Introduction
Among butterflies, two types of species generate the most interest, classically those that are large and colourful (mainly Papilionidae and some Nymphalidae) and those that are “different”. This last rather vague term covers species that, while not necessarily especially attractive, possess evolutionary, ecological, or behavioural particularities that are not yet fully understood. For example, the Asian Liphyra brassolis Westwood (Lepidoptera: Lycaenidae) for their unusual carnivorous behaviour and its unresolved phylogenetic relationships (Fiedler Reference Fiedler2012), or the Pseudopontia Plötz (Lepidoptera: Pieridae) complex of species in Africa for their unresolved phylogenetic placement and unique un-clubbed antennae among Rhopalocera (Mitter et al. Reference Mitter, Larsen, De Prins, De Prins, Collins and Vande Weghe2011). Among Papilionidae, phylogenetic relationships are still not totally elucidated for genera Teinopalpus Hope and Meandrusa Moore, even though recent work has somewhat clarified their relationships in this family (Simonsen et al. Reference Simonsen, Zakharov, Djernaes, Cotton, Vane-Wright and Sperling2011).
Baronia brevicornis Salvin (Lepidoptera: Papilionidae: Baroniinae) remains one of the most enigmatic species of butterfly in the world. This localised insect, which is strictly endemic to Mexico (Soberón and Townsend-Peterson Reference Soberón and Townsend-Peterson2005), is considered to be a “living fossil” (Eisner Reference Eisner2003) but the term panchronic species (i.e., with a stable phenotype through time) seems more appropriate. Its distribution (Fig. 1) centres on the south of the Morelos and the north of the Guerrero States with peripheral populations from Jalisco to Oaxaca States (Soberón and Townsend-Peterson Reference Soberón and Townsend-Peterson2005). An isolated population, identified as a subspecies: B. brevicornis rufodiscalis De la Maza and White occurs in Chiapas State (De la Maza et al. Reference De la Maza, White and White1987).

Fig. 1 Distribution of Baronia brevicornis: Black circles for the nominal subspecies, white circles for subspecies Baronia brevicornis rufodiscalis from Chiapas. In grey the distribution of the host plant Acacia cochliacantha (modified from Soberón and Townsend-Peterson Reference Soberón and Townsend-Peterson2005).
Apart from its unresolved phylogenetic position, one of the most striking particularities of B. brevicornis, in opposition to all other Papilionidae in the world (except very rare records for Papilio demoleus Linnaeus and Papilio demodocus Esper (Aubert et al. Reference Aubert, Legal, Descimon and Michel1999)), is that their caterpillars feed strictly on Acacia cochliacantha Humboldt and Bonpland (Fabaceae) (Pérez Reference Pérez1967, Reference Pérez1972) with exceptional (N.A.M., personal observation) or regional records (Chiapas subspecies: León-Cortés et al. Reference León-Cortés, Pérez-Espinoza, Marín and Molina-Martínez2004) on Acacia pennatula (Schlechtendal and Chamisso) Bentham.
The phylogenetic position of this species is unclear with a paraphyletic position between two primitive tribes of Papilionidae: Parnassiini and Zerynthiini (Reed and Sperling Reference Reed and Sperling2006; Michel et al. Reference Michel, Rebourg, Cosson and Descimon2008). According to Nazari et al. (Reference Nazari, Zakharov and Sperling2007) and Michel et al. (Reference Michel, Rebourg, Cosson and Descimon2008) the lineage leading to B. brevicornis may date from the late Cretaceous (70 million years ago). The Papilionidae species closest to B. brevicornis are known to feed on Crassulaceae, Aristolochiaceae, or occasionally on Zygophylaceae (such as the Iranian butterfly, Hypermnestra helios Nickerl (Lepidoptera: Papilionidae: Parnassiinae) (Michel et al. Reference Michel, Rebourg, Cosson and Descimon2008)). Photographs of all stages of B. brevicornis, including biota and a few videos, are available from Warren et al. (Reference Warren, Davis, Grishin, Pelham and Stangeland2010).
Acacia cochliacantha is a widespread and extremely common shrub from Mexico to Guatemala, other records from further south of Central America are doubtful and at least need more extensive studies including molecular work (Seigler and Ebinger Reference Seigler and Ebinger1988; Clarke et al. Reference Clarke, Downie and Seigler2000). A key point is that the overall distribution of B. brevicornis is far smaller than that of its host plant (Soberón and Townsend-Peterson Reference Soberón and Townsend-Peterson2005) (Fig. 1). Even though this butterfly is recognised as being extremely particular, very few elements concerning its ecological constraints are available except data coming from old studies published in Spanish (Vázquez and Pérez Reference Vázquez and Pérez1962; Pérez Reference Pérez1967, Reference Pérez1972, Reference Pérez1979; Pérez and Sánchez Reference Pérez and Sánchez1986) and two more recent works published in English, but they focused on the isolated populations of Chiapas (León-Cortés et al. Reference León-Cortés, Pérez-Espinoza, Marín and Molina-Martínez2004) and on the global distribution of the species (Soberón and Townsend-Peterson Reference Soberón and Townsend-Peterson2005). The chemistry of leguminous trees and, more particularly of Acacia species, has been extensively studied. They are known to be rich in tannins, flavonoids, and cyanogenic glycosides (Vetter Reference Vetter2000; Seigler Reference Seigler2003; Wink Reference Wink2003). However, the chemical composition of A. cochliacantha is little known but Heil et al. (Reference Heil, Delsinne, Hilpert, Schürkens, Andary and Linsenmair2002) classified it as a “toxic” Acacia species.
Our goal is to clarify some aspects of the general natural history and ecology of B. brevicornis. We determined (i) the abiotic factors necessary to host a population; (ii) the minimum area of suitable vegetation to host a viable population; (iii) the host-plant density required to sustain a population; (iv) the density of adults in a population; (v) the number of generations per year for B. brevicornis; (vi) the level of predation on adults and caterpillars, and (vii) a model for the potential distribution.
These elements will all be important in future interpretation of molecular and chemical ecology studies but also in making decisions on the phylogenetic position and conservation status of this particular species. Modelling abiotic conditions with presence/absence of occurrences of B. brevicornis gave us the possibility to propose a potential complete distribution in Mexico (i.e., niche occurrence). Congruence between real occurrences and our model will be discussed.
