Introduction
Colonisation of North America by European settlers has been accompanied by a shift in the composition of the insect assemblage associated with cattle dung. Of the estimated 300 species that comprise this assemblage in Canada, perhaps 50% are of European origin (Floate Reference Floate2011). Many of these species likely first arrived in maritime ports when ships from Europe discharged their ballasts of soil or sand upon arrival (Brown Reference Brown1940). From these initial introductions, the species have continued to spread across the continent and become established members of the cow dung community (Blume Reference Blume1985).
Species of dung beetles (Coleoptera: Scarabaeidae) are of particular interest. Their tunnelling, feeding, and breeding activities in cattle dung accelerate the cycling of nutrients in pasture ecosystems. These activities return nitrogen to the soil, fragment pats that otherwise block the growth of new forage, and reduce the suitability of pats as breeding sites for pest flies and parasites that affect cattle. The economic value of these activities in North America may exceed several hundred million dollars per year (Fincher Reference Fincher1981; Nichols et al. Reference Nichols, Spector, Louzada, Larsen, Amequita and Favila2008).
The most effective species of dung-degrading beetles are “tunnellers” and “rollers”. Depending upon their numbers, members of these functional groups can scatter and bury a dung pat in less than a week. Adults arrive at fresh pats to remove small pieces of manure. Tunnellers bury this manure in chambers that they excavate beneath the pat (e.g., see fig. 1 in Bornemissza Reference Bornemissza1970). Rollers bury the manure in chambers excavated more distant from the pat. Females lay an egg in a cavity within the buried manure to form a brood ball that nourishes the beetle grub when it hatches. Tunnelling and dung burial enhances soil fertility, aeration, and water permeability. “Dwellers” form a third functional group that degrade dung pats over a period of weeks and do not tunnel in the soil. Instead, eggs are laid within the pat where subsequent larval feeding slowly degrades it to the consistency of sawdust. Presently, dung beetle assemblages on northern pastures in North America are dominated mainly by European species of dwellers (Floate and Gill Reference Floate and Gill1998; Lobo Reference Lobo2000; Floate and Kadiri Reference Floate and Kadiri2013; Kadiri et al. Reference Kadiri, Lumaret and Floate2014).
The current paper reports results of three studies to determine the likelihood of the exotic tunnelling species Onthophagus taurus (Schreber) establishing in southern Alberta, Canada. In the first study, we determined egg-to-adult development times at constant temperatures of 10–32 °C. These data were used to estimate the minimum temperature threshold for development and the accumulative degree days (DD) needed by O. taurus to complete one generation. We also collected these data on Onthophagus nuchicornis (Linnaeus) and Digitonthophagus gazella (Fabricius). The former is a European species already established in the region and served as a “positive” control. The latter is an Afro-Asian species and served as a “negative” control. In the second study, we tested predictions arising from the temperature development study by introducing populations of O. taurus and D. gazella into outdoor field cages to assess reproduction and overwintering survival. In the third study, we compared soil temperatures in southern Alberta to a site at the northern distributional limits of O. taurus. The combined results show that O. taurus can reproduce and overwinter in southern Alberta, but long-term persistence of populations in the region is unlikely.
Materials and methods
Distribution and source of species
The establishment and spread of O. nuchicornis, O. taurus, and D. gazella in North America is reviewed by Hoebeke and Beucke (Reference Hoebeke and Beucke1997) and updated by MacRae and Penn (Reference MacRae and Penn2001). Onthophagus nuchicornis became established in northeastern North America before the 1840s. It was first reported in western North America in British Columbia in 1945 and is now a common member of the cow dung community across southern Canada and adjacent states (Floate and Gill Reference Floate and Gill1998; Tinerella and Fauske Reference Tinerella and Fauske1999; Rounds and Floate Reference Rounds and Floate2012; Floate and Kadiri Reference Floate and Kadiri2013). Onthophagus taurus was first recovered in North America in 1971 in Florida (Fincher and Woodruff Reference Fincher and Woodruff1975). It has rapidly expanded its distribution northward and recently was reported near Lake City, Michigan, United States of America (Rounds and Floate Reference Rounds and Floate2012). Digitonthophagus gazella (formerly Onthophagus gazella) was deliberately introduced into the United States of America in the early 1970s (Blume and Aga Reference Blume and Aga1978). Within North America, it now occurs in the southern states from Florida to Arizona as far north as northwestern Mississippi (Hoebeke and Beucke Reference Hoebeke and Beucke1997) and further south into Mexico (Montes de Oca and Halffter Reference Montes de Oca and Halffter1998).
