Introduction
Carrots (Daucus carota subsp. sativus (Hoffmann) Schübler and Martens; Apiaceae) are the largest field vegetable crop in Canada, generating over $120 million (CAD) in farm gate value from roughly 8600 ha of production in 2016 (Mukezangango Reference Mukezangango2017). Over 75% of all Canadian carrot production occurs in Ontario and Québec, with other provinces generating < 10% of the national production (Agriculture and Agri-Food Canada 2014). The carrot weevil (Listronotus oregonensis (LeConte); Coleoptera: Curculionidae) and carrot rust fly (Psila rosae (Fabricius); Diptera: Psilidae) are the most important carrot insect pests in Canada (Boivin Reference Boivin, Howard, Garland and Seaman1994). The carrot weevil is a severe issue in Québec, with widespread infestations and high populations, whereas in Ontario, the carrot weevil pest pressure is generally more localised, though populations can still be high (Agriculture and Agri-Food Canada 2014). Based on research predominately performed in the 1980s (see Madder and McEwen Reference Madder and McEwen1982; Stevenson Reference Stevenson1983a; Boivin Reference Boivin1985, Reference Boivin1987, Reference Boivin1988), there are established integrated pest management recommendations for the management of carrot weevil and carrot rust fly in carrots.
Carrot weevil larvae feed on carrot roots, tunnelling typically in the upper one-third of the carrot root, which renders the root unmarketable and is a significant issue for carrot production in Canada (Fig. 1A, Boivin Reference Boivin1988; Joseph et al. Reference Joseph, Cutler and Blatt2018). Adult carrot weevils are monitored using pairs of Boivin traps, which consist of tightly spaced wooden slats and a carrot bait, placed around field margins (Boivin Reference Boivin1985). Two action thresholds have been established when monitoring with the Boivin trap: capturing 1.5 and five cumulative adult carrot weevils/trap justify a first and second insecticide application, respectively (Boivin Reference Boivin, Howard, Garland and Seaman1994). It is recommended that insecticides be applied at the second and fourth true-leaf stage, when carrot weevils are entering the fields and when carrot plants have developed enough to stimulate carrot weevil oviposition, respectively (Boivin Reference Boivin, Howard, Garland and Seaman1994). These thresholds are designed to keep damage below the economic injury level of 2% (Stevenson and Barczcz Reference Stevenson and Barszcz1997). Carrot weevil oviposition begins at 147 ± 9DD7 °C and 90% of oviposition should have occurred at 456 ± 47DD7 °C (Boivin Reference Boivin1988) There is an established degree day model (base 7 °C) for carrot weevil oviposition (Boivin Reference Boivin1988) and after the threshold for 90% oviposition has occurred, additional insecticide applications are not recommended as carrot weevil generally produces only a single generation in Canada (Stevenson and Boivin Reference Stevenson and Boivin1990). Based on this oviposition model, carrot weevil monitoring typically is completed near the end of June. Currently, there are three registered active ingredients for carrot weevil control in Canada: phosmet (Imidan 50WP/Imidan 70WP, Gowan Company, Yuma, Arizona, United States of America), lambda-cyhalothrin (Matador 120EC, Syngenta Canada, Guelph, Ontario, Canada; Silencer 120, Adama Agricultural Solutions Canada, Winnipeg, Manitoba, Canada), and novaluron (Rimon 10EC, Adama Agricultural Solutions Canada, Winnipeg, Manitoba, Canada). Lambda-cyhalothrin and novaluron were only recently registered for use on carrot weevil in 2014 and 2015, respectively. Phosmet, registered since the 1980s, is considered the primary insecticide for carrot weevil control in Canada, with phosmet applications occurring on over half of Canadian carrot acreage each year (Agriculture and Agri-Food Canada 2009). The reliance on phosmet for carrot weevil control justifies concerns that phosmet resistance may be developing in Canadian carrot weevil populations. In addition, there are recent concerns that carrot weevil may now be developing a second generation in Canada, complicating management (Boivin Reference Boivin, Parker and Gillespie2013).

Fig. 1. Carrot damage. A, Carrot weevil damage on a carrot root: tunnels are often > 1 cm in width and depth and start at the crown of the carrot root and move downwards; B, carrot rust fly damage: tunnels < 1 cm in width and depth and primarily on the bottom two-thirds of the carrot root.

Fig. 2. Relationship between cumulative carrot weevil captures in a season using the Boivin trap to observed carrot weevil damage from the University of Guelph – Muck Crops Research Station integrated pest management programme, Holland Marsh, Ontario, from 2009 to 2015. Observed values are represented with circles and the projected model is represented with the grey dashed line.