Materials and methods
Study sites
The main population of B. brevicornis is centred between the south of Morelos State and the north of Guerrero State. The Sierra de Huautla Biosphere Reserve (REBIOSH) is a recently created (2006) UNESCO (United Nations Educational, Scientific and Cultural Organization) Biosphere Reserve including the whole of the south of Morelos State. The zone under conservation corresponds to the upper valley of the Balsas River and most of its territory is under tropical dry forest. With the recent proposal to increase the protected area by 600 km2 in the southwest of Puebla State, the REBIOSH could cover 1200 km2 (600 km2 in Morelos and 600 km2 in Puebla) representing one of the largest world reserves for topical dry forest. According to Soberón and Townsend-Peterson (Reference Soberón and Townsend-Peterson2005) the REBIOSH covers a large part of the main range of B. brevicornis distribution.
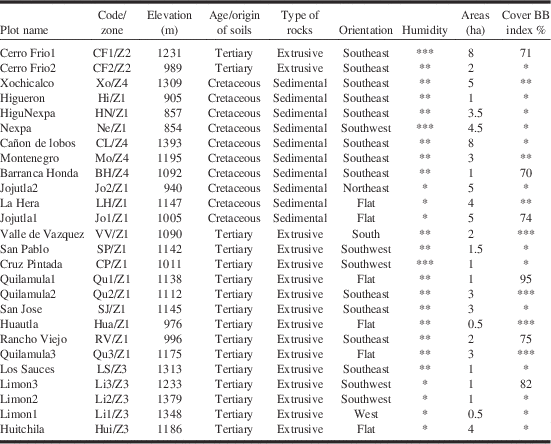
Twenty-six independent plots of occurrence of B. brevicornis, in four different zones in Morelos State (central-south Mexico) were identified and studied after eight years of field work. Baronia brevicornis was present in these 26 plots over at least four consecutive years. The four zones (Z1–4) were delimited on the basis of geographical barriers; Z1 is separated from Z2 by the Amacuzac River, from Z3 by a cordillera of average elevation of 1500–1600 m, and from Z4 by the central plateau mainly occupied by agriculture (Table 1 and Fig. 2). Most of our results take into account all 26 plots, but some more precise experiments were performed in six specific plots chosen for their accessibility, their diversity in terms of abiotic conditions and the widest range of human disturbance (from well conserved plots to very disturbed ones) and that at least one plot was present in each of the four main zones where B. brevicornis occurs (Table 1 and Fig. 2). All six plots presented shrubs covering a wide range of ages (natural turn-over of this Acacia species). Four of the six plots were located inside the UNESCO Biosphere Reserve of the “Sierra de Huautla”, which covers the south of Morelos State: (1) an abandoned field colonised by A. cochliacantha close to the village of Quilamula (Zone 1), (2) the Biological Station of “El Limon” (Zone 3), (3) above the village of Rancho Viejo (Zone 1) and, (4) a conserved area on the slopes of the volcanic area of “Cerro Frio” (Zone 2). The two other plots were outside the UNESCO Biosphere Reserve of the “Sierra de Huautla”: (5) along the road leading from the city of Jojutla to the village of Chinameca (Zone 1) and (6) close to the village of Barranca Honda (Zone 4). As B. brevicornis is especially sought by private collectors, exact locations are not given (The Scientific Committee of CIByC (e-mail: cibyc@uaem.mx), Universidad Autónoma del Estado de Morelos, Morales, Mexico can be contacted to provide precise localities to institutional scientists).

Fig. 2 Map of Morelos (south-central Mexico), Spot Image 2012 (Toulouse, France). Dotted ellipses indicate the four main zones (Z1–4) where we recorded population patches of Baronia brevicornis; thick ellipses (Z1 and Z2)/light ellipses (Z3 and Z4), respectively corresponding to highest densities of plots and abundances of individuals versus lower densities. The thick black lines correspond to our two transects T1 and T2. North of the thick white line is elevations above 1400 m (note that some mountains are above 1400 m south of this line but not covering large and continuous areas). Arrows represent the main possibilities of contact (based on similar abiotic conditions) with neighbouring states.
Table 1 Plots with presence of Baronia brevicornis (classified from west to east).

Highlighted in grey our six reference locations (see Materials and methods).
Four zones were discriminated under criteria explained in Materials and methods. Average elevations±SD: 1124.52±153.36 m. Average area of plots where B. brevicornis was found±SD: 2.83±2.08 ha. Humidity: *dry; **medium; ***humid (see Materials and methods for more details). Covering Braun-Blanquet in % or comparative estimation (see Materials and methods).
Abiotic conditions and surface areas concerned
For each of the 26 plots studied, the areas were calculated using a GPS device (SIRF Star III; San Jose, California, United States of America) and confirmed by aerial photography using Google Earth©; Mountain View, California, United States of America. The relative humidity was determined mainly by the plant associations occurring in each area (O.D., unpublished results), but also using rare rainfall data collected by local farmers. Data from 20 meteorological stations from the south of Morelos were also used to evaluate local precipitation in the study sites. Annual rainfall ranges in the 26 locations where B. brevicornis was found were divided into three groups: between 800 and 900 mm (considered “dry”), 900–1000 mm (considered “medium”), and 1000–1100 mm (considered “humid”) (Torres et al. Reference Torres, Osorio-Beristain, Mariano and Legal2009). Soil composition was assessed using local geological maps and for chalky areas was verified directly by the release of gas bubbles after depositing a few drops of hydrochloric acid. The orientation of the main slope was checked with a standard compass. The complete abiotic data are provided in Table 1.
Host plant: Acacia cochliacantha
At least two botanists corroborated the identification of the plant species for the field study. In former studies, inaccuracies most likely arose from the numerous synonyms and possible confusions among the various species of Acacia of the “A. macracantha clade” (all named “Cubata” in Mexico), the precise naming of the host plants is then always difficult (Siegler and Ebinger Reference Seigler and Ebinger1988; Clarke et al. Reference Clarke, Downie and Seigler2000; Dorado et al. Reference Dorado, Arias, Ramírez and Sousa2005). Old A. cochliacantha specimens are almost entirely devoid of the large red spines, which are its most noticeable characteristic and thus may be confused with a close species A. pennatula by non-specialists (note that numerous natural hybrids between these two species were suspected and discarded from the present study). However, in old specimens, the shape is very different with a single trunk for A. pennatula (tree pattern) while A. cochliacantha has a multiple trunk (shrub pattern) (see picture in Warren et al. Reference Warren, Davis, Grishin, Pelham and Stangeland2010).
