the five effects of the interaction between the components of the quadratic response surface and population, and initially a random vial effect. In the cold tolerance experiment of mid-April 2014 (experiment 2B) in which only eggs from the Québec population were tested, the initial model was similar to that constructed for the 2013 cold tolerance data, except that all effects involving population vanished and the reference parameters in logit(1 – C) and logit(θ) were associated with the Québec population.
In both experiments, the number of developed eggs per vial was assumed to follow a binomial distribution with probability of egg development π = (1 – C)θ. Initial models were reduced based on Akaike’s information criterion and likelihood ratio tests. Population effects were assessed from odds ratios of the form [ϕ1/(1−ϕ1)]/ [ϕ2/(1−ϕ2)], where ϕ is the probability of interest and the indices 1 and 2 refer to the two arms of the comparison. When ϕ1 = ϕ2, the odds ratio is 1. Mean probabilities were estimated on the logit scale, back-transformed to the appropriate scale, and multiplied by 100 for presentation as percentages in the text or figures. The LTT50 curve was computed from the response surface models at π = 0.5 replacing C and θ by their estimated values, which are functions of population for C and of population, temperature, and exposure duration for θ. The equation was solved for exposure duration, and approximate 95% confidence bands for the LTT50 curve were estimated with the delta method (Agresti Reference Agresti2013). These models were fit with the NLMIXED procedure of SAS 9.2.
Experiment 3: winter egg survival
In 2012, winter egg survival experiments were conducted at four sites: Armagh (46°46′N, 70°39′W, 268.0 m), Sainte-Foy (near our outdoor insectary) (46°46′N, 71°17′W, 92.2 m), Saint-Nicolas (46°40′N, 71°21′W, 89.0 m), and Épaule (near the infested site of the Laurentian Mountains) (47°18′N, 71°11′W, 785.0 m), all located in the province of Québec (Fig. 1). Throughout the season, air temperatures at each site were recorded every 15 minutes with two dataloggers (HOBO H8 Pro Series; Hoskin Scientific, Montréal, Québec, Canada) attached 3 m above the ground to the trunk of two trees standing 5 m apart.
To assess winter egg survival, oviposition discs containing approximately 50 eggs from each population were stapled inside the interior walls of Delta sticky traps (Scentry Biologicals, Billings, Montana, United States of America). At each site, in the fall of 2012, 10 A. balsamea fir trees were randomly selected. Two traps, one per population, were attached to the trunk of each tree. The two traps on the same tree were positioned one below the other, 3.0 and 3.5 m above the ground, respectively. A total of 20 traps were set up at each site.
In the fall of 2013, the experiment was repeated in two of the four experimental sites, Sainte-Foy and Épaule. At each site, sticky traps containing oviposition discs (approximately 50 eggs) from the Québec population were attached to 10 randomly selected A. balsamea trees. Traps containing oviposition discs from the Newfoundland population were attached to only half of the 10 trees at each site, due to the limited number of oviposition discs available. When discs from both populations were present on a tree, the two traps on each tree were positioned as described above. This experiment involved a total of 30 traps.
Each year, from mid-May to mid-July, the number of newly hatched larvae found on the sticky surface of the Delta trap was recorded daily at each site. At the end of the hatching period, eggs that had not hatched were also counted, including those that contained a visible dead pharate larva.
The number of developed eggs out of the total number of eggs per trap was assumed to follow a binomial distribution with probability of development π. Logit(π) = log[π/(1 – π)] was modelled as the sum of a reference parameter and fixed population, site, and site by population interaction effects. Analysis of the number of developed eggs per trap from the 2014 experiment involved only data from the Sainte-Foy site because none of the eggs developed at Épaule. Models were fit with the GLIMMIX procedure of SAS 9.2. In the 2013 analysis, pairwise comparisons among sites were adjusted for multiplicity by the Holm-simulated method (Westfall et al. Reference Westfall, Tobias and Wolfinger2011).