Carrot rust fly larvae also cause tunnelling damage on carrot roots, although the tunnels are typically smaller in diameter and lower on the carrot root compared with the carrot weevil tunnelling (Fig. 1B). However, this damage also renders the carrots unmarketable (Burn Reference Burn1984; Muehliesen et al. Reference Muehleisen, Bary, Cogger, Miles, Johnson, Ostrom and Carkner2003). As the tunnels are very small, any level of damage is concerning to a carrot packager or processor as it is very difficult to grade out. There are two generations of carrot rust fly in the Holland Marsh (Ontario, Canada), with a partial third generation occasionally developing in late September or October (Stevenson Reference Stevenson1983b). Monitoring relies on the use of yellow-orange sticky traps placed along one or two field margins slightly above the carrot canopy to capture adult carrot rust flies (Ontario Ministry of Agriculture, Food, and Rural Affairs 1996). Monitoring is performed in conjunction with a degree-day emergence model established by Boivin (Reference Boivin1987) that predicts carrot rust fly emergence should begin at 363 ± 33 DD3 °C and 1555 ± 56 DD3 °C for the first and second carrot rust fly generation, respectively. The action threshold for insecticide application is 0.1 carrot rust fly/trap/day for fresh market carrots and 0.2 carrot rust fly/trap/day for processing carrots (Ontario Ministry of Agriculture, Food, and Rural Affairs 1996). Since the phase out of diazinon (Diazinon 500 E, Loveland Products Canada, Dorchester, Ontario, Canada) in 2016, two active ingredients are registered for carrot rust fly control: cypermethrin (Mako, Engage Agro Corp., Guelph, Ontario, Canada; Up-Cyde, United Phosphorus, King of Prussia, Pennsylvania, United States of America) and lambda-cyhalothrin (Matador 120EC, Syngenta Canada, Guelph, Ontario, Canada; Silencer 120, Adama Agricultural Solutions Canada, Winnipeg, Manitoba, Canada) are registered for carrot rust fly control. Diazinon and cypermethrin were both applied to approximately 25% of Canadian carrot hectarage before diazinon was phased out of use (Agriculture and Agri-Food Canada 2009).
In this study, field trials were performed to evaluate the efficacy of integrated pest management recommendations for carrot weevil and carrot rust fly. Trapping methods for both pests were assessed using historical data from an established integrated pest management programme run by the University of Guelph – Muck Crops Research Station (King, Ontario, Canada; 44.0229°N, 79.3554°W) and related to damage and climatic data. An Ontario population of carrot weevils was evaluated for phosmet resistance in the laboratory, and field trials were conducted to examine the efficacy of current integrated pest management recommendations for control of carrot weevil and carrot rust fly.
Methods
Analysis of historical data from the University of Guelph – Muck Crops Research Station integrated pest management programme for carrot weevil
Carrot weevil monitoring and damage data from 2009 to 2015 were examined in order to assess the field-level efficacy of the existing carrot weevil integrated pest management programme run by the Muck Crops Research Station. Carrot weevil activity was monitored using three pairs of Boivin traps in all fields registered to receive integrated pest management programme services, ranging from 10 to 35 carrot fields in any given year with a field size of four to eight ha within a 28 km2 region. The number of carrot weevil adults trapped in each participating field was assessed twice per week. Boivin traps were monitored from early May until shortly after the degree day model predicts that 90% of carrot weevil oviposition is complete, typically late June to early July. Prior to harvest in the fall, 100 carrots (10 carrots subsampled from 10 areas in a field) were collected from each field. These samples were washed in a small vegetable drum washer and assessed for carrot weevil damage, in addition to other pest and growth issues that impact marketability. The number of carrot weevils captured and their associated damage was determined for each field. The number of recommended insecticide applications was calculated for each field using the carrot weevil integrated pest management thresholds for cumulative adult weevil counts: (1) no insecticide spray at < 1.5 carrot weevils/trap; (2) one insecticide application at 1.5 to five carrot weevils/trap; and (3) two insecticide applications at more than five carrot weevils/trap.
Assessment of carrot weevil resistance to phosmet
Adult carrot weevils were collected from commercial carrot fields in Ontario from early May to late June 2015. The weevils were returned to the University of Guelph (Guelph, Ontario, Canada) and reared following methods described by Martel et al. (Reference Martel, Svec and Harris1975) for six to eight months prior to testing. As these carrot weevils were taken directly from commercial fields, they were a wild population that had experienced insecticide exposure and will hereafter be referred to as the field strain. A laboratory strain of carrot weevil was obtained from Dr. Guy Boivin (Agriculture and Agri-Food Canada, St. Jean-sur-Richelieu, Québec, Canada) who has maintained this culture following the same rearing protocol. This laboratory strain originated from an experimental farm in Québec that had not received insecticide applications and had been reared for > 10 years in the colony without the addition of wild-type genes and was considered a susceptible population, hereafter referred to as the laboratory strain.