Shrub density
In each of the six specific locations (see above), we found no significant variations of densities or coverage of A. cochliacantha per ha (data not shown). Therefore, to estimate the total number of A. cochliacantha, we randomly chose 1 ha in each of the six locations. In each ha an estimation of the coverage was made following the Braun-Blanquet abundance scale (Mueller-Dombois and Ellenberg Reference Mueller-Dombois and Ellenberg1974). Three nested quadrats following Barbour et al. (Reference Barbour, Burk and Pitts1987) were performed (Table 1). On the remaining 20 plots (except on transects, see next section) no measurements of cover were made but a rough comparative estimation with reference plots was established: *between 65% and 75%; **between 75% and 90%; ***above 90% corresponding to a pure covering of A. cochliacantha named “cubateras” in Mexico.
Baronia brevicornis
Density of Baronia brevicornis occurrence plots
In order to estimate the density and distribution of occurrence plots over a limited area where the host plant is abundant (at least 45% cover using the Braun-Blanquet abundance/cover scale; Mueller-Dombois and Ellenberg Reference Mueller-Dombois and Ellenberg1974) and regularly distributed, two transects were performed: (1) first along the road from Jojutla/Chinameca to Rancho Viejo the area covered was estimated at around 8 km2 using Google Earth©: 16 km in a direct line but, about 21 km by road exploring a strip 200 m wide on either side of the tarmac. Average elevation: 1072 m, minimum elevation: 933 m, maximum elevation: 1342 m, (code T1 and situated in Zone 1), and (2) a second transect from the village from Huitchila to the locality of “El Limon”. The total area covered was estimated at around 6.5 km2 using Google Earth©: 12 km in a direct line but around 16 km by road exploring a strip 200 m wide on either side of the track. Average elevation: 1294 m, minimum elevation: 1169 m, maximum elevation: 1490 m (code T2 and situated in Zone 3). Areas where covering of A. cochliacantha exceeds 65% were also measured. The geographic location of these transects is given in Fig. 2. Each of the plots (checked by the presence of adults and/or caterpillars) was numbered and geo-referenced using a GPS device and the altitudinal pattern of the two transects was determined using tools available in Google Earth© (Fig. 3).

Fig. 3 Transects for two zones where Braun-Blanquet covering index of Acacia cochliacantha was 50% for transect 1 and 45% for transect 2. Codes of plots where Baronia brevicornis is present correspond to those given in Table 1. Coordinates are elevation in metres. More details in Materials and methods.
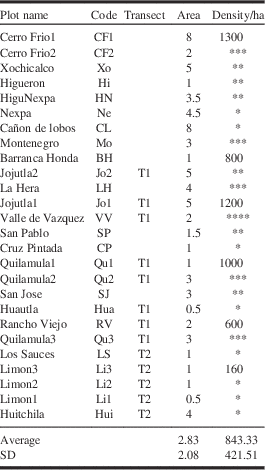
Density of adults
For each of the six study reference plots, adult density was estimated by counting the number of foraging individuals per shrub over a 15-minute period: 10 mature (exceeding 4 m high) shrubs per ha, two independent ha/location when possible otherwise 20 shrubs/ha. This information combined with counting the number of shrubs per ha (see “Shrub density” above) provided an estimate of adult B. brevicornis density. Note that to avoid counting foraging individuals twice on different shrubs, counting was done simultaneously by four different people. We calculated the mean number of individuals on the six plots sampled/ha×average area of the six studied sites. For the 20 other plots only a rough estimation, individuals on five shrubs/15 minutes per plot were counted.
Number of generations per year
This point was mainly checked to provide information on the dynamics of the butterfly, even though this is not directly related with its ecology. Furthermore, numerous individuals were recorded outside the period covered by the single generation described in the literature (early June). In order to measure the emergence of adults, 200 fifth-stage caterpillars were collected in the Cerro Frio locality (July 2006) and followed in the laboratory for two years. Half of the resulting chrysalides were placed in a terrarium under “natural” conditions (outside, aerated, and exposed to precipitation) and the other half were kept in similar conditions but protected from the rain (called “dry” conditions). The “dry” treatment was applied to ensure that the level of humidity in the terrarium was not too high but also to determine the resistance of the species to desiccation in exceptionally dry years (“El Niño” years are usually very dry in this part of Mexico; Badan Reference Badan2003). In parallel, occurrence of adults and caterpillars in the six field locations was checked over two consecutive years (2007–2008).
Predation and mortality
Over five years we noted all mortality, predation, and parasitism events observed in the field.
Modelling distribution
Climate data were used to determine a model of the current range of B. brevicornis using the database from WorldClim (Hijmans et al. Reference Hijmans, Cameron, Parra, Jones and Jarvis2005). We considered the following set of variables: (1) annual mean temperature, (2) mean diurnal range (mean of monthly, maximum and minimum temperature), (3) isothermality, (4) temperature seasonality, (5) maximum temperature of warmest month, (6) minimum temperature of coolest month, (7) annual temperature range, (8) mean temperature of wettest quarter, (9) mean temperature of driest quarter, (10) mean temperature of warmest quarter, (11) mean temperature of coldest quarter, (12) annual precipitation, (13) precipitation of wettest month, (14) precipitation of driest month, (15) precipitation seasonality, (16) precipitation of wettest quarter, (17) precipitation of driest quarter, (18) precipitation of warmest quarter, and (19) precipitation of coldest quarter. We used the best resolution available in the WorldClim database of 30 arc/second (926 m2 at the equator) to model the ecological niche of B. brevicornis.