For the current study, adult O. taurus and D. gazella were field-collected in North Carolina and shipped to the Lethbridge Research Centre (CFIA Import Permit No. P-2008–01907). Upon receipt, beetles were continuously reared in laboratory culture using plastic pails (11 L, 27 cm diameter×24 cm depth) with a 20-cm layer of tightly packed soil. The soil comprised a mix of fine vermiculite, peat moss, washed sand, and Cornell mix in a ratio of 1.6:1.5:1.3:1. Cornell mix is a soilless potting medium developed by Cornell University (Boodley and Sheldrake Reference Boodley and Sheldrake1982). The mixture was moistened sufficiently to allow formation into a ball when firmly gripped, but without exuding water; i.e., damp, not wet. Ten to 15 pairs of beetles were added to each pail and fed two to three times weekly with fresh dung (~300 mL per feeding) from cattle fed a diet of grass hay or silage (80% barley silage, 17% rolled barley, 3% supplement). Cattle treated with parasiticides can faecally excrete insecticidal residues for several months post-treatment (Floate et al. Reference Floate, Wardhaugh, Boxall and Sherratt2005; Floate et al. Reference Floate, Bouchard, Holroyd, Poulin and Wellicome2008). Thus, dung was collected from cattle that had not been treated with parasiticides in the previous four months. Each pail was fitted with a lid into which was cut a screened opening (~7 cm diameter) for ventilation. Pails were held under constant conditions of 27 °C and 16:8 light:dark photoperiod.
Adult O. nuchicornis were collected in the spring using dung-baited pitfall traps on pastures near Lethbridge, Alberta. They were held in pails of soil as were the other two species. In contrast to O. taurus and D. gazella, we only were able to maintain O. nuchicornis for one generation in laboratory culture.
Temperature development study
To assess the effect of temperature on development time, reproductively mature beetles were placed in fresh pails of soil and provided with dung. After two days, the soil in these pails was sifted to recover brood balls. Three to seven brood balls were placed in individual plastic containers and covered with a layer of moist vermiculite. Containers then were placed in temperature cells (one container per cell) on a 100-cell thermal gradient plate (McLaughlin et al. Reference McLaughlin, Bowes, Thomas, Dyck, Lindsay and Wise1985) and held at constant (±0.5 °C) temperatures of 10 °C, 12 °C, 14 °C, 16 °C, 18 °C, 20 °C, 22 °C, 24 °C, 26 °C, 28 °C, 30 °C, and 32 °C (seven to eight cells per temperature). Temperatures were monitored periodically during the study to ensure accuracy. This process was repeated for each species. Because of the greater availability of brood balls, measurements for O. taurus were repeated at temperatures of 22 °C and higher.
Containers were examined daily to detect the emergence of new adults. In such cases, the beetle was removed and its sex and time to emergence were recorded. For this purpose, it was assumed that eggs were laid on the day that brood balls were recovered from pails, and that adults emerged from brood balls on the day that they completed development. When two weeks had passed at a given temperature with no further emergence of adults, remaining brood balls held at that same temperature were broken apart to assess the fate of the occupant. Upon examination, many brood balls were found to lack a hollow cavity, or which contained a cavity with no indication of an egg. This was attributed to the disruption of nesting behaviour and oviposition at the time brood balls were removed from the laboratory colonies.
The effect of sex on developmental time first was assessed with analysis of variance (ANOVA) tests (P=0.05) using data for the temperature from which the greatest number of adults emerged. These data were normally distributed (Shapiro–Wilk test, P>0.11) except for a small deviation with data for male O. taurus (Shapiro–Wilk test, P=0.038). Because ANOVA tests are robust against deviations from normality, data were not transformed before analyses. Data were then combined across temperatures to increase sample sizes. With one exception, these combined data sets deviated greatly from normality (Shapiro–Wilk test, P<0.001). Hence, Kruskal–Wallis tests (P=0.05) were employed.
Minimum threshold temperatures and the accumulative DDs required for development were estimated for each species with two approaches. We first used the x-intercept method (Arnold Reference Arnold1959). Egg-to-adult development times (time) were plotted as 1/time on the y-axis with temperature (°C) on the x-axis. We then determined the equation that described the linear function that best fit the plotted data. Development times at the highest temperatures for O. taurus (32 °C) and O. nuchicornis (30 °C and 32 °C) indicated that these temperatures were beyond the optimal range for development. Hence, these data were excluded from calculations. The equations were used to calculate the minimum temperature for development (the x-intercept) and accumulative DDs (1/slope).
We then estimated the minimum threshold and required DDs based directly on observations of egg hatch and time to development at the lowest temperature for which adult emergence was observed.
Field cage study
To assess overwintering survival in southern Alberta, field cages were established at the Lethbridge Research Centre in 2009 (49.698°N, 112.770°W). Cages comprised plastic tubs (61×40×42 cm deep) buried in the ground with the top 10 cm projecting above the soil surface. Tubs were filled with firmly packed soil to a depth of 30 cm and provisioned with fresh cattle dung (~500 mL) each week until winter (October). The soil was of the same type described for the rearing of the dung beetles in laboratory culture. Screened windows in the bottom of tubs allowed for drainage. Lids with screened openings (20×40 cm) allowed for ventilation. Two cages were set up every second week from June through August (eight cages in total) with one cage each stocked with adult O. taurus or adult D. gazella (50–95 per tub).