Experiment 4: spring egg parasitism
To determine the incidence of spring egg parasitism by Telenomus species, oviposition discs (sentinel traps), each containing approximately 50 L. fiscellaria eggs from the Québec population produced in the fall of 2012, were placed in a polystyrene box covered with several layers of nylon to prevent parasitism or predation and kept in an outdoor insectary for acclimatisation until the next spring. Three sites located within the infested area of the Laurentian Mountains (47°20′N, 71°13′W, 783.0 m) were selected for this experiment. At each site, sentinel traps were stapled 3–4 m above the ground on the trunks of 10 trees spaced 10 m apart along a single row. Each week, from 29 April to 21 June 2013, 10 sentinel traps in each site were removed and replaced with new traps. Given that first attacks by female Telenomus species can occur as early as 24 April in southern Québec (Legault et al. Reference Legault, Hébert, Blais, Berthiaume, Bauce and Brodeur2012), 10 extra sentinel traps were installed in one of the three sites from 15 March to 29 April 2013, representing six consecutive weeks of sampling. To protect eggs against bird or small mammal predation, each sentinel trap was covered with a homemade circular wire mesh cage (15 cm in diameter × 3 cm in height) nailed to the tree trunks. Each week, sentinel traps were brought back to the laboratory and eggs were removed from the oviposition disk under a stereomicroscope using fine forceps. Eggs were counted, placed in individual clear gelatine capsules (size 00; PCCA, London, Ontario, Canada), and incubated at 15 °C, 65% relative humidity, under a 16:8 light:dark photoperiod until hatching or parasitoid emergence. Parasitoids were sexed and identified at the species level (Pelletier and Piché Reference Pelletier and Piché2003). Over the season, 10 885 L. fiscellaria eggs from 220 sentinel traps (10 traps × three sites × seven weeks + 10 extra traps) were exposed to Telenomus attacks.
Experiment 5: seasonal flight activity
To measure seasonal changes in male and female moth density in the L. fiscellaria-infested area of the Laurentian Mountains (47°19′N, 71°12′W, 820 m), three light traps – one from Bioquip (Compton, California, United States of America) and two from Leptraps LLC (Georgetown, Kentucky, United States of America) – were suspended 4 m above the ground from a rope attached at mid-crown to the trunks of two trees that stood 2 m apart. The light traps were 100 m apart and 50 m from the nearest main road. Each light trap was connected to a marine battery for power and the batteries were replaced every 48 hours to prevent full discharge. A 12 W (Bioquip) or 15 W (Leptrap) neon tube was used as a source of light. Given that L. fiscellaria adults are strictly nocturnal (Delisle et al. Reference Delisle, West and Bowers1998, Reference Delisle, Bernier-Cardou and Laroche2016), each light trap was automatically switched on at dusk (19:00) and turned off at dawn (05:00).
Each year, from early August to late October, moths captured in each trap were collected three times a week: on Monday, Wednesday, and Friday. On each harvest day, moths were brought back to the laboratory where they were sexed and counted. The seasonal flight activity of both sexes was recorded in this manner during two consecutive years, 2012 and 2013. It was also monitored during two more years (from mid-August to late October) using one Bioquip light trap in 2014 and three Multipher traps (BIOCOM, Ville de Québec, Québec, Canada) (Jobin and Coulombe Reference Jobin and Coulombe1988) in 2015, each replacing the three light traps used in 2012 and 2013. The Multipher traps were baited with a red rubber septum (S5509; Sigma, St. Louis, Missouri, United States of America) impregnated with 10 µg of a 1:1 (vol:vol) ratio of 5,11-dimethylheptadecane and 2,5-dimethylheptadecane, the two-component blend of the eastern L. fiscellaria female sex pheromone (Gries et al. Reference Gries, Li, Gries, Slessor, Bowers and West1991), which is also recommended as a commercial lure to monitor Lambdina fiscellaria lugubrosa (Hulst) (Lepidoptera: Geometridae) in North America, using the same dosage (Evenden et al. Reference Evenden, Borden, van Sickle and Gries1995). Two strips of Vapona insecticide (dichlorvos) (Vaportape II; Hercon Environmental, Emigsville, Pennsylvania, United States of America) were placed inside the bucket of each light or pheromone trap to kill the moths.
Results
Supercooling point determination
None of the L. fiscellaria eggs that supercooled survived. The estimated mean supercooling point of L. fiscellaria eggs was 4.4 °C (2.0, 6.8) lower in winter than in spring (F = 27.7; df = 1, 18.3; P adj = 0.0002; Fig. 2); the supercooling point averaged −40.2 °C (−41.7, −38.8) in winter (mid-February), and −35.9 °C (−36.8, −34.9) in spring. Means per spring date were: −38.8 °C (−40.4, −37.1) in April, −35.1 °C (−36.8, −33.5) in early May, and −33.7 °C (−35.3, −32.0) in mid-May. The mean supercooling point apparently differed among spring dates (F = 10.86; df = 2, 18.0;P = 0.0008). The difference was mainly due to the lower supercooling points in April relative to those in May (F = 20.04; df = 1, 18.0; P adj = 0.0012); there was no indication that the mean supercooling point differed between early and mid-May (F = 1.70; df = 1, 18.0; P adj = 0.49). The mean supercooling point did not appear to differ between the two populations in either winter (F = 0.75; df = 1, 18.4; P adj = 0.63) or spring (F = 0.18; df = 1, 18.0; P adj = 0.68). This was true at all dates (F = 0.52; df = 2, 18.0; P = 0.61 for the population × date interaction within season). A minimum supercooling point of −51 °C was observed in both mid-February and early April; its maximum of −19 °C was observed in early May.