To model species distributions where only presence data are available, we used an ensemble model implemented in the BIOMOD2 package (Thuiller et al. Reference Thuiller, Lafourcade, Engler and Araújo2009) in R 2.15.2 (R Development Core Team 2012), which generates pseudo-absences randomly over the whole study area. BIOMOD2 is a powerful method compared with single-SDMs to model species distribution as it uses an ensemble forecasting approach, building a map based on the consensus evaluation of multiple SDM models (Grenouillet et al. Reference Grenouillet, Buisson, Casajus and Lek2011). Because the output is strongly affected by the number of pseudo-absences selected (Barbet-Massin et al. Reference Barbet-Massin, Jiguet, Albert and Thuiller2012), and also following the kind of algorithm used, ensemble forecasting was implemented through three algorithms: generalised boosting models (GBM), random forest (RF), and classification tree analysis (CTA), which required the same number of pseudo-absences selected (Barbet-Massin et al. Reference Barbet-Massin, Jiguet, Albert and Thuiller2012). We used a random subset of 70% of the data to calibrate the model and the remaining 30% to evaluate its predictive performance. To evaluate model performance, we used the area under the receiver operating characteristic (ROC) curve (AUC) (Fielding and Bell Reference Fielding and Bell1997) and the true skill statistic (TSS) (Allouche et al. Reference Allouche, Tsoar and Kadmon2006). The AUC values ranged from 0.5 for models with random predictions to 1 for models perfectly fitting the data, while the TSS values varied between 0 for a random model and 1 for a model with an excellent agreement. The data splitting approach was replicated 30 times to give a mean for the AUC and TSS by cross validation. The final projections used 100% of the available data for calibration. Projections resulting from different algorithms were averaged, and we used a decay of 1.6 to take into account their predictive performance (Marmion et al. Reference Marmion, Parviainen, Luoto, Heikkinen and Thuiller2009). To select models with the strongest predictions, both ROC and TSS scores were used with a minimal TSS value of 0.8 and a minimal ROC value of 0.9 (both considered as excellent predictions of accuracy) (Thuiller et al. Reference Thuiller, Lafourcade, Engler and Araújo2009). In order to transform the consensus distribution from ensemble modelling to a presence/absence distribution, we used the TSS threshold that maximises the sum of sensitivity and specificity.
Results
Abiotic conditions
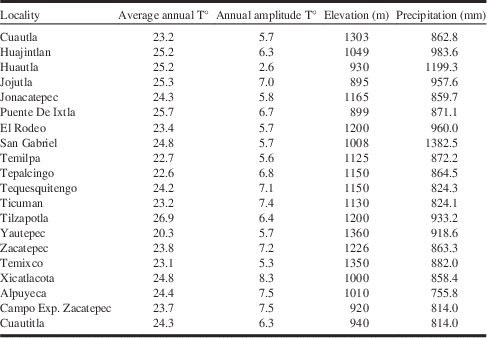
For 20 meteorological stations, over five years (SEMARNAT data, http://www.semarnat.gob.mx), situated close to or directly within the zones of occurrence of B. brevicornis, the values include (mean±SD): an annual temperature of 24.1±1.4 °C, amplitude of temperature differences over the year 6.3±1.2 °C, and annual precipitation of 915±143.2 mm (Table 2). We did not find any occurrences when precipitations exceeded 1200 mm. The average elevation of the meteorological stations was 1100±148 m. According to the climate classification system of Köppen–Geiger (and updates; Peel et al. Reference Peel, Finlayson and McMahon2007), the climatic characteristics in our study area correspond to the most humid range of arid climates (BS1) and to the driest of the tropical rainy climates (Aw0). The average elevation of the 26 B. brevicornis plots in Morelos was 1124±153 m but never exceeded 1400 m. This information indicates an optimal precipitation level for B. brevicornis of around 920 mm/year. Similarly, optimum annual temperature was estimated to be around 24 °C not exceeding 18 °C for the coolest months and 30 °C for the hottest. These characteristics correspond to the optimum climate type Aw0(w)(i')g.
Table 2 Average abiotic data collected in 20 meteorological stations in the south of Morelos State (five years data set 2006–2010).

The geomorphology of this part of Mexico is complex, forming a mosaic of volcanic soils, calcareous areas and even a few metamorphic spots (mainly granitic) (E14 series of maps of INEGI, “Instituto Nacional de Estadística y Geografía”, Mexico). No clear relationship between the type of soil and the presence/absence of B. brevicornis was found. However, there was a trend for a more regular distribution in chalky and extrusive (volcanic) areas (Table 1).
Orientation, and thus incident sunshine, seems to be an important factor with most population patches being found on southeast facing slopes, adults are mostly active in the mornings that could be an explanation but also a consequence of this point (Table 1).
Surface areas of population patches
Baronia brevicornis is strictly distributed close to its host plant, A. cochliacantha. Its flight is vigorous especially in males, which always return to the same Acacia branch, guarding their territory (extremely strong territorial behaviour as in many Hesperiidae/Eudaminae, Pyrginae, or Heteropterinae (Ravenscroft Reference Ravenscroft1994)). Such species may survive in very small patches and the average areas found for our 26 plots was 2.83±2.08 ha (mean±SD). The smallest stable plots (over five years) encountered, such as “El Limon 1” (“Li1” see Table 1), were no larger than 0.5 ha, which, for a flying insect, is a tiny area (Cowley et al. Reference Cowley, Wilson, León-Cortés, Gutiérrez, Bulman and Thomas2000). Some temporarily occupied plots, just one or two years, are frequently observed especially when a few isolated old trees are present. Even though they do not represent stable settlements, they surely play an important role in the connectivity between the stable plots described above.
Host-plant densities
Acacia cochliacantha is by far the most widespread and commonest shrub in the south of Morelos below 1400 m. It is almost impossible not to see it except perhaps in the wettest areas or in deep primary forest (Dorado et al. Reference Dorado, Arias, Ramírez and Sousa2005). Baronia brevicornis is much more localised than its host plant.
The average distance between our 26 plots was 16.5±10.9 km but the median value was lower: 11.3 km indicating that the number of plots (density) was not homogeneous in the region. The average distance between our six reference plots was 26.8±13.7 km. Two main distribution areas (Zones 1 and 2), with a high density of plots where B. brevicornis occurs and is numerous, are separated by the Amacuzac River and situated in the centre south part of Morelos State (Fig. 2). Two other areas (Zones 3 and 4) are noticeable; they showed a much lower density of plots and hosted lower densities of individuals. One is southeast of the Morelos State and the other centred around “Lobos Cañon”/Sierra de Montenegro in the centre of the State (Fig. 2).