Each carrot weevil strain was assessed for susceptibility to phosmet (Imidan 50WP, Gowan Company, Yuma, Arizona, United States of America) at one time and two times the recommended rate of 2.25 kg formulated product/ha, 1125 ppm and 2250 ppm, phosmet respectively. Stock concentrations of phosmet were prepared in deionised water and diluted to the desired concentrations. Insecticide treatments were applied using a custom-built miniature (one-ninth scale) Potter spray tower (Potter Reference Potter1952) placed in a fumehood. The one-ninth scale Potter spray tower uses a mounted airbrush with an air compressor, delivering 103.4 kPa to apply the insecticides. Each insecticide application consisted of a 13–14 second spray containing 1 mL of treatment solution. For the applications, 10 male or 10 female carrot weevils, two to four weeks old, were distributed randomly ventral-side up in the bottom of a 50-mm glass petri dish lined with filter paper (Sartorius AG, Bohemia, New York, United States of America). To reduce carrot weevil mobility during treatments, each group of 10 carrot weevils was held in a refrigerator at 6 ± 2 °C for three to five hours and then shaken gently prior to application to limit movement, as carrot weevil feign death when disturbed (Pepper and Hagmann Reference Pepper and Hagmann1938). Post-application, the tested group of carrot weevil was transferred immediately to a clean 30-mL plastic cup with a lid (Solo Cup Company, Lake Forest, Illinois, United States of America) containing a fresh slice of carrot and held at 48 ± 2 °C for 48 hours. The control treatment consisted of 1 mL of distilled water, applied to groups of 10 carrot weevils in the same manner as the insecticide applications. Between changes in treatment or rate, the spray tower was flushed with three alternating sprays of 1 mL of 1% Liquinox (Alconox, White Plains, New York, United States of America) solution and 1 mL of acetone, followed by a single flush of distilled water to ensure no insecticide residue remained in the tower. At 48 hours post-application, carrot weevils were assessed for mortality by gently squeezing each carrot weevil with forceps. Weevils that exhibited no response (no movement) were considered dead. In order to produce sufficient replication (n = 7), this experiment took place over two separate dates.
Assessment of relationships between weather and carrot rust fly activity
Hourly weather data were recorded from a weather station (CR3000 Micrologger, Campbell Scientific, Edmonton, Alberta, Canada) established at the Muck Crops Research Station from January 2005 to December 2014. Variables calculated from this data were daily degree days (DD3 °C), cumulative degree days starting 1 April (CDD3 °C), daily average, minimum, and maximum air temperature (°C), daily soil temperature at 5 cm below ground (°C), and daily precipitation (mm). Carrot rust fly damage data were obtained from the annual carrot cultivar trial performed at the Muck Crops Research Station and reported annually in the Muck Vegetable Cultivar Trial and Research Report (www.uoguelph.ca/muckcrop/annualreport.html). Carrot rust flies were monitored using yellow-orange sticky traps, with five traps established 50 m apart along the field margin. Traps were changed twice weekly, with the number of captured rust flies counted at replacement. One location was always the Muck Crops Research Station. The second location for each year (hereafter referred to as off-station) varied from 2005 to 2008. From 2009 to 2014, the off-station location was the same field. All off-station sites were 1–3 km away from the Muck Crops Research Station and roughly 4 ha in size.
Evaluation of existing carrot insect integrated pest management programme
Three field trials were conducted to assess the efficacy of integrated pest management recommendations for carrot weevil and carrot rust fly by comparing untreated carrot plots with carrot plots receiving insecticide applications according to the integrated pest management programme recommendations. Two trials were conducted at an off-station site (King, Ontario) with a moderate carrot weevil infestation based on previous monitoring (Z.T., unpublished data) in 2015 and 2016, and one trial was conducted at the Muck Crops Research Station in 2016. Carrots (cultivar Enterprise) were triple-seeded (70 seeds/m) directly onto raised beds using a precision seeder (Stanhay Webb, Bourne, United Kingdom) at the off-station site on 28 May 2015 and 24 May 2016 and at the Muck Crops Research Station on 28 May 2016. A randomised complete block design was used with each plot sizes of (experimental unit) 25 × 14 m in 2015 and 15 × 14 m in 2016 with three replications. In 2016, these plots were established with the addition of soybean plots of the same size as carrot plots at each distal end and in the centre of the trial for another investigation only at the off-station site. Soybean (Glycine max (Linnaeus) Merrill; Fabaceae; cultivar S04-D3) was seeded on 18 June 2016. Three pairs of Boivin traps and five yellow-orange sticky traps were used to monitor carrot weevil and carrot rust fly abundance, respectively, in each field and were examined twice per week. All captured carrot weevils were removed from the field. Based on carrot weevil monitoring and established recommendations, a single application of phosmet (Imidan 70 WP) at a rate of 1.1 kg AI/ha was applied to the plots receiving the integrated pest management treatments for all three trials: one on 19 June 2015 and two on 21 June 2016, as the carrots had reached the second true-leaf stage. Despite reaching the second action threshold in all trials, the 90% carrot weevil oviposition threshold was reached before the carrots developed to the fourth true-leaf stage, meaning the second insecticide application is not recommended according to the integrated pest management programme. Trapping for carrot rust fly exceeded the action threshold of 0.1 carrot rust fly/trap/day three times in 2015 and twice in 2016. Cypermethrin (Ripcord 400 EC, BASF Canada, Mississauga, Ontario, Canada) was applied at 71.2 g AI/ha within a week of each exceeded action threshold on 19 June, 4 August, and 25 August 2015 and 4 and 16 August 2016 (Muck Crops Research Station plots only). The off-station trial in 2016 never reached the action threshold for carrot rust fly; therefore, no insecticides were applied targeting carrot rust fly. All insecticides were applied using a tractor-mounted sprayer fitted with TeeJet Air Induction Even Flat spray tips (AI9503 EVS) at 415 kPa calibrated to deliver 500 L water/ha.