Cold tolerance assays
In the final analysis of the data from the mid-February 2013 cold tolerance experiment, logit(1 – C) was modelled as α0 + αQC and logit(θ) as β0 + βQC + βtt + βdd + βt2t 2, where t and d represent temperature and exposure duration, respectively. The probability, expressed as a percentage, that control eggs develop successfully under favourable conditions was estimated as 95.6% (93.5, 97.7) in the Québec population and 99.0% (97.8, 100) in the Newfoundland population (χ 2 = 8.80; df = 1; P = 0.003 for the main population effect on C). The odds of survival among control eggs from the Newfoundland population were 4.6 times (1.3, 16) higher than among those from the Québec population (χ 2 = 19.78; df = 1; P < 0.0001 for the test that αQC = 0). However, the positive estimate of βQC suggests that viable eggs from the Québec population submitted to cold temperatures had better odds of development than those from the Newfoundland population (Table 1).
Table 1. Parameter estimates for the final nonlinear model of the probability of either hatching or producing a pharate larva in the cold tolerance experiment of mid-February 2013.

C, probability that an egg aborts naturally under standard conditions; θ, probability that an egg hatches or produces a pharate larva given that it is viable.
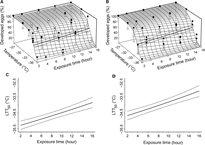
The probability, π, that eggs develop successfully decreased as temperature decreased and exposure duration increased (Fig. 3; Table 1). At the 50% response level in the Québec population, the equation giving exposure duration (d) as a function of temperature (t) is:
 $$\eqalign{d = {1 \over {{\beta _d}}}\Bigg[ {\log \Bigg( {{{\exp ( {{\alpha _0} + {\alpha _{{\rm{QC}}}}} ) + 1} \over {\exp ( {{\alpha _0} + {\alpha _{{\rm{QC}}}}} ) - 1}}} \Bigg)} \cr {- {\beta _0} - {\beta _{{\rm{QC}}}} - {\beta _t}t - {\beta _{t2}}{t^2}} \Bigg]}$$
$$\eqalign{d = {1 \over {{\beta _d}}}\Bigg[ {\log \Bigg( {{{\exp ( {{\alpha _0} + {\alpha _{{\rm{QC}}}}} ) + 1} \over {\exp ( {{\alpha _0} + {\alpha _{{\rm{QC}}}}} ) - 1}}} \Bigg)} \cr {- {\beta _0} - {\beta _{{\rm{QC}}}} - {\beta _t}t - {\beta _{t2}}{t^2}} \Bigg]}$$
where the parameters must be replaced by their estimates (Table 1). The corresponding LTT50 equation for eggs from the Newfoundland population is the same except that the parameters αQC and βQC vanish. The LTT50 ranged from −35.3 °C (−35.5, −35.0) to −33.8 °C (−34.2, −33.5) for the Québec population (Fig. 3C) and from −34.7 °C (−34.9, −34.4) to −33.1 °C (−33.5, −32.8) for the Newfoundland population (Fig. 3D). In the Québec population, for example, 9.8 hours (7.7, 12.2) of exposure to −34.5 °C prevented the development of half the eggs, whereas only 3.6 hours (< 2.0, 5.8) at −34.5 °C resulted in the same proportion of undeveloped eggs in the Newfoundland population. At temperatures greater than −33 °C, > 50% of the eggs developed at all tested exposure durations, and at temperatures less than –35 °C, egg development was < 50% for all tested exposure durations. Egg mortality reached almost 100% after two hours of exposure to –37 °C in both populations.