In order to illustrate this point, two transects were performed along road/tracks, as the shrub is much more common in disturbed areas, (Jojutla-Rancho Viejo: transect 1 and Huitchila-Limon: transect 2; Fig. 2). On the two transects the Braun-Blanquet covering of A. cochliacantha was 50% for transect 1 and 45% for transect 2. Abiotic conditions were fairly similar except elevation (Fig. 3) with an average of 1072 m for transect 1 and 1294 m for transect 2. Along transect 1 we found eight plots of B. brevicornis and only five for transect 2 (Fig. 3). Note that no occurrences were observed above 1275 m for transect 1 and a single one around 1350 m for transect 2. Average distance among occurrences along transect 1 was 2.8±1.67 km and for transect 2 it was 2.3±1.74 km. The main result of this transect study is that no occurrences were noted when Braun-Blanquet coverage by the Acacia was below 65–70%. Areas where coverage exceeded 65% were measured giving 10.6% for transect 1 and 5.3% for transect 2. Coverage data are reported in Table 1. In terms of landscape occupancy, taking into account the presence of the host plant, for transect 1 occupancy reached 21.5 ha for 8.24 km2 (occupancy: 2.6%) and for transect 2 occupancy was 7.5 ha for 6.36 km2 (occupancy: 1.2%). Occupancy, when taking into account only zones where the coverage of A. cochliacantha exceeded 65%, was 24.7% for transect 1 and 22.25% for transect 2.
Density of adults in each of the plots
Among the six reference plots, the number of adults observed was 160 individuals/ha in Barranca Honda (Zone 4; Fig. 2 and Table 3) and up to 1300 individuals/ha in Cerro Frio (Zone 2; Fig. 2 and Table 3) with an average of 840±423 individuals/ha. Our estimates indicated that average population patches of this butterfly included at least 2500 individuals (mean of the six plots sampled: 840 individuals/ha×average surface of the six studied sites: 3 ha). When extrapolating these data to the average area encountered for our 26 plots B. brevicornis population patches are seen to include between 400 and 6500 individuals with an average of 2400 (which is almost the value obtained using only our six reference plots). Most of the plots with high densities of individuals (>600/ha) were located along the sides of the Amacuzac River in south-central Morelos State (Zones 1 and 2; Fig. 2).
Table 3 Number of Baronia brevicornis adults per ha.

Highlighted in grey our six reference plots where counting was performed more precisely than for the other 20 plots (see Materials and methods for details). *Estimation of 150–300 individuals/ha; **300–600 individuals/ha; ***600–1000 individuals/ha; ****more than 1000 individuals/ha.
How many generations per year?
Hatching occurred throughout the rainy season (from late May to November), with one generation every six to eight weeks (Table 4). However, only the first generation was abundant, the others being partial, becoming progressively smaller with time. Some pairing was observed in the relatively abundant second generation, from late July to the beginning of August (see picture in Warren et al. Reference Warren, Davis, Grishin, Pelham and Stangeland2010) but the following generations gave few individuals (Table 4). Counting individuals was performed from 2006 to 2008 but presence/absence was noted at the end of July every year from 2005 to 2013. A maximum of four occurrence periods per year were recorded (Table 4). As we have no record of caterpillars for occurrences in September and November, only the occurrence in July is considered as a second partial effective generation (followed by reproduction). Mating occurred mainly on the highest branches of the host plant and very rarely on the ground as in most Parnassiini.
Table 4 Occurrence of Baronia brevicornis along two years.

*Hatching in captivity from 200 caterpillars collected in the field (100 submitted to local precipitations “Natural” and 100 kept sheltered from the rain “Dry”); **observations in the field in the six locations studied. When not specified, observation of adults.
In captivity, among the 100 chrysalides placed in “natural” conditions, 90% successfully produced an adult, versus 73% in “dry” conditions (Table 4) showing statistically significant differences (test of proportions, χ2=8.49, df=1, P=0.0036). A few of the chrysalides emerged after two years in “natural” conditions, and one emerged after two years in “dry” conditions.
Predation and parasitism
As very few events were recorded, only a short description of the main events is provided. Surprisingly, amongst the 200 caterpillars collected in the field (fifth instar), no hatching of parasitoids was observed in captivity. The only parasitoid observed in the field was a Trichogramma Westwood (Hymenoptera: Trichogrammatidae) species found on B. brevicornis eggs (see picture in Warren et al. Reference Warren, Davis, Grishin, Pelham and Stangeland2010). These tiny wasps may not have occurred naturally, as they are released in large numbers for biological control of certain pests in sugarcane fields close to where they were observed (road Jojutla/Chinameca). Trichogramma chilonis Ishii (Hymenoptera: Trichogrammatidae) is the most commonly used in the region.
Very few natural predators of adults, or caterpillars, were observed in the locations studied: a very common stinkbug (Brochymena Amyot and Serville or Parabrochymena Larivière (Heteroptera: Pentatomidae), see picture in Warren et al. Reference Warren, Davis, Grishin, Pelham and Stangeland2010) and two lizards identified as Aspidoscelis communis Cope and Aspidoscelis deppeii Wiegmann (Squamata: Teiidae) (Chávez-Juárez et al. Reference Chávez-Juárez, Dorado and Arias2010). The stinkbug and these species of lizard were observed everywhere in the region independently of the presence of either B. brevicornis or A. cochliacantha. In some localities (mainly Quilamula), some Calosoma sayi Dejean (Coleoptera: Carabidae) individuals were observed eating caterpillars. Curiously, no birds were observed eating either adults or caterpillars.
Modelling distribution
When comparing the potential distribution of B. brevicornis (Fig. 4B) with the distribution of the species in the literature (Fig. 1) we note the almost perfect geographical congruence even though the population densities are fairly different depending on the region. Almost all the populations deduced by modelling are situated among the Balsas and Papaloapan river basins (Fig. 4A) and only few points are outside this range. The resolution of this modelling approach is high with every single pixel (isolated point on the map), corresponding to <1 km2, only three of these points occur outside the Balsas basin (circled in red in Fig. 4B). From west to east, the first two are situated in the south Pacific division of the Mexican dry tropical forest (Fig. 4A); a first spot that corresponds to Chamela Reserve (Jalisco State) (Beutelspacher Reference Beutelspacher1982), a second situated south of Colima volcanoes and corresponding to known populations of Mixcoate (Colima), and the third isolated between the Isthmus of Tehuantepec and Chiapas central depression (Fig. 4A). In addition, two isolated points were included in the Balsas basin corresponding to known populations of the “Boqueron Cañon” in Oaxaca State. Except for the Chiapas spot (situated far south of “Sumidero Cañon” where the local Chiapas subspecies, B. brevicornis rufodiscalis occurs) all the geographic predictions of our model correspond strictly to known populations of B. brevicornis (Fig. 1).