In May 2010, the soil in all cages was removed and carefully examined to recover adult beetles and brood balls, which were broken apart to determine the fate of their occupants. In June 2010, field cages were filled with fresh soil, stocked with O. taurus and weekly provisioned with fresh dung. Soil in the cages was removed in May 2011 and carefully examined to recover adult beetles and brood balls. Based on preliminary results from the temperature development study, D. gazella was excluded from the second year of the overwintering study.
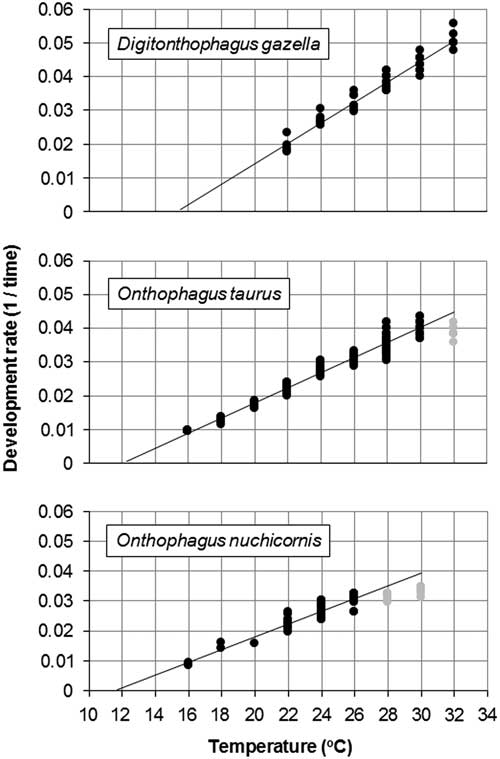
Soil temperatures at depths of 0, 10, and 20 cm in one cage were recorded hourly from 6 June 2009 to 5 June 2010 using a HOBO data logger (Onset, Cape Cod, Massachusetts, United States of America). These hourly values were used to determine if sufficient DDs would accumulate during the year to allow egg-to-adult development for the test species. Digitonthophagus gazella develops in brood balls at depths of 5–51 cm (Fincher and Hunter Reference Fincher and Hunter1989). Onthophagus taurus was assumed to develop at similar depths. Onthophagus nuchicornis develops in brood balls at depths of 5–15 cm (Burmeister Reference Burmeister1930).
Results
Temperature development study
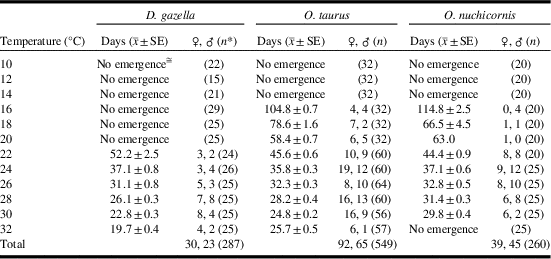
The greatest number of adults emerged from temperatures of 28 (D. gazella) and 24 °C (O. taurus, O. nuchicornis) (Table 1). Results of ANOVA tests on these data did not detect an effect of sex on development time; i.e., D. gazella (F(1,13)=0.909, P=0.358), O. taurus (F(1,29)=1.627, P=0.212), O. nuchicornis (F(1,19)<0.001, P=0.984). Similarly, no effect of sex on development time was detected when data were combined across temperatures; e.g., D. gazella (H=0.824, P=0.364), O. taurus (H=0.828, P=0.363), O. nuchicornis (H=1.450, P=0.229). Data for male and female beetles were therefore combined for subsequent analyses.
Table 1 Egg-to-adult development times for the dung beetles Digitonthophagus gazella, Onthophagus taurus, and Onthophagus nuchicornis when held at constant temperatures of 10–32 °C. Means are calculated for both sexes combined.
Notes:
* n=number of brood balls from which adults could potentially have emerged.
≅ No adult emergence. Brood balls may have contained dead immature stages or may not have contained an egg.
Digitonthophagus gazella
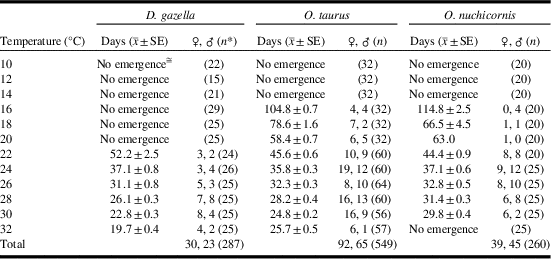
Egg-to-adult development occurred from 22–32 °C (Table 1). Average development time ranged from 20 days at 32 °C to 52 days at 22 °C. Development time was predicted by the equation: y=93720x −2.452 (R 2=0.9391, n=53) with y=time and x=temperature (Fig. 1A). The predictive power of the equation was weakest at 22 °C, for which the estimated development time was four days shorter than what was observed.