In all trials, each plot was sampled twice: once in the middle of the growing season (14 August 2015 and 25 July 2016) and again at harvest (15 October 2016 and 3 October 2016) to assess differences in first-generation and second-generation carrot rust fly damage, while also assessing the possibility of increased carrot weevil damage after the first generation. This midseason sampling date was timed based on degree days at a point where second-generation carrot weevil were unlikely, based on the approximately 630DD7 °C needed for full development (Simonet and Davenport Reference Simonet and Davenport1981). Each sampling effort consisted of three (in 2015) or five (in 2016) randomly chosen 1.5 m row sections per replicate. Within a week of each sampling date, carrots were washed in a small drum washer and visually inspected for carrot weevil and carrot rust fly damage (Fig. 1A–B). The number of damaged and marketable carrots (marketable was defined as no insect damage) was recorded. Insect damage was differentiated primarily based on size, form, and location of the tunnelling as shown in Figure 1A–B. At the second sampling effort in each year, the weight of carrots in each assessment group was also recorded to determine yield.
Statistical analysis
All analyses were performed in Proc Mixed using SAS University Edition version 9.4 (SAS Institute, Cary, North Carolina, United States of America) unless otherwise noted. All trials set α = 0.05. Means separation was performed using Tukey’s honestly significant difference test.
Analysis of historical data from the University of Guelph – Muck Crops Research Station integrated pest management programme for carrot weevil
Using the historical integrated pest management data for carrot weevil collected from the University of Guelph – Muck Crops research programme between 2009 and 2015, a Spearman’s rank correlation was performed using Proc Freq to examine relationships between number of carrot weevil per trap, number of recommended insecticide applications (based on carrot weevil action thresholds), per cent of carrot with carrot weevil damage, and year. Spearman’s rank correlation was used as a preliminary examination for trends as several variables were not normally distributed.
Assessment of carrot weevil resistance to phosmet
In the laboratory trial examining phosmet resistance in Ontario carrot weevils, control treatment mortality was below 15%, and mortality in treatments was adjusted using Abbott’s formula (Abbott Reference Abbott1925). Mixed analysis of variance was used to determine the fixed effects of carrot weevil strain, sex, and dose on carrot weevil mortality, and trial date as a random effect. Tukey’s honestly significant difference was used for means separation.
Assessment of relationships between weather and carrot rust fly activity
All correlations were performed in R version 2.3.5 (R Core Team 2016) using the Picante package version 1.6.2. Spearman’s rank coefficient was used to determine relationships between carrot rust fly trap counts or carrot rust fly damage to the weather parameters, as the weather data were not normally distributed.
Evaluation of existing carrot insect integrated pest management programme
No trials were pooled based on year or location. In both years, carrot rust fly numbers were extremely low. For all trials, analysis of variance was performed to examine the fixed effect of treatment on carrot rust fly damage using only the second sampling date, including block as a random effect. Repeated-measures analysis of variance was performed to examine the fixed effect of treatment, and sampling date on carrot weevil damage. Sample date was assessed as repeated measures and block as a random effect.
Results
Analysis of historical data from the University of Guelph – Muck Crops Research Station integrated pest management programme for carrot weevil
Several significant correlations were found in the examination of data from the carrot weevil integrated pest management programme at the Muck Crops Research Station. Observed carrot weevil damage was positively correlated with the number of carrot weevils trapped in a field (Figure 2, Rs = 0.25, P = 0.001) and the number of recommended insecticide applications (Rs = 0.23, P = 0.002). Year was positively correlated with number of carrot weevils trapped (Rs = 0.44, P < 0.001) and number of insecticide applications (Rs = 0.2, P < 0.001). Overall, the integrated pest management programme recommendations and associated grower practices failed to keep carrot weevil damage below the economic injury level of 2% in 42% of participating fields.
Assessment of Ontario carrot weevil resistance to phosmet
In the laboratory trial examining phosmet resistance, the laboratory strain of carrot weevil showed significantly greater mortality at 48 hours when exposed to phosmet, compared with the field strain (Fig. 3, F 1,110 = 209.33, P < 0.001); field strain survival under 75% only occurred in one experimental unit throughout the entire trial. Male carrot weevil exhibited significantly greater mortality than females (F 1,110 = 25.80, P < 0.001), with mortality increasing with an increased dose of phosmet (F 2,110 = 87.19, P < 0.001). There was a significant interaction with the rate of phosmet and carrot weevil strain, with mortality in the laboratory strain increasing more compared to the field strain in response to increases in phosmet rate (F 2,110 = 91.46, P < 0.001). Female carrot weevil from the laboratory strain exhibited lower mortality than their male counterparts (F 2,110 = 14.62, P = 0.001) with no interaction between the rate of phosmet and sex (F 2,110 = 0.31, P = 0.582). Overall, there was a significant interaction between rate of phosmet, sex, and strain (Fig. 3, F 2,110 = 4.54, P = 0.040) indicating the multiple factors contribute to phosmet susceptibility are not independent of one another.

Fig. 3. Mean (± standard error) per cent mortality of adult male (M) and female (F) carrot weevils from a laboratory strain (Lab) and a field-collected (Ontario) strain (Field) after exposure to phosmet (Imidan 50 WP) at one time and two times the recommended rate (RR) (1125 and 2250 ppm, respectively). Mortality was adjusted using Abbott’s formula. Bars with different letters (a-d) are significantly different according to Tukey’s test (α = 0.05).