Fig. 4 Subdivisions of the dry tropical forest in Mexico and modelling distribution of Baronia brevicornis. (A) Main subdivisions of the tropical dry forest of Mexico according to Trejo-Vazquez (Reference Trejo-Vazquez1999) modified from Secretaria de Programación y Presupuesto (1981). Scale in km. (B) Potential distribution of B. brevicornis using Bioclim2 script in “R” under conditions described in Materials and methods. Each pixel (dot on the map) corresponds to a square of 926 m2. (C) Error representation of our model based on receiver operating characteristic (ROC) and true skill statistic (TSS) threshold (dark green: 6–8%; light green 4–6%; light orange 2–4% and dark orange, <2%).
Using ROC and TSS (see Materials and methods) thresholds, we obtained a map (Fig. 4C) corresponding to the error risk of our model. Most of the green zones (Fig. 4C), (indicating the highest error range of our model) are situated in zones where the host plant, A. cochliacantha, is not recorded in the field especially in the California, Peninsula and south of Mexico (Fig. 1).
Discussion
Abiotic constraints
Many of our data are in accordance with those published in the 1960s and 1970s (Vázquez and Pérez Reference Vázquez and Pérez1962; Pérez Reference Pérez1967, Reference Pérez1972, Reference Pérez1979; Pérez and Sánchez Reference Pérez and Sánchez1986) but at the local scale of Morelos, our study provides new and important information about the natural history and ecology of B. brevicornis.
Our results concerning abiotic conditions are fully in accordance with those of Pérez (Reference Pérez1979). Their conclusions were that the association of Baronia with Acacia Miller (Fabaceae) (over the whole range of this species) occurs mainly in the zones with “Awo” type climate: precipitations from 600 to 1100 mm, temperature of 22 °C with a maximum annual oscillation of 7 °C and elevation between 505 and 1335 m. As the lowest locality in Morelos is around 700 m, we were unable to confirm the lowest value but only three of the 26 population patches (average elevation: 1125 m) were slightly above the upper limit of 1350 m and no occurrences were found above 1400 m. No plots of B. brevicornis occurrence were found along the north of Morelos State, which is above this elevation (Fig. 2). Like Pérez (Reference Pérez1979), we had to use the records of the nearest meteorological stations and not exact measurements from the localities where B. brevicornis occurs. Our values of 24 °C with an annual oscillation of 6 °C are perfectly compatible with those of Pérez (Reference Pérez1979). Rather than average temperature, oscillations between the coldest month (January in Morelos) and hottest one (April/May depending on the year) seems to be critical for this butterfly which was totally absent when the temperature variation amplitude was > 6 °C.
The orientation of the slope also seems to be a key factor since this species seems to be absent or rare when the morning sun (orientation south-east) is not present. This is in accordance with a major peak of activity in the morning for males while females fly later in the day, as mentioned by Vázquez and Pérez (Reference Vázquez and Pérez1962).
Adult distribution and densities
As butterflies are flying insects, a common view is to imagine great dispersal possibilities over a large territory. However, studies have regularly shown that many species occupy tiny patches (Hanski et al. Reference Hanski, Alho and Moilanen2000; Ricouart et al. Reference Ricouart, Cereghino, Gers, Winterton and Legal2013). Among Nymphalidae, the dispersal ability per day, of most species, is not more than 900 m and more likely around 200 m (Ribeiro et al. Reference Ribeiro, Batista, Prado, Brown and Freitas2012; Tufto et al. Reference Tufto, Lande, Ringsby, Engen, Sæther and Walla2012) even though notable exceptions exist for Hesperiidae and other families (Shapiro Reference Shapiro1977). Baronia brevicornis seems to be among the very static species as few individuals were found outside of the limits of their population patches. Even though we found few plots of 1 ha or less, most population patches were about 3 ha (often around 175×175 m, which are values compatible with the most static Nymphalidae species; Ribeiro et al. Reference Ribeiro, Batista, Prado, Brown and Freitas2012; Tufto et al. Reference Tufto, Lande, Ringsby, Engen, Sæther and Walla2012). We observed a dynamic system of colonisation/extinction between stable plots, forming temporary settlements. Such a dynamic system is characteristic of a metapopulation structure, and seems to be frequent for extremely localised butterflies such as the Palaearctic species Euphydryas aurinia Doubleday (Lepidoptera: Nymphalidae) (Hula et al. Reference Hula, Konvicka, Pavlicko and Fric2004; Fric et al. Reference Fric, Hula, Klimova, Zimmermann and Konvicka2010; Zimmermann et al. Reference Zimmermann, Blazkova, Cizek, Fric, Hula and Kepka2011). As with B. brevicornis, these species possess some restricted territories but often with huge local densities of individuals (often>100 individuals/ha).