Fig. 3. Estimated and observed percentage of developed eggs of Lambdina fiscellaria from (A) Québec and (B) Newfoundland populations as a function of temperature and exposure duration in mid-February 2013. Observed points plotted as filled circles lie above the response surface, and a solid line drops vertically from each point to the surface. Observed points plotted as empty circles lie below the response surface, and a dashed line rises vertically from each point to the surface. The thick curve is the contour line where 50% of eggs develop (LTT50) and the thin curves on either side define a 95% confidence band. Two-dimensional projections of LTT50 of egg development for (C) Québec and (D) Newfoundland eggs with 95% confidence bands are also shown in the plane of temperature and exposure duration.
In the cold tolerance experiment conducted in mid-April 2014 on eggs from the Québec population, the probability of successful development π increased as temperature increased and exposure duration decreased (Fig. 4; Table 2). The probability that a control egg contained a developed larva or hatched, 100(1 – C), was estimated at 86% (80, 91). Among eggs treated with cold temperatures for various durations, the linear form for logit(θ) was β0 + βtt + βdd + βt2t2 + βd2d2 + βtdtd, which could not be reduced without loss of information.

Fig. 4. A, Estimated and observed percentage of developed eggs of Lambdina fiscellaria from the Québec population as a function of temperature and exposure duration in mid-April 2014. Observed points plotted as filled circles lie above the response surface, and a solid line drops vertically from each point to the surface. Those plotted as empty circles lie below the response surface, and a dashed line rises vertically from each point to the surface. The thick curve is the contour line where 50% of eggs develop (LTT50) and the thin curves on either side define a 95% confidence band. B, A two-dimensional projection of LTT50 of egg development for Québec eggs with 95% confidence bands is also shown in the plane of temperature and exposure duration.
Table 2. Parameter estimates for the final nonlinear model of the probability of either hatching or producing a pharate larva in the cold tolerance experiment of mid-April 2014.

C, probability that an egg aborts naturally under standard conditions; θ, probability that an egg hatches or produces a pharate larva given that it is viable.
The equation giving exposure duration (d) as a function of temperature (t) at the 50% response level in the spring of 2014 is (Fig. 4B):
 $$\eqalign{d = - \left( {{{{\beta _{td}}t + {\beta _d}} \over {2{\beta _{d2}}}}} \right) \cr \hskip 12 - {\bigg\{ {{1 \over {{\beta _{d2}}}}\bigg[ {\log \bigg( {{{\exp ( {{\alpha _0}} ) + 1} \over {\exp ( {{\alpha _0}} ) - 1}}} \bigg) }}} \cr {{{\hskip14pt - {\beta _0} - {\beta _t}t - {\beta _{t2}}{t^2} + {{{{( {{\beta _{td}}t + {\beta _d}} )}^2}} \over {4{\beta _{d2}}}}} \bigg]} \bigg\}^{{\raise0.2ex\hbox\!{$\scriptstyle{1}$} \!\mathord{/ {\vphantom {1 2}}\right.}\!\!\lower0.5ex\hbox{$\scriptstyle{2}$}}}}.}$$
$$\eqalign{d = - \left( {{{{\beta _{td}}t + {\beta _d}} \over {2{\beta _{d2}}}}} \right) \cr \hskip 12 - {\bigg\{ {{1 \over {{\beta _{d2}}}}\bigg[ {\log \bigg( {{{\exp ( {{\alpha _0}} ) + 1} \over {\exp ( {{\alpha _0}} ) - 1}}} \bigg) }}} \cr {{{\hskip14pt - {\beta _0} - {\beta _t}t - {\beta _{t2}}{t^2} + {{{{( {{\beta _{td}}t + {\beta _d}} )}^2}} \over {4{\beta _{d2}}}}} \bigg]} \bigg\}^{{\raise0.2ex\hbox\!{$\scriptstyle{1}$} \!\mathord{/ {\vphantom {1 2}}\right.}\!\!\lower0.5ex\hbox{$\scriptstyle{2}$}}}}.}$$
The equation is only valid for exposures of 5–16 hours because the observed proportion of developed eggs exposed to any test temperature for two or four hours was almost always > 50%, and LTT50 could not be estimated in that range. Eggs from the Québec population died at temperatures higher than their mean supercooling point (−38.8 °C (−40.4, −37.1)) when exposed for a sufficiently long period. On average, LTT50 was reached after 7.0 hours (5.4, 9.4) of exposure to −34.5 °C, or after 9.9 hours (7.6, 13.7) at −33.8 °C. As observed in mid-February 2013, at temperatures above −33 °C, > 50% of the eggs developed at all tested exposure durations even though survival among control eggs was slightly lower in 2014 (86% (80, 91)) than in 2013 (95.6%) for unknown reasons.