Assessment of relationships between weather and carrot rust fly activity
Overall, there were few significant correlations between carrot rust fly activity and weather parameters (Table 1). Both locations had significant negative correlations of carrot rust fly activity with calendar day and cumulative degree days. At the Muck Crops Research Station, precipitation was negatively correlated with carrot rust fly activity and off-station rust fly activity was positively correlated with soil temperature. Trap counts ranged from 0 to 2.2 carrot rust fly/trap/day across years and sites. Mean carrot rust fly damage ranged from 0.1% to 19.3% but was below 5% in all years except 2011.
Table 1. Spearman’s rank correlation of weather parameters and carrot rust fly trap counts from the University of Guelph – Muck Crops Research Station (MCRS) integrated pest management programme for the years 2005–2014. Counts were made “at MCRS” and “off MCRS” (not at MCRS) fields. Significant correlations (α = 0.05) are indicated in bold. For all analyses, n = 10.

* DD3 °C = Degree days with a base temperature of 3 °C.
† CDD3 °C = Cumulative degree days with a base temperature of 3 °C, starting 1 April.
‡ Soil temperature in °C at 5 cm below the surface.
To compare historical trapping records to the two published degree day models (Stevenson Reference Stevenson1983b; Boivin Reference Boivin1987), carrot rust fly trap counts on and off station were compared against CDD3 °C, summing trap counts across years (Fig. 4A–B). First carrot rust fly captures with respect to degree days are reported in Table 2. Overall, the first capture of carrot rust fly for the first and second generation, based on degree day accumulations, were much more variable when examining traps on- and off-station as compared with the established degree day models. Across the 10 years of trapping, there was a range of > 200 DD3 °C and > 400 DD3 °C for the first generation at the station and off-station sites and > 500 DD3 °C and > 600 DD3 °C, whereas all models reported a range of < 100 degree days, except for the second-generation prediction in the Boivin model at > 300 DD3 °C.
Table 2. Degree-day ranges for first captures of carrot rust fly adults from 2006 to 2014 at the University of Guelph – Muck Crops Research Station and an off-station field (King, Ontario) compared to values reported in the literature (Stevenson Reference Stevenson1983b; Boivin Reference Boivin1987).

Table 3. Mean per cent damage from carrot weevil and carrot rust fly, and yield in the 2015 and 2016 integrated pest management (IPM) evaluation trials in a commercial field and at the University of Guelph – Muck Crops Research Station (MCRS) in the Holland Marsh, Ontario. Mid (August) and late (October) season samples were pooled using repeated measures. Trials were analysed separately by year and location. Different letters within each column, or between the mid and late season sample columns, in the same trial location and year are significantly different according to Tukey’s honestly significant difference (α = 0.05).

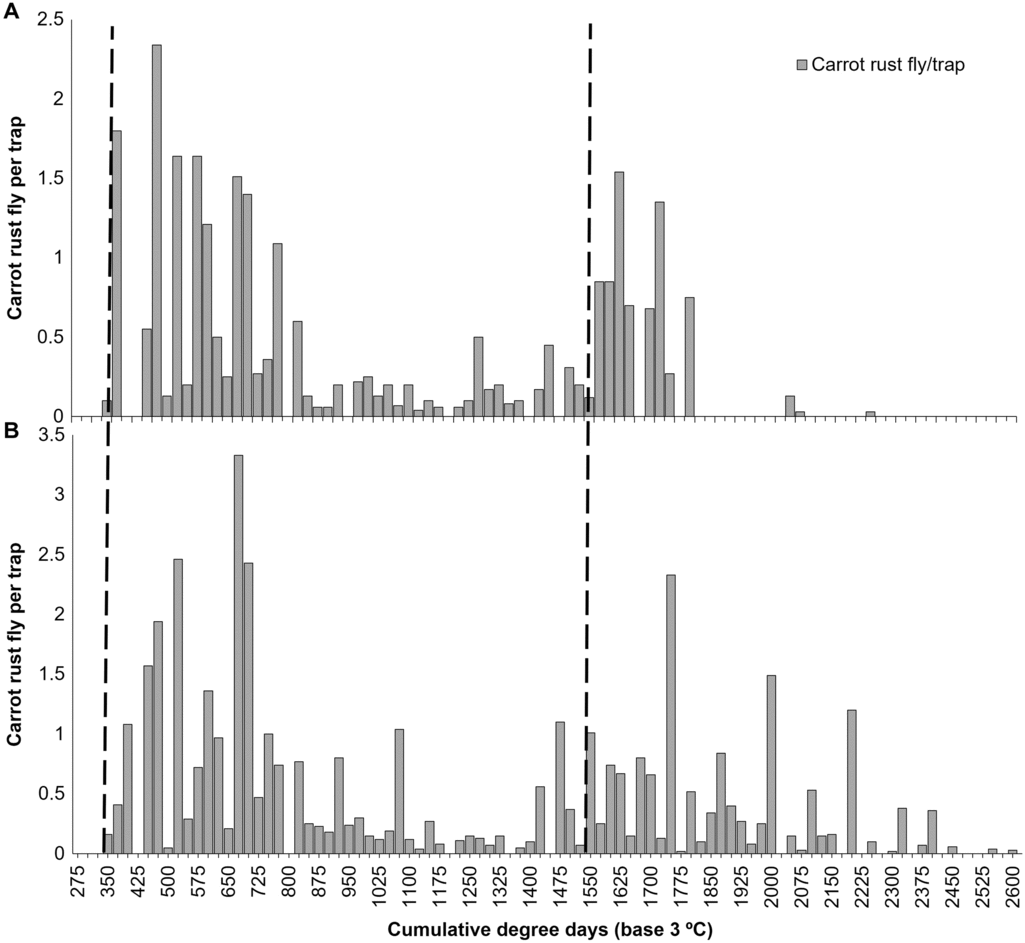
Fig. 4. Carrot rust fly trap counts from A, the University of Guelph – Muck Crops Reserach Station, and B, an off-station field, Holland Marsh, Ontario by cumulative degree days (base 3 °C) from 2005 to 2014. Trap counts across all years were summed stepwise each 25 CDD3 °C to visualize carrot rust fly trapping frequency throughout the season. Dashed lines represent the predicted emergence based on modelling from Boivin (Reference Boivin1987).