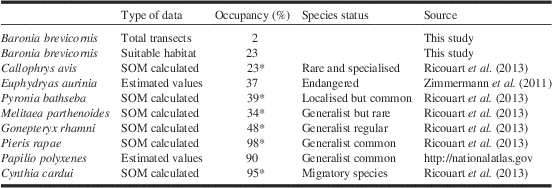
Plant density is often considered as an important factor. It has been demonstrated that the greater the quantity of host plants available the lesser the movement of female Euphydryas anicia (Odendaal et al. Reference Odendaal, Turchin and Stermitz1989). However, the counter effect leading to female dispersion due to sexual harassment by the males, described by Odendaal et al. (Reference Odendaal, Turchin and Stermitz1989), was not observed for B. brevicornis. Our results indicate that a population patch of B. brevicornis needs at least 70% coverage by its host plant (average 78%) as reported by Léon-Cortés et al. (2004). Our total sample covered around 2000 km2 but a special effort was made in the central part of the REBIOSH (central South of the Morelos State) on an area of 500 km2 where we found 20 of our 26 population patches (Table 1 and Fig. 2) and where we located our two transects (Figs 2 and 3). Earlier studies report that B. brevicornis occupies no more than 5–10% (depending on the models or studies) of the total distribution of its host plant (Léon-Cortés et al. 2004; Soberón and Townsen-Petersen Reference Soberón and Townsend-Peterson2005). In the present study we found occupancy of 2.6% for transect 1 with high numbers of individuals/patch and 1.2% for transect 2 with much lower numbers. These values are lower than those from earlier studies (Léon-Cortés et al. 2004; Soberón and Townsen-Petersen Reference Soberón and Townsend-Peterson2005) due mainly to the greater accuracy of our sampling (Fig. 2 and Table 3). Anyway, it is important to introduce the concept of “real” suitable habitat. Presence of the host plant is a necessary criterion, but not the only one. To host a plot of B. brevicornis food plant coverage of greater than 70% was shown to be a key condition. When performing occupancy calculations, taking into account the area effectively representing a suitable habitat for this species, we reached values of around 23%. Such values can be compared with those obtained for the endangered Palaearctic nymphalid, E. aurinia (Hula et al. Reference Hula, Konvicka, Pavlicko and Fric2004; Fric et al. Reference Fric, Hula, Klimova, Zimmermann and Konvicka2010; Zimmermann et al. Reference Zimmermann, Blazkova, Cizek, Fric, Hula and Kepka2011). This species occupies 1.44 km2 of a study zone in the Czech Republic covering 3316 km2 (occupancy 4/10 000), but when measuring the total area of suitable habitat (depending on the presence of the host plant and other ecological factors), occupancy reaches 37% (area of suitable habitat: 3.87 km2, 0.11% of the studied region). In other words, measuring occupancy implies calculating the probability of collecting a species in a suitable habitat (these data are generally available a posteriori, based on exhaustive sampling in specific study sites). We proposed this approach in a recent paper (Ricouart et al. Reference Ricouart, Cereghino, Gers, Winterton and Legal2013) and obtained values from 25% for rare and/or specialised species to 100% for common generalist species. We have summarised a selection of these results Table 5.
Table 5 Occupancies (%) in suitable habitat or Footnote *probabilities of collection in suitable habitat (SOM=Neuron network calculation) for a sampling of butterfly species.

* Probabilities of collection in suitable habitat for a sampling of butterfly species.
Comparison with B. brevicornis; Total transect: criteria presence of host-plant in considered area; suitable habitat: criteria covering of host-plant>70% in considered area.
Baronia brevicornis is a species that lives in locations where the drought stress is high with no rain at least from December to April (some years from early October to the end of May). Such conditions require the species to be adapted to survive during the dry season as a chrysalis in the ground with few weeks to achieve larval development at the beginning of the rainy season. Earlier studies claimed that there is only a single generation per year, in early June (Vázquez and Pérez Reference Vázquez and Pérez1962; Léon-Cortés et al. 2004), it was thus a surprise to find adults flying in Morelos throughout the rainy season, and also mating. Our conclusion is that this species possesses a kind of “safety mechanism” with an extremely abundant first generation early June but with partial generations later during the rainy season (only the second generation – end of July beginning of August – quite numerous, as verified in the field from 2005 to 2013). Another key point is that a certain proportion of chrysalides hatched after two years in the ground even with zero rainfall. Although we found significantly lower hatching under “dry” conditions the still high percentage of success in the terrarium indicates elevated resistance to desiccation. These two elements are important in terms of population dynamics but also for conservation considerations.
An aspect that can play an important role in the presence, or at least in the density of a species, is the level of predation and parasitism. For example, in Europe, experiments on Limenitis populi Linnaeus (Lepidoptera: Nymphalidae) have shown that 90% of overwintering caterpillars are eaten by the great tit (Parus major Linnaeus) and blue tit (Cyanistes caeruleus Linnaeus) (Aves: Paridae) (Ligue Suisse pour la Protection de la Nature 1987). Up to 95% of the caterpillars of Arctia flavia Fuessly (Lepidoptera: Arctiidae) collected in the field were reported to have parasites (Leraut Reference Leraut2006). A totally unexpected result here was that, of the 200 fifth-instar larvae collected in the field, none showed the presence of parasites. A first suspicion of an efficient chemical defense is therefore proposed. In the case of B. brevicornis, it seems that both predation and parasitism remain at very low levels. A few carnivorous insects and lizards but no birds, were observed preying on the caterpillars.
Chemical clues and evolutionary perspectives
Fabaceae are known to produce a wide range of cyanogenic glycosides (Vetter Reference Vetter2000; Seigler Reference Seigler2003; Wink Reference Wink2003; Wink and Mohamed Reference Wink and Mohamed2003). These compounds are mainly toxic by the release of hydrogen cyanide, which binds to cytochromes, blocking the respiratory chain and thus muscular contraction (Poulton Reference Poulton1983). Cyanogenic glycosides are present in a large number of plant families (Francisco and Pimenta-Pinotti Reference Francisco and Pimenta-Pinotti2000) and interestingly are also abundant in Crassulaceae, Fumariaceae, and Saxifragaceae (Nishida Reference Nishida1995, Reference Nishida2002; Lechtenberg and Nahrstedt Reference Lechtenberg and Nahrstedt1999), which are the main host plants for Parnassiini; the butterfly tribe closest to B. brevicornis. The origins of the Crassulaceae lineage are contemporary to that of B. brevicornis (69–77 million years ago) and interestingly a centre of distribution of one basal tribe (Echeverioideae and some Sedum Linnaeus (Crassulaceae)) of this family is strictly limited to arid/dry areas of Mexico (Van Ham and Hart Reference Van Ham and Hart1998; Forest and Chase Reference Forest and Chase2009). Note that the starting point of the lineage leading to the Acacia genus is dated at around 40 million years ago (Clarke et al. Reference Clarke, Downie and Seigler2000) so is much more recent than all the estimated ages of B. brevicornis even the revised value proposed recently by Simonsen et al. (Reference Simonsen, Zakharov, Djernaes, Cotton, Vane-Wright and Sperling2011): 68 million years ago. Therefore, it must be assumed that the shift to Acacia as host plant is a derived adaptive character as hypothesised for the phylogenetically nearest species from Iran, H. helios, on Zygophylaceae (Clarke et al. Reference Clarke, Downie and Seigler2000; Michel et al. Reference Michel, Rebourg, Cosson and Descimon2008). Host-plant specificity and shifts for some Lepidoptera species/tribes have already been described as being more dependent on chemistry than on plant taxonomy (Aubert et al. Reference Aubert, Legal, Descimon and Michel1999; Wahlberg Reference Wahlberg2001).