Winter egg survival
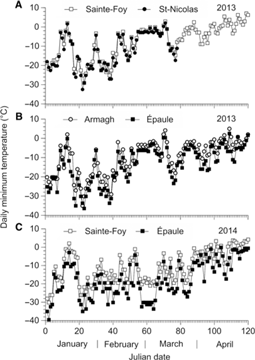
Daily minimum temperatures recorded throughout the winter of 2013 differed between the four experimental sites: at Sainte-Foy and Saint-Nicolas, the lowest daily minimum temperatures recorded were −29.1 °C (23 January) and −32.4 °C (22 January), respectively (Fig. 5A), whereas it was −35.1 °C in Armagh (22 January) and −36.5 °C in Épaule (23 January) (Fig. 5B). The hourly pattern of sub-zero temperatures recorded at these two coldest sites throughout January 2013 shows that the temperature dropped to −35 °C for only 15 minutes in Armagh (Fig. 6A), but in Épaule, temperature remained at or below −35 °C for at least 6.5 hours if we combine the durations of the two cold spells that occurred on 18 January and 23 January (Fig. 6B–C).

Fig. 5. Daily minimum temperature recorded from January to the end of April 2013 at the (A) Sainte-Foy and (B) Armagh and Épaule sites, and from January to mid-March 2013 at the Saint-Nicolas site (A). Daily minimum temperature recorded from January to the end of April 2014 at the (C) Sainte-Foy and Épaule sites is also shown.

Fig. 6. Hourly patterns of temperatures to which eggs of Lambdina fiscellaria were exposed on (A) 22 January 2013 at the Armagh site, on (B,C) 18 and 23 January 2013 at the Épaule site, and on (D) 2 January 2014 at the Épaule and Sainte-Foy sites.
As indicated in Figure 5C, on 2 January 2014, temperature at the Épaule site dropped as low as −39.6 °C for at least five hours (Fig. 6D), whereas at the Sainte-Foy site, temperature fluctuated near −30 °C for at least 10 hours (Fig. 6D) on that day and remained well above that level throughout the winter of 2014 (Fig. 5C). It is of interest to note that for both years, the rate at which temperature decreased from its highest to lowest value in each region was estimated as < 1 °C per hour.
The estimated probability that eggs develop successfully in the spring of 2013 was higher in Sainte-Foy, Saint Nicolas, and Armagh than in Épaule (F = 63.6; df = 3, 72; P < 0.0001 for the main effect of site, and P adj < 0.0001 for these three pairwise comparisons; Table 3A). On average, the odds of egg development were 65 times (35, 121) larger in the first three sites [mean, 84% (81, 87)], where winter temperature remained above −35 °C, than in Épaule. The mean probability that eggs develop in the spring was also higher in Sainte-Foy than in Armagh (P adj < 0.0001) or Saint-Nicolas (P adj = 0.0079). These estimated probabilities were consistent with the lowest temperatures recorded in each region. The odds that eggs develop successfully in the spring were 1.9 times (1.3, 3.0) higher in the Québec population than in the Newfoundland population (F = 9.12; df = 1, 72; P = 0.0035 for the test that this ratio is 1; Table 3B). There was no indication that the effect of population differed among sites (F = 1.27; df = 3, 72; P = 0.29).
Table 3. Mean estimated probability (×100) that eggs develop successfully per site (A) and per population (B) in the spring of 2013, and mean probability (×100) that eggs develop successfully per population at the Sainte-Foy site in the spring of 2014 (C).

Among the 580 eggs from the Québec population and the 145 eggs from the Newfoundland population that overwintered at the Épaule site in the winter of 2014, where the lowest temperature recorded was close to their supercooling point, none developed or hatched the following spring. Consistent with the results obtained in the spring of 2013, the odds that eggs from the Québec population develop at the Sainte-Foy site were 5.0 times (2.0, 12.3) greater than those of eggs from the Newfoundland population (F = 15.1; df = 1, 12; P = 0.0021; Table 3C).