Evaluation of current carrot insect integrated pest management programme
In all field trials in 2015 and 2016, some unusual carrot weevil activity was noted. Carrots were dying early in the season, with further examination showing extensive tunnelling of carrot roots (Fig. 5A). In 2016, carrot weevil oviposition was detected on second and third true-leaf stage carrots (Fig. 5B), although oviposition is not supposed to occur until plants are at the fourth true-leaf stage (Boivin Reference Boivin1988). It is possible that the earlier oviposition results in carrot weevil larvae feeding on critical vascular tissues in the carrot root, resulting in plant death. Unfortunately, this phenomenon is currently anecdotal as no structured sampling efforts took place to quantify these observations.

Fig. 5. Carrots at the University of Guelph – Muck Crops Research Station, Holland Marsh, Ontario, in 2016. A, Young, damaged carrot – the root has been tunnelled out, likely by carrot weevil larvae; B, carrot weevil oviposition pit on a second true-leaf stage carrot.
In 2015, applying insecticides according to the recommendations of the carrot weevil and carrot rust fly integrated pest management programmes did not significantly reduce carrot weevil damage (F 1,6 = 3.27, P = 0.12), or carrot rust fly damage (F 1,6 = 1.21, P = 0.48), nor improve yield (F 1,6 = 5.94, P = 0.051) compared with the untreated control (Table 2). There was no significant difference between carrot weevil damage observed in the first (August) and second (October) sampling in 2015 (F 1,6 = 1.18, P = 0.32).
At the off-station site in 2016, carrot weevil damage was not significantly different between the integrated pest management and control plots (F 1,6 = 1.36, P = 0.29). No carrot rust fly damage was found in any plots, and there were no differences in marketable yield (F 1,6 = 0.26, P = 0.62). Carrot weevil damage increased significantly between the first (July) and second (October) sampling dates at both the Muck Crops Research Station (F 1,6 = 47.04, P = < 0.001) and off-station trials (F 1,6 = 21.16, P = 0.004).
At the Muck Crops Research Station field trial in 2016, insecticides applied according to the integrated pest management programme recommendations significantly reduced carrot weevil damage compared with the control (F 1,6 = 6.83, P = 0.040) but did not significantly reduce carrot rust fly damage compared with the control (F 1,6 = 14.15, P = 0.06). When examining a specific sampling date, no difference in carrot weevil damage between the untreated control and integrated pest management recommended insecticides was discernable (Table 3). Marketable yield was significantly increased by 4.6–26.2% in plots following integrated pest management recommendations (F 1,6 = 42.94, P = < 0.001).
Discussion
Overall, this research suggests that the current Canadian integrated pest management recommendations for carrot weevil and carrot rust fly control require revision, at least in Ontario, where these trials were performed. Current monitoring methods for carrot weevil do not correlate well with expected damage and the efficacy of phosmet for control of carrot weevil is decreasing. For carrot rust fly, the degree day model predicting first-generation emergence may no longer be accurate, and the action threshold could potentially be increased. This research re-emphasises the importance of monitoring and evaluating the functioning of established integrated pest management programmes.
Examination of data from the current carrot weevil integrated pest management programme at the Muck Crops Research Station generated concerns that the Boivin trap is no longer particularly effective for monitoring carrot weevil or that currently used thresholds need to be modified. The products registered during the data collection period (phosmet and lambda cyhalothrin) failed to provide carrot weevil control in field trials (Telfer et al. Reference Telfer, Lemay, McDonald and Scott-Dupree2018), and phosmet was generally ineffective in trials reported here. Carrot weevil captures should be strongly correlated to damage if chemical controls are ineffective, and the Boivin trap accurately reflects the threat of carrot weevil damage in the field. There were some fields where no insecticide applications were recommended; yet, carrot weevil damage exceeded 10%, whereas fields with > 30 carrot weevils per trap never exceeded 7% damage. The economic injury level for carrot weevil is 2% (Stevenson and Barscsz Reference Stevenson and Barszcz1997) and 15% of fields with no recommended insecticide applications had surpassed this economic injury level. Based on these results, it is likely the monitoring method and associated integrated pest management recommendations need to be improved to ensure effective carrot weevil management.