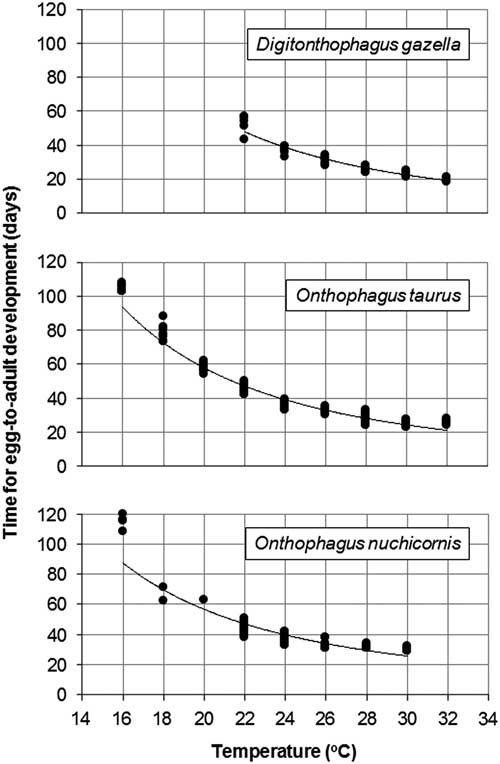
Fig. 1 Egg-to-adult development time for Digitonthophagus gazella, Onthophagus taurus, and Onthophagus nuchicornis at constant temperatures of 10–32 °C (see Table 1).
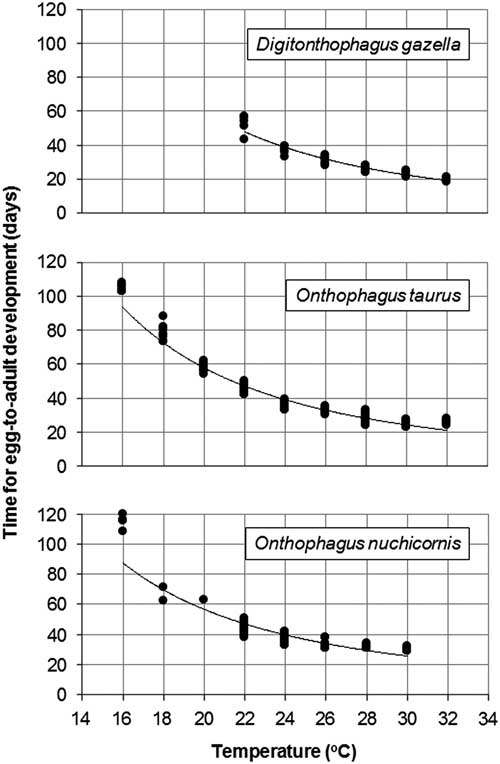
The x-intercept method identified a minimum developmental threshold of 15.5 °C and a requirement of 333 DDs for egg-to-adult development (Fig. 2A). Based on hourly field cage temperatures (Fig. 3) and this threshold, a total of 150 and 82 DD accumulated at soil depths of 10 and 20 cm, respectively, between 6 June 2009 and 5 June 2010.
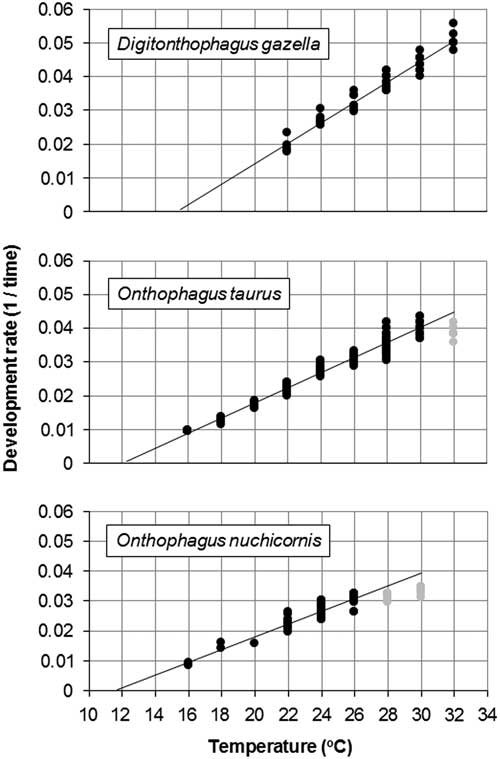
Fig. 2 Minimum development thresholds (x-intercepts) for Digitonthophagus gazella, Onthophagus taurus, and Onthophagus nuchicornis based on development rates at constant temperatures of 10–32 °C (see Table 1). Development rate is 1/egg-to-adult development (days). Data points in grey are excluded in calculations of linear regressions to increase accuracy of predicting minimum development thresholds.