Seasonal flight activity and spring egg parasitism
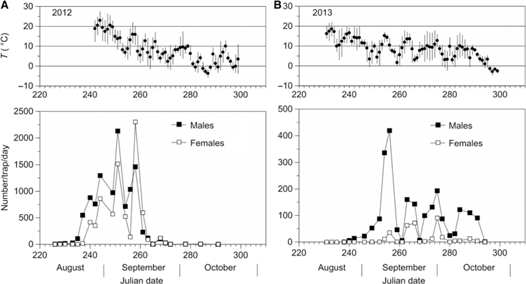
In the fall of 2012, the mean density of L. fiscellaria was > 43 000 adults per light trap. In 2013, it was 6400 adults per trap, a 6.7-fold reduction from the previous year (Table 4), even though parasitism by Telenomus was virtually absent from the area that was heavily infested by L. fiscellaria in 2012. The proportion of trapped males was 59% in 2012; the next year, it was 88%. In 2012, male flight was initiated about four days earlier (13 August, Julian date 226) than female flight (17 August, Julian date 230) (Fig. 7A). Trap catches reached their maximum on 7 September (Julian date 251) for males and on 14 September (Julian date 258) for females. For both sexes, peaks of activity were generally preceded by higher temperature conditions (male, Julian date 242–247; female, Julian date 255–258). In 2013, a slight increase in female trap catches was observed on 23 September (Julian date 266), and male activity peaked on 13 September (Julian date 256) (Fig. 7B); for each sex, this represents a five-to-eight-day delay in peak activity relative to that observed in 2012. Such differences were likely the result of lower temperatures in the summer of 2013 (approximately 11 °C) compared with those of 2012 (approximately 15 °C), particularly in August when L. fiscellaria females initiated their activity (20 August, Julian date 240). A total of 20 males and 3 females were caught in the single light trap set up in 2014 for a period of two months. In the three pheromone traps set up in 2015, 14, 27, and 32 males were caught (median density 27).

Fig. 7. Mean number of male and female moths of Lambdina fiscellaria per light trap caught during the (A) 2012 and (B) 2013 flight periods. Mean daily (•) temperatures (T) during these two flight periods are illustrated above each graph; vertical bars span the range of daily temperatures.
Table 4. Total number of adults per trap and proportion of males per trap.

* Censored observation.
† Ratio of totals per trap or ratio of mean totals per trap.
Discussion
This study demonstrates that the supercooling capacity of L. fiscellaria eggs originating from the Québec and Newfoundland populations varied similarly over the seasons. Their mean supercooling points increased from −40.2 °C (−41.7, −38.8) in February to −33.7 °C (−35.3, −32.0) in mid-May. As L. fiscellaria diapause is generally completed by the end of December (Delisle et al. Reference Delisle, Royer, Bernier-Cardou, Bauce and Labrecque2009), the lower capacity of the eggs to supercool or withstand low sub-zero temperatures with the return of higher temperatures in spring was likely the result of an increase in their metabolic rate associated with the resumption of embryonic development (Leather et al. Reference Leather, Walters and Bale1993). Interestingly, the mean supercooling point of L. fiscellaria eggs from the Newfoundland population remained quite stable over the years: in January and February 2010, the supercooling points averaged −40.1 °C (−40.7, −39.6) (Delisle et al. Reference Delisle, Labrecque, Royer, Bernier-Cardou, Bauce and Charest2013), whereas in February 2013, it was −39.6 °C (−41.7, −37.6) (current study). This finding suggests that the continuous propagation of L. fiscellaria eggs under seminatural conditions did not affect substantially their ability to supercool.
Among insects, two categories of freeze-intolerant species are widely recognised: insects that die as soon as they reach their supercooling point and those that die at sub-zero temperatures well above their supercooling point depending on the duration of exposure to low sub-zero temperatures (Bale Reference Bale1996; Lee Reference Lee, Denlinger and Lee2010). For instance, the cold tolerance assay conducted in mid-February 2013 showed that almost 100% of the eggs died after a two-hour exposure to −37 °C, even though their mean supercooling point was estimated at −40.2 °C. This example and several others from the response surfaces of Figures 3–4 support the view that L. fiscellaria eggs from the infested site of the Laurentian Mountains belong to the second category, as previously reported by Rochefort et al. (Reference Rochefort, Berthiaume, Hébert, Charest and Bauce2011) and Delisle et al. (Reference Delisle, Labrecque, Royer, Bernier-Cardou, Bauce and Charest2013) for eggs from other L. fiscellaria populations in eastern Canada.