This proposed shift of host plant can be explained by the sudden radiation of legume trees in the New World (Clarke et al. Reference Clarke, Downie and Seigler2000), which provided an unexpected and abundant source of attractive chemical compounds for B. brevicornis. Our hypothesis is that the current distribution of B. brevicornis can be explained by the evolutionary radiation of the Acacia genus 40 million years ago.
Baronia brevicornis is assumed to be a Nearctic element (Pérez Reference Pérez1979) and may represent the last surviving species of the lineage that led to Parnassiini and Zerynthiini and/or even all the Papilionidae (Simonsen et al. Reference Simonsen, Zakharov, Djernaes, Cotton, Vane-Wright and Sperling2011). Taking into account larval morphological characteristics (Pérez Reference Pérez1967), the chemical clues described above and some molecular studies (Michel et al. Reference Michel, Rebourg, Cosson and Descimon2008), we favour a closer proximity with Parnassiini. Anyway, some ecological traits of life, such as the occurrence of a single main generation at the very beginning of the most favourable period of the year, the habitat restricted to dry hills below 1400 m and reproduction events (mainly) on host plants are elements that place this species closer to most of the Zerynthiini.
Congruence field distribution and modelling
As for B. brevicornis, the major distribution of A. cochliacantha is situated in the Balsas and Papaloapan river basins (Figs 1 and 4A). However, populations of the host plant are found in most of the regions where subdivisions of the tropical dry forest occur in Mexico (Figs 1 and 4A). During the Pleistocene, the influence of glaciation events was relatively mild in this region but with changes in levels of precipitations, a very dry period occurred 6000 years before present and another less marked one around 1000 years before present (Metcalfe et al. Reference Metcalfe, O’Hara, Caballero and Davies2000). This meant that tropical dry forest cover was greater in Mexico and that optimal conditions for B. brevicornis were possibly more widely distributed. Such events, may explain of the current isolated populations such as those of Jalisco, Colima, Oaxaca, and Chiapas. Following our model (Fig. 3A), current conditions are still optimal in the first three regions but in Chiapas, at least not in the zone where small populations of the subspecies B. brevicornis rufodiscalis occur. We may hypothesise that optimal conditions for this subspecies already differ for nominal subspecies as already suspected by Pérez (Reference Pérez1979) (this is confirmed by the absence or errors in our model in the area close to Sumidero cañon, Fig. 4C), but we cannot ignore the possibility that accidental or deliberate introduction occurred (followed by a bottleneck effect to explain the rare original female forms encountered in Chiapas) around the 1960s. More specific observations and genetic work is therefore necessary to reach a decision as to the likelihood of an artificial introduction or more probably to search for bias in our model design. It is interesting to note that a model using only climatological parameters can be so close to real/field distribution of a flying insect.
Conservation perspectives and conclusions
Baronia brevicornis lives on a pioneer plant. This means that areas of human disturbance are readily colonised by A. cochliacantha (e.g., abandoned fields or roadsides). Therefore, the distribution of the host plant of B. brevicornis is frequently related to human activity (Léon-Cortés et al. 2004). As the plant is almost absent from primary forest, the question that arises is “What was the original biotope of this shrub?” On the basis of historical local reports, confirmed by the oldest farmers of the region, it seems that before the development of agriculture and urbanisation, the shrub occupied all the flat lowland areas of the State of Morelos. When checking on the few conserved flat areas of Morelos (Área de Conservación Ecológica “El Texcal”, Jiutepec) and those situated in the adjacent more conserved State of Guerrero, this assertion was verified.
All the necessary restoration programs in progress in the Sierra of Huautla Reserve may in fact lead to a strong reduction of suitable environments for B. brevicornis as the areas covered by A. cochliacantha will slowly contract. This conclusion was also drawn for the populations in Chiapas (Léon-Cortés et al. 2004). Our recommendations are to keep these restoration programmes in the mountainous areas and extend the protected areas to the adjacent plateau in order to fix and maintain stable populations of both A. cochliacantha and B. brevicornis.
It seems clear that B. brevicornis has a large population from the centre of the State of Morelos to Guerrero, Puebla and few isolated ones in the extreme southwest of the State of Mexico (Warren et al. Reference Warren, Davis, Grishin, Pelham and Stangeland2010). However, only small and restricted population plots subsist, but still with high density, as demonstrated on some other Lepidoptera species (Thomas et al. Reference Thomas, Bulman and Robert2008; Zimmermann et al. Reference Zimmermann, Blazkova, Cizek, Fric, Hula and Kepka2011). The current situation seems to correspond to a metapopulation structure without the possibility of distinguishing if it represents the original population structure for B. brevicornis or an intermediate step due to accelerated fragmentation of a single widespread ancestral population. In a restricted area, we showed that B. brevicornis occupies around 25% of what we defined to be its suitable habitat. This value is among the lowest found using comparable measurements for European species. The issue is to determine whether B. brevicornis belongs to the most endangered species of butterflies or if our definition of “real” suitable habitat needs to be revised. We noticed that females seem to almost exclusively prefer to lay eggs on older trees (data not shown), an additional constraint in relation with the age of Acacia settlement that is suspected to explain the restricted distribution of this insect and also our low values of suitable habitat occupancies. Therefore, considering the present findings, the International Union for Conservation of Nature (IUCN) categorisation as “Lower Risks/Near Threatened” is highly questionable and could be updated to “Vulnerable” even though we agree that the protection of biotopes should be favoured over protection of single species.
Acknowledgements
Thanks are due to the two directors of Centro de Educación Ambiental e Investigación Sierra de Huautla (CEAMISH) and Centro de Investigación en Biodiversidad y Conservación (CIByC), Dulce Arias and David Valenzuela, for their constant encouragement and for providing us administrative and field facilities to carry out this study. Many thanks to all those who helped us in the field, Marcela Osorio, Cecilia Torres, many students of the University of Morelos (Universidad Autónoma del Estado de Morelos, UAEM), and farmers living in the REBIOSH. Special thanks to Víctor Toledo Hernández who provided us local authorisations under the authority of the SEMARNAT FAUT-0178 (Secretaría de Medio Ambiente y Recursos Naturales) permit. We would like to thank Holger Weissenberger of El Colegio de la Frontera Sur (ECOSUR) for drawing the map of Fig. 1. Special thanks to Kalina Bermudez-Torres for her critical assessment of early versions of this manuscript and all the valuable information provided on the structure and dynamics of the Mexican tropical dry forest.