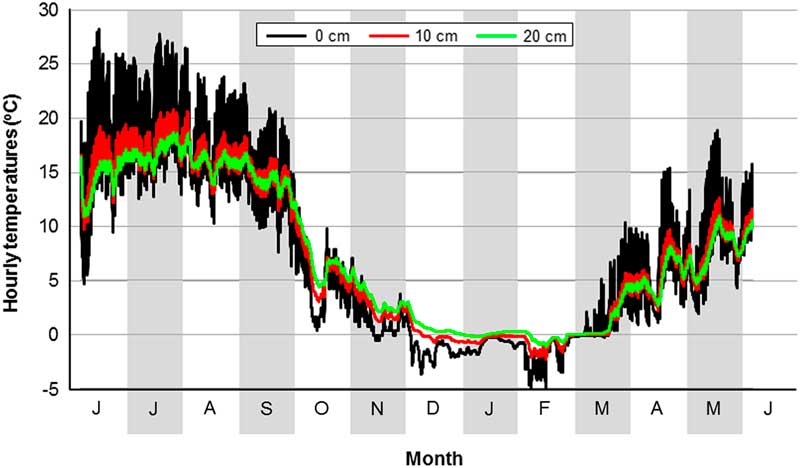
Fig. 3 Hourly temperatures in field cages at soil depths of 0, 10, and 20 cm at Lethbridge, Alberta, Canada (6 June 2009 to 5 June 2010).
Based on our temperature development study, a minimum development threshold of 18 °C may be more appropriate. Dissection of intact brood balls after 80 days at temperatures below 22 °C recovered five second-instars and one third-instar at 20 °C. One first-instar and one second-instar were recovered at 18 °C. All of these immature stages were dead. There was no indication of egg hatch below 18 °C. A development time of 52.2 days at 22 °C (Table 1) with a minimum development threshold of 18 °C equates to 209 DD. For a development threshold of 18 °C, accumulated DDs in field cages ranged from 18 (10 cm) to 3 (20 cm).
Onthophagus taurus
Egg-to-adult development occurred from 16–32 °C (Table 1). Average development time ranged from 25 days at 30 °C to 105 days at 16 °C. Between 16 °C and 32 °C, development time was predicted by the equation: y=36561x -2.153 (R 2=0.9526, n=157) (Fig. 1B). The strength of the relationship between predicted versus observed development was weakest at 16 °C for which the former (93) was 12 days shorter than the latter (105).
The x-intercept method identified a minimum developmental threshold of 12.3 °C and a total requirement of 455 DDs (Fig. 2B). Using this threshold and hourly field cage temperatures (Fig. 3), accumulated DDs ranged from 446 (10 cm) to 372 (20 cm) between 6 June 2009 and 5 June 2010.
Conversely, a minimum threshold of 14 °C may be more appropriate. Brood ball dissections identified only four cases of egg hatch below 16 °C. Two live teneral adults were recovered from brood balls in a temperature cell set to operate 14 °C, but which subsequently was determined to be operating at 15.3 °C. Two dead second-instars were recovered from a second cell operating at 14 °C. There was no evidence of egg hatch below this temperature. Using 14 °C as the minimum threshold, and based on an average development time of 105 days at 16 °C (Table 1), 210 DDs are required for immature development. For a development threshold of 14 °C, accumulated DDs in field cages ranged from 268 (10 cm) to 191 (20 cm).
Onthophagus nuchicornis
Egg-to-adult development occurred from 16–30 °C (Table 1). No adults emerged from brood balls held at 32 °C. Average development time ranged from 30 days at 30 °C to 115 days at 16 °C. Within this temperature range, development time was predicted by the equation: y=19362x -1.947 (R 2=0.8654, n=53) (Fig. 1C). The strength of the relationship between predicted versus observed development was weakest at 16 °C for which the former (87) was 28 days shorter than the latter (115).
The x-intercept method identified a minimum development threshold of 11.7 °C and a total requirement of 476 DDs (Fig. 2C). Using this threshold and hourly field cage temperatures (Fig. 3), accumulated DDs ranged from 514 (10 cm) to 439 (20 cm) between 6 June 2009 and 5 June 2010.
Similar to O. taurus, a minimum threshold of 14 °C may be more appropriate. Brood ball dissections detected only four cases of egg hatch below 16 °C. From cells operating at 14 °C, a dead third-instar and three dead second-instars were recovered. There was no evidence of egg hatch below 14 °C. A development time of 115 days at 16 °C (Table 1) for a threshold of 14 °C equates to 230 DDs. With this threshold, accumulated DDs in field cages ranged from 268 (10 cm) to 191 (20 cm).
Field cage study
Onthophagus taurus, but not D. gazella, completed egg-to-adult development in outdoor field cages. A total of 119 adult O. taurus were placed in cages in summer of 2009. Eight live adults were recovered in May 2010. Their pristine condition and the presence of brood balls with emergence holes identified the specimens as the F1 progeny of adults placed in cages in 2009. A total of 146 adults were placed in cages in summer of 2010, but no live adults were recovered in May 2011. Only dead adult D. gazella were recovered from cages. These were in various states of decay and were undoubtedly individuals placed in cages the previous summer.