The model for the probability that embryos develop within the eggs as a function of temperature (above the supercooling point) and exposure duration (expressed in hours) shows that for each population (Québec and Newfoundland), the probability that eggs complete their embryonic development decreased as temperature decreased and as exposure duration increased (Fig. 3A–B). However, at similar sub-zero temperatures, eggs from the Newfoundland population reached their LTT50 at a faster rate than those from the Québec population (Fig. 3D), suggesting that island eggs were less cold-tolerant than mainland eggs, despite their similar supercooling capacity. This inter-population difference in the cold tolerance of L. fiscellaria eggs did not appear to be the result of a bias in preselecting the eggs or an effect of cold storage given that in both populations, > 95% of the control eggs developed successfully. Instead, it may simply reflect local adaptations to different thermal regimes (Bradshaw and Holzappel Reference Bradshaw, Holzappel, Denlinger and Lee2010; Sinclair et al. Reference Sinclair, Williams and Terblanche2012), given that winters on the island are generally milder than on the mainland. Consistent with the results obtained under controlled laboratory conditions, the winter egg survival experiment (experiment 3) conducted in 2013 in various bioclimatic regions of the province of Québec also revealed that eggs from the Québec population had a higher survival rate (72% (66, 76)) than those from the Newfoundland population (57% (48, 65)). Overall, these results show that there is no direct relationship between the supercooling capacity of L. fiscellaria eggs and their degree of tolerance to low sub-zero temperatures.
The ability of L. fiscellaria eggs to overwinter also differed among regions. At the Sainte-Foy and Saint-Nicolas sites, where daily minimum winter temperatures were consistently above −33 °C (Fig. 5A), or at the Armagh site, where temperature fell below −35 °C for no more than 15 minutes (Fig. 5B), 84% (81, 87) of the eggs from the two populations combined developed successfully the following spring. This clearly shows that this temperature–time range was not harmful to L. fiscellaria eggs. In contrast, at the Épaule site, only 7.3% (4, 12) of the eggs from the two populations completed their embryonic development after 6.5 hours of exposure to temperatures as low as −35 °C or −36 °C in January 2013 (Fig. 6B–C). Based on the cumulative distribution of supercooling points obtained in mid-February 2013 (Fig. 2), 95% of the egg population tested at the Épaule site should have survived under these conditions given that their supercooling point was lower than −36 °C. However, as predicted by our models, a five-hour exposure to −35 °C or −36 °C was sufficient to kill > 50% of the L. fiscellaria eggs (Fig. 3), which clearly demonstrates a strong correspondence between laboratory and field tests.
Interestingly, in a similar experiment conducted at the Épaule and Armagh sites in the fall/winter of 2008–2009, none of the eggs from the Newfoundland and Baie-Trinité, Côte-Nord populations (Fig. 1) hatched the following spring, likely because they had been exposed to −40 °C for at least two hours in January 2009 (Delisle et al. Reference Delisle, Labrecque, Royer, Bernier-Cardou, Bauce and Charest2013). The −40 °C temperature reached that winter corresponds to the mean supercooling point of both populations (Delisle et al. Reference Delisle, Labrecque, Royer, Bernier-Cardou, Bauce and Charest2013); hence, it was not possible to demonstrate conclusively that L. fiscellaria eggs may die at temperatures above their mean supercooling point under field conditions. The fact that only 7% of the egg population survived after 6.5 hours of exposure to −35 °C/−36 °C at the Épaule site in the current study provides the first evidence in favour of this hypothesis.
Between mid-February and early April 2013, the supercooling point of L. fiscellaria eggs from the Québec population increased from −40 °C to −38.8 °C (Fig. 2), so these eggs were still very cold hardy in early spring. Similarly, the cold tolerance assay conducted in mid-April 2014 revealed that 50% of Québec eggs were still alive after exposure to −34.5 °C for seven hours (Fig. 4B). In the Laurentian Mountains (L’Étape, 47º33′N, 71º13′W, 791.2 m), temperatures in early April may drop as low as −27 °C as recorded in 2002 (Environment Canada 2018), but this was not the case in 2013, when temperatures remained stable at around −15 °C for most of the month (Fig. 5B; Épaule). This observation suggests that the high mortality rate observed among eggs that overwintered around the infested area of the Laurentian Mountains was not due to cold spring weather, nor was it due to egg parasitism by Telenomus, given that these parasitoids were apparently not present near the infested site of the Laurentian Mountains at that time that year. Therefore, it would appear that the two cold spells recorded at the Épaule site in January 2013 were mostly responsible for the high mortality rate (> 90%) among L. fiscellaria eggs placed in this site of the Laurentian Mountains in the fall of 2012, resulting in the collapse of the population the following spring.