One potential complication impacting the ability for the Boivin trap to predict damage is natural enemies. There are several Carabidae (Coleoptera) species that are abundant within the region (Lemay et al. Reference Lemay, Telfer, Scott-Dupree and McDonald2018); however, semifield studies suggest that these enemies may not be particularly efficient at preying upon carrot weevils within the crop (Zhao et al. Reference Zhao, Boivin and Stewart1990). There are some carrot weevil egg parasitoids in the region as well (Cormier et al. Reference Cormier, Stevenson and Boivin1996); however, recent attempts to collect these parasitoids have been generally unsuccessful (Z.T., unpublished data). One alternate method of carrot weevil monitoring may be carrot root sections. Carrot root section monitoring uses 20 35 × 60 mm sections of carrot roots placed in 4–5 groups on a transect across a field to measure carrot weevil oviposition (Stevenson Reference Stevenson1985). Carrot root sections measure the active carrot weevil oviposition occurring in a field rather than population as determined when using the Boivin trap. Originally, recommendations from carrot root sections monitoring used action thresholds similar to the Boivin trap and produced similar recommendations for insecticide application (Stevenson and Barscsz Reference Stevenson and Barszcz1997). However, it may be possible to use carrot root sections to create a dynamic action threshold, which could recommend insecticide applications outside of the structured second and fourth true-leaf stage currently used in conjunction with the Boivin trap. This may be increasingly important if the carrot weevil is ovipositing on plants earlier than the fourth true-leaf stage, as seen during these field trials, since recommendations based on a dynamic action threshold would allow for insecticide application as soon as the carrot weevil is actively ovipositing in the field. With the registration of novaluron, which has recently shown efficacy in the region (Telfer et al. Reference Telfer, Lemay, McDonald and Scott-Dupree2018) and has a mode of action that can be ovicidal, carrot root sections could allow insecticide applications to occur when the target life stage of the pest (egg or early instar) is most prevalent. Alternatively, identifying an aggregation or sex pheromone used by the carrot weevil could allow for better trapping efficiency.
When insecticides were applied using a miniature spray tower, the field strain of carrot weevil exhibited significantly lower sensitivity to phosmet than a non-insecticide exposed laboratory strain. Males exhibited greater susceptibility to phosmet, which in part could be explained by a difference in size; female carrot weevils are on average slightly larger than males (Martel et al. Reference Martel, Svec and Harris1976), meaning the dose required for mortality could be slightly different. Discussions with growers involved with the Muck Crops Research Station integrated pest management programme found that growers have reduced confidence in the efficacy of phosmet for carrot weevil control. In the current study, the applications of phosmet significantly reduced carrot weevil damage in only one of three trials with treated plots in that trial showing over 20% carrot weevil damage at final harvest.
Phosmet was effective in Ontario at the time of registration (Stevenson Reference Stevenson1983a), and Pree et al. (Reference Pree, Stevenson and Barszcz1996) reported phosmet applications to effectively control carrot weevil damage in Ontario in 1990–1994. Into the 2000s, phosmet was the most widely used insecticide in Canadian carrot production with around 5000 kg of phosmet applied on just over 2500 ha (Agriculture and Agri-Food Canada 2009). Since label calls for 1.1 kg of phosmet/ha for each application, meaning most fields received two, the maximum amount, of phosmet applications. No alternative insecticides were registered for carrot weevil control until 2010. Therefore, it is likely that the 30 years of widespread phosmet applications in Canada have resulted in the reduction of efficacy of phosmet in the endemic carrot weevil population, at least in Ontario fields where this study was conducted. Further research needs to be done to determine the degree and prevalence of this reduction of susceptibility to phosmet in carrot weevils where infestations are occurring. The mechanism conferring decreased phosmet susceptibility to the field strain of carrot weevil is currently not known. Identifying how carrot weevil tolerates insecticide exposure is critical to avoid potential issues of cross-resistance development and to ensure chemistries being investigated for improved management can be effective.
It is evident from the results of the field trials that the existing carrot weevil integrated pest management programme requires modification to improve efficiency. It is not completely clear if the modifications should be focussed on carrot weevil monitoring methods, action thresholds, more efficacious insecticides, or all three. The reduction in efficacy of phosmet as reported here suggests new carrot weevil control products are needed in Canada. All integrated pest management evaluation trials presented here had significant carrot weevil damage despite following integrated pest management recommendations. Insecticide applications targeting carrot weevil may need to occur earlier than currently recommended, at the second and fourth true leaf stage (Boivin Reference Boivin, Howard, Garland and Seaman1994), as our field trials found evidence of carrot weevil oviposition into carrots earlier than the fourth true leaf stage. However, future research is needed to evaluate the efficacy of earlier insecticide applications for carrot weevil control, and these trials should be conducted to evaluate insecticides as an alternative to phosmet for carrot weevil control.
Additionally, there was a significant increase in carrot weevil damage between sampling dates in two out of three field trials, suggesting a second generation of carrot weevil in Ontario. Currently, there are no management methods that target the second generation of carrot weevil. It is believed the second generation is now occurring in Québec due to increased summer temperatures associated with climate change (Boivin Reference Boivin, Parker and Gillespie2013). Confirming the second generation and developing effective management methods are integral to ensure the overall effectiveness of the integrated pest management programme in reducing carrot weevil damage. Measuring the size of the second generation is difficult as all baits used for monitoring currently rely on carrots, which get outcompeted as the crop develops (Boivin Reference Boivin1999).