The field cage study also assessed overwintering survival. Adult D. gazella placed in cages in autumn hypothetically could have survived to emerge in the following spring. Our a priori prediction was that this would not occur and indeed this was the case. As an Afro-Asian species, D. gazella was expected to have little tolerance for cold temperatures. For example, Fincher and Hunter (Reference Fincher and Hunter1989) reported that adults were unable to survive for more than a few days when held in soil at temperatures below 7 °C. During the first year of our field cage study, soil temperatures at depths of 10–20 cm were below 7 °C for about six months (Fig. 3).
The recovery of live adult O. taurus identifies a much higher level of cold tolerance than that exhibited by D. gazella. For most of the period from December through March, temperatures at a depth of 10 cm were below 0 and −3 °C and only slightly warmer at a depth of 20 cm (Fig. 3).
Discussion
Developmental times and degree-day models
The development times that we obtained in our temperature study were faster than previous reports for D. gazella, but similar to reports for O. taurus. We found egg-to-adult development of D. gazella to average 23 days at 30 °C, whereas Blume and Aga (Reference Blume and Aga1978) reported development in 30 days at 29 °C. We observed an average development time of 32 days for O. taurus at 26 °C, similar to a report of 33 days at this temperature (Wardhaugh et al. Reference Wardhaugh, Holter and Longstaff2001). We are unaware of any previous reports for development times of O. nuchicornis.
Degree-day models support our a priori expectation that D. gazella, which we used as a negative control, cannot establish in southern Alberta. For a minimum development threshold of 15.5 °C (x-intercept method), the estimated DD requirement for egg-to adult development was 333 DD. During one year of measurements, the maximum accumulated DD calculated with this threshold at a soil depth of 10 cm were only 150. For a threshold of 18 °C (brood ball dissections), an estimated 209 DDs were required. With this threshold at the same soil depth, maximum accumulated DDs were 18. In summary, there are insufficient DDs for D. gazella to reproduce in the region. This conclusion is consistent with the absence of new adults in field cages.
Degree-day models support the hypothesis that O. taurus can reproduce in Alberta. For a threshold of 12.3 °C (x-intercept method), an estimated 455 DDs were needed for development. For this threshold, accumulative DDs were 446 (at 10 cm). For a threshold of 14 °C (brood ball dissections), an estimated 210 DDs were needed. For this threshold, accumulated DDs were 268 (at 10 cm). Thermal requirements for O. taurus to reproduce in the region are met with either threshold and are verified by the recovery of F1 adults in field cages.
Degree-day models are consistent with the ability of O. nuchicornis to establish in Alberta. This species, which we used as a positive control, was first recorded in Alberta in the 1960s and is now among the most abundant members of the local dung beetle assemblage (Floate and Gill Reference Floate and Gill1998; Floate and Kadiri Reference Floate and Kadiri2013). For a threshold 11.7 °C (x-intercept method), an estimated 476 DDs were needed for complete development. Accumulated DDs for this threshold were 514 (at 10 cm). For a threshold of 14 °C (brood ball dissections), an estimated 230 DDs were needed. For this threshold, maximum accumulated DDs were 268 (at 10 cm).
Likelihood of establishment
Although O. taurus and O. nuchicornis share many attributes, it remains uncertain whether the former can establish viable populations in southern Alberta. They have comparable DD requirements and minimum development thresholds. They both can reproduce and overwinter in the region, and they share similar patterns of seasonal activity. In regions of co-occurrence, overwintered adults emerge in spring to lay eggs that produce an autumn peak of F1 adults (Rounds and Floate Reference Rounds and Floate2012). However, the low recovery of F1 adults from field cages shows O. taurus to be less cold-tolerant than O. nuchicornis. A pair of O. taurus will produce a lifetime total of about 15–30 brood balls under ideal conditions (Lee and Peng Reference Lee and Peng1982; Hunt et al. Reference Hunt, Simmons and Kotiaho2002). We added about 150 females to field cages in 2009 and 2010, but recovered only eight F1 progeny. This negative rate of replacement would preclude local establishment of O. taurus.
Lack of an obligatory diapause is a key difference between O. taurus and O. nuchicornis that may limit the northern distribution of the former species. Onthophagus taurus can be reared continuously in laboratory culture and may have four to five generations per year in southern regions of the United States of America (Bertone et al. Reference Bertone, Green, Washburn, Poore, Sorenson and Watson2005). In contrast, we determined by accident that O. nuchicornis appears to have an obligatory diapause. For the temperature development study, we obtained brood balls from overwintered adult O. nuchicornis field-collected in spring. We obtained an F1 generation in laboratory culture, but F1 progeny did not produce brood balls. In an unrelated study, we subsequently sent 2400 adults obtained in autumn field collections to eight institutions. Although the shipments were received in good condition, our colleagues also failed to observe reproductive behaviour in these non-overwintered individuals (Floate et al. Reference Floate, Blanckenhorn, Coghlin, Davies, Gray and Höhn2011). Thus, an adult overwintering period seems to be a requirement for O. nuchicornis reproduction.