Consistent with these results, a 6.7-fold decline in light trap catches occurred during the fall of 2013 (Table 4; Fig. 7A–B). Overall, mean daily temperature was much cooler in the fall of 2013 than in the fall of 2012; this may explain the approximate one-week delay observed in the peak flight activity of both sexes in 2013. Furthermore, by the time the first females were captured in the light traps in 2013 (6 September, Julian date 249), daily minimum temperatures had fallen below 10 °C (Fig. 7B), a condition that could hamper the development of the remaining female pupae (the proportion of trapped females was only 12%) or disrupt the mating of newly emerged adults as recently demonstrated by Delisle et al. (Reference Delisle, Bernier-Cardou and Laroche2016). Based on these findings, a further decline in L. fiscellaria population was expected in 2014, particularly if winter temperatures reached low levels similar to those of 2013.
The winter egg survival experiment that started in the fall of 2013 revealed that 91% (87, 95) of eggs from the two L. fiscellaria populations that overwintered at the Sainte-Foy site hatched the following spring, even though they had been exposed to air temperatures of −30 °C for nearly 10 hours in January 2014 (Fig. 6D). This high survival rate was somewhat expected, as this temperature–time range was not lethal for L. fiscellaria eggs based on our cold tolerance assay (Fig. 3). In contrast, none of the eggs from the two populations survived at the Épaule site, when temperatures dropped to −39.6 °C for at least five hours on 2 January 2014 (Fig. 6D), providing conditions quite similar to those of the 2009 winter at the same site (Delisle et al. Reference Delisle, Labrecque, Royer, Bernier-Cardou, Bauce and Charest2013). Consequently, very few moths (20 males and three females) were captured in the single light trap deployed in the fall of 2014, which clearly indicates that the L. fiscellaria population in the Laurentian Mountains had already returned to an endemic level two years after its sudden rise in 2012. Moth density was still very low in 2015: only 27 males per pheromone trap were caught over the fall season. This low density was somewhat predictable given that the winter of 2015 was as cold as the two previous ones. For instance, our dataloggers indicated that on 8 January 2015, temperature at the Épaule site decreased to −35 °C and −36 °C for seven consecutive hours (data not shown). Although the amplitude of daily thermal fluctuations can vary on multiple scales depending on the season and the habitat (reviewed by Colinet et al. Reference Colinet, Sinclair, Vernon and Renault2015), there was no reason to believe that these amplitudes changed abruptly during the course of our winter egg survival experiments. Indeed, the rate of change between minimum and maximum temperatures, estimated as > 1 °C per hour in each region and for both years, was comparatively much slower than the conventional rate of 1 °C per minute required to measure the supercooling point without causing cold shock injury to the insect. Above all, our results highlight the importance of considering not only the daily minimum winter temperature but also the duration of exposure to low sub-zero temperatures as major ecological factors affecting L. fiscellaria population dynamics.
Based on historical climate data, the three-year period of mild winters recorded in the Laurentian Mountains (Forêt Montmorency, 47º19′N, 71º08′W, 672.8 m) from 2010 to 2012 (Environment Canada 2018) apparently provided ideal climatic conditions for the L. fiscellaria population, which reached an epidemic level during the summer of 2012. This fast and unexpected rise of the L. fiscellaria population in the Laurentian Mountains was likely the result of a gradual increase in the number of moths dispersing from the border to the interior of the province of Québec because of the recent warming trends registered in eastern Canada (Bush et al. Reference Bush, Loder, Mortsch, Cohen, Warren and Lemmen2014). Interestingly, a similar period of at least three years was required for the geometrid moth, E. autumnata, to reach outbreak levels in the birch forests of Fenno-Scandia (Virtanen et al. Reference Virtanen, Neuvonen and Nikula1998).
In light of the results reported herein, the temperature–time range to which L. fiscellaria eggs are exposed in January and February is a factor that forest managers should consider carefully when they decide, several months in advance (March or April), whether or not Bacillus thuringiensis Berliner serovar kurstaki (Bacteria: Bacillaceae) treatments should be applied to control rising populations the following spring. This is especially true if egg samples collected early in the season reveal a low incidence of parasitism by Telenomus. Furthermore, recording the daily minimum temperature and its total duration (in hours) throughout the winter months and estimating egg mortality from the response surface generated by our cold tolerance assay for the Québec population should help predict the occurrence of an outbreak or changes in the range distribution of L. fiscellaria populations in the Laurentian Mountains.
Acknowledgements
We would like to acknowledge Dr. B.J. Sinclair and an anonymous reviewer for their constructive comments on an earlier version of the manuscript. We also thank Mr. Martin Charest for his assistance in supercooling point determination and Ms. I. Lamarre for her editorial work. This research was supported by the Canadian Forest Service of Natural Resources Canada.