To improve monitoring, identifying baits that are more attractive to adult weevils than a carrot root is critical. In Ohio, United States of America, the Boivin trap is ineffective for carrot weevil monitoring in parsley (Petroselinum crispum (Miller) Fuss; Apiaceae) fields (Torres and Hoy Reference Torres and Hoy2002); therefore, future research should assess other Apiaceous plants or volatiles produced within the Apiaceae as a carrot weevil attractant. However, even if the second generation of carrot weevil can be quantified, the dense canopy created by a carrot crop makes it difficult to apply insecticides to reach the base of the carrot where the carrot weevil oviposits or the soil where the weevil resides. Currently, there are no established recommendations for monitoring or controlling second-generation carrot weevils; effective control of the first generation is critical to keep the population size of the second generation low.
Very few weather parameters had significant correlations with carrot rust fly activity in this study, and seasonal weather was not related to observed carrot rust fly damage. It is surprising that so few relationships were found, as adult carrot rust fly activity is temperature dependant with only females entering fields from the hedgerows for oviposition on cool windless evenings (Wakerly Reference Wakerley1963). In Ontario, the degree day model predicting carrot rust fly emergence appears to require revision. Stevenson (Reference Stevenson1983b) found carrot rust fly flight to occur between 237–277 DD5 °C (first generation) and 1107–1191 DD5 °C (second generation) in Ontario between 1972 and 1980, while Boivin (Reference Boivin1987) predicted carrot rust fly emergence should begin at 363 ± 33 DD3 °C (first generation) and 1555 ± 56 DD3 °C (second generation). Our results were closest with Boivin (Reference Boivin1987), although slight later, with the mean first-generation emergence at 436.4 ± 29.1 DD3 °C on station and 506.2 ± 50.2 DD3 °C off-station. The exact timing of the second generation is difficult to identify as carrot rust fly trapping frequency is highly variable between the first and second generation, but may start earlier than expected based on the Boivin (Reference Boivin1987) model. In all cases, the Stevenson (Reference Stevenson1983b) predictions were much earlier than seen in our trials; however, direct comparison is difficult as the Stevenson model used a slightly higher base air temperature.
Carrot rust fly populations have a high level of population variability, particularly compared with the carrot weevil (Lamb and Boivin Reference Lamb and Boivin2017) meaning that population size could be high in future years and emphasises the importance of effective trapping and threshold recommendations. The high variability could be decreasing the ability to identify trends in carrot rust fly activity and weather. Through the Muck Crops Research Station integrated pest management programme, we have anecdotally noticed carrot rust fly issues tend to be highly localised (Z.T., unpublished data). An improved understanding of the abiotic factors that improve carrot rust fly survival could help identify regions with high risk of carrot rust fly infestations. Carrot rust fly adults do not live within crop fields, rather in field-adjacent habitats such as weeds and hedgerows (Petherbridge et al. Reference Petherbridge, Wright and Davies1942), and a better understanding of the factors that promote a suitable carrot rust fly habitat could present opportunities for cultural control of carrot rust fly populations through the removal of habitat.
It is difficult to assess how the carrot rust fly integrated pest management programme performed in field trials because carrot rust fly pressure was low, carrot rust fly damage on carrots was negligible, and thresholds were rarely reached. This indicates that the recommendations to not apply insecticides for carrot rust fly under the action threshold are likely accurate, but we cannot conclude that the carrot rust fly integrated pest management programme recommendations are effective when intervention is needed. Our results suggest the action threshold of 0.1 carrot rust fly/trap/day for fresh market carrots and 0.2 carrot rust fly/trap/day for processing carrots could be increased as there was no benefit to insecticide applications in our trials, but this requires additional research to confirm. In British Columbia, Canada, carrot rust fly integrated pest management programmes use the same monitoring methods as at the Muck Crops Research Station but the action threshold is set at 0.25 carrot rust fly/trap/day (Judd et al. Reference Judd, Vernon and Borden1985), while in Denmark, the action threshold ranges from 0.3 to 1 carrot rust fly/trap/day (Esbjerg et al. Reference Esbjerg, Jorgensen, Neilsen, Philipsen, Zethner and Ogaard1983), so increasing the action threshold would not be unprecedented. It is likely different biotic and abiotic factors are affecting the carrot rust fly populations in different regions, and as such, the action threshold may need to be developed for each region.
Overall, this research suggests that the existing carrot weevil integrated pest management programme in Canada is currently not providing optimal recommendations for effective management of this insect pest. Further research needs to focus on improving carrot weevil monitoring, changing action thresholds, and identifying effective insecticides for carrot weevil control. During these field trials, novaluron was registered for carrot weevil control and has some reported efficacy; however, relying on a single mode of action is not a sustainable pest management practice. For carrot rust fly, future research should focus on examining a higher carrot rust fly action threshold to avoid unnecessary insecticide applications.
Acknowledgements
The authors thank staff at the University of Guelph – Muck Crop Research Station for technical advice and assistance, all growers participating in the University of Guelph – Muck Crops Research Station integrated pest management programme, and Dr. Angela Gradish – postdoctoral fellow (University of Guelph – School of Environmental Sciences). Funding for this project was received from the Ontario Ministry of Agriculture, Food, and Rural Affairs-University of Guelph Partnership, the Bradford Co-Op Storage, Bayer CropScience, Engage Agro, E.I. DuPont Canada, and the Fresh Vegetable Growers of Ontario.