We foresee the lack of a diapause in O. taurus as having two main consequences. First, reproductive activity prior to winter would reduce population fitness. Eggs or immature stages arising from eggs laid in autumn would likely not survive to spring. Oviposition also would reduce the energy reserves of adults and their overwintering survival. In contrast, oviposition by O. nuchicornis begins only after overwintered adults have emerged in spring. Second, diapause is often linked with cold-tolerance (Pullin Reference Pullin1996). Hence, it is reasonable to assume that adult O. taurus will have higher overwintering mortality than O. nuchicornis.
Field cage effect
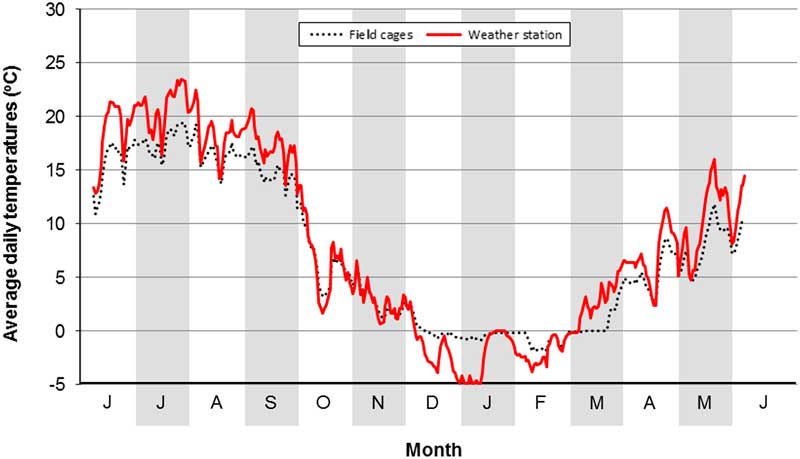
A confounding factor in our field study was the presence of a cage effect. This was identified by comparing soil temperatures at a depth of 10 cm in a field cage with soil temperatures at the same depth and period obtained from a weather station at the Lethbridge Research Centre (Fig. 4). The comparison showed that soil temperatures in cages were cooler in summer and warmer in winter. The former is attributed to a shading effect of the lids on field cages. The latter is attributed to greater snow cover in cages than in surrounding sites. Differences in soil type and moisture also may be contributing factors.
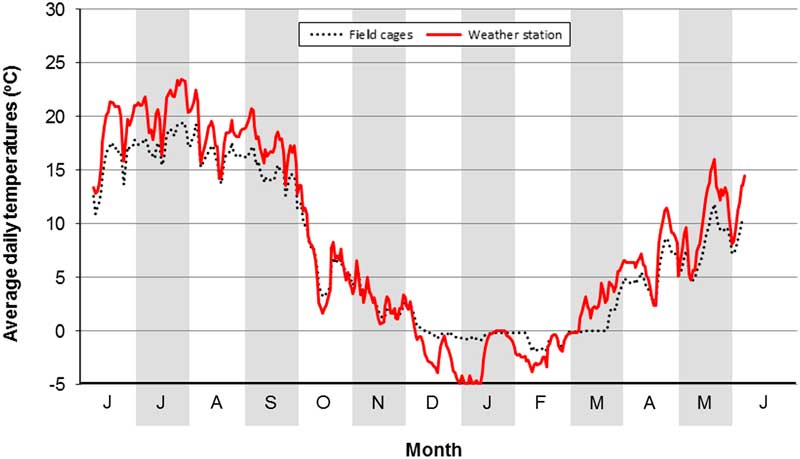
Fig. 4 Evidence of a field cage effect. Values are average daily temperatures at a soil depth of 10 cm at Lethbridge, Alberta, Canada (6 June 2009 to 5 June 2010). Data are from a field cage versus a weather station at the Lethbridge Research Centre, Alberta, Canada.
This confounding factor has contrasting implications. Because of warmer summer soil temperatures, the number of cumulative DDs outside of field cages is higher than is identified in our cage study. This provides further confidence that O. taurus can complete a generation in southern Alberta. Conversely, because of colder winter soil temperatures, overwintering survival outside of field cages is likely lower than indicated in our cage study. Thus, the small number of F1 adults recovered in cages in spring of 2010 may be a field cage artefact.
Temperature comparisons with Lake City, Michigan
The study by Rounds and Floate (Reference Rounds and Floate2012) provides an indirect way to address the likelihood of O. taurus establishing in southern Alberta. In a study near Lake City, Michigan, the authors reported the co-occurrence of O. nuchicornis and O. taurus and provided data on seasonal activity. To our knowledge, this is the northernmost report of O. taurus populations in North America.
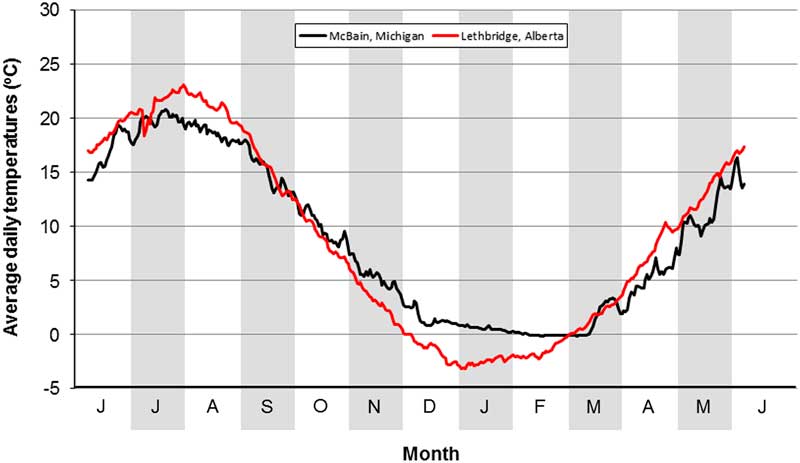
If overwintering mortality is the key factor that limits the distribution of O. taurus, it is instructive to compare soil temperatures between the Michigan and Lethbridge locations. For this purpose, we obtained data records for the period 2009–2014 for McBain, Michigan (http://www.agweather.geo.msu.edu/mawn/station.asp?id=mcb&rt=24). McBain is 11 km SE of Lake City (44.194°N, 85.213°W). We also obtained data records from a weather station at the Lethbridge Research Centre (49.698°N, 112.770°W) for the period 1981–2010. Data comprised average daily temperatures at a soil depth of 10 cm, calculated as the (daily maximum+daily minimum)/2.
The O. taurus that overwintered in our field cage study survived average daily soil temperatures of −2.1 °C for one day, of −1.8 °C for one week, and of −1.0 °C for one month (Fig. 4). At McBain, February is the coldest month, during which daily soil temperatures average −0.1 °C (minimum=−0.2 °C in 2014; maximum=0.3 °C in 2012) (Fig. 5). These values are above those experienced by overwintered beetles at Lethbridge, which is consistent with the presence of O. taurus at McBain. At Lethbridge, January is the coldest month, during which daily soil temperatures average −2.4 °C (minimum=−7.3 °C in 1982; maximum=0.9 °C in 1994) (Fig. 5). These values are below those experienced by the overwintered beetles and predict the inability of O. taurus to establish in the region.
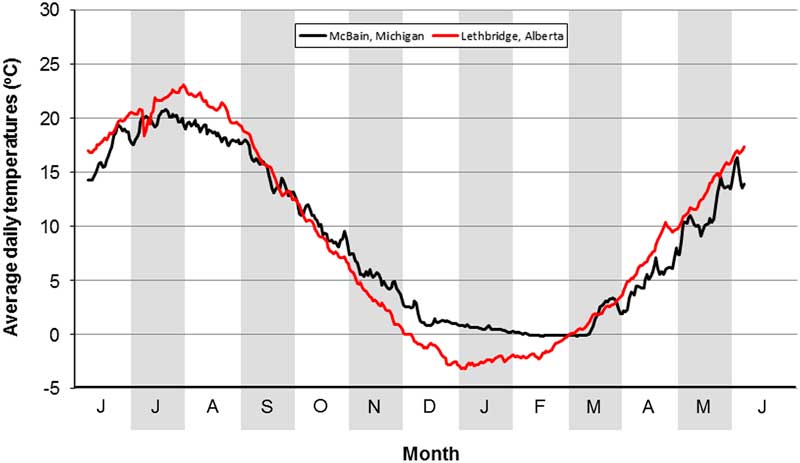
Fig. 5 Long-term average daily soil temperatures at a depth of 10 cm at the Lethbridge Research Centre, Alberta, Canada (1981–2010) and McBain, Michigan, United States of America (2009–2014).
Soil temperatures are not recorded by most weather stations across North America, but use of air temperatures provides an indirect method of predicting where O. taurus might establish elsewhere in Canada. The United States Department of Agriculture has produced a temperature map that categorises regions of the United States of America into zones based on 30-year averages (1976–2005) of annual extreme minimum air temperatures (http://planthardiness.ars.usda.gov). An equivalent map is available for Canada (http://planthardiness.gc.ca/index.pl?m=1&lang=en). The population of O. taurus at Lake City is in Zone 5a (−28.9 °C to −26.1 °C). In Canada, Zone 5a and warmer zones encompass the coastal and interior regions of southern British Columbia, southern Ontario, Prince Edward Island, and parts of Newfoundland and Nova Scotia, Canada. Assuming that air temperature is a good correlate of soil temperature and that overwintering mortality is a key factor limiting its northern distribution, these regions may be more suited to future efforts to establish O. taurus in Canada.
Acknowledgements
The authors thank R. Barrett, S. Denning, P. Fletcher, M. Youssef, K. Tiberg, J. Cote, and M. Thompson for their contributions in the collection and rearing of the dung beetles used in this study. This is Lethbridge Research Centre Publication Number 38714020.