Introduction
Carabid beetles play an important role in agro-ecosystems because of their predatory and/or granivorous habits (Zhang Reference Zhang1993; Kromp Reference Kromp1999). Examples of carabid beetles contributing to pest control include Pterostichus melanarius (Illiger) for the control of earwigs and possibly Lepidoptera caterpillars, and Poecilus lucublandus (Say) for the control of Lepidoptera caterpillars (Larochelle and Larivière Reference Larochelle and Larivière2003). In no-till (NT) corn, greater armyworm damage was observed when carabids were removed (Clark et al. Reference Clark, Luna, Stone and Youngman1994). Carabid beetles can have an impact on weed population dynamics by consuming large quantities of seed, and thus significantly reduce the size of the weed seedbank (Zhang et al. Reference Zhang, Drummond, Liebman and Hartke1997; Westerman et al. Reference Westerman, Wes, Kropff and Van der Werf2003). Because of feeding preferences (Lundgren and Rosentrater Reference Lundgren and Rosentrater2007; White et al. Reference White, Renner, Menalled and Landis2007), seed predation by carabids can also affect weed emergence, weed community structure, and possibly weed–crop interactions resulting in crop yield loss (House Reference House1989; Hartke et al. Reference Hartke, Drummond and Liebman1998; Menalled et al. Reference Menalled, Smith, Dauer and Fox2007).
Biotic and abiotic conditions created by agricultural practices strongly influence carabid community structure and population size by determining available food and shelter at various times during their life cycle. Tillage intensity affects the amount, quality, and location of the crop residues, and alters soil physical properties such as porosity, soil moisture content, and temperature, which are important for soil organisms (Kromp Reference Kromp1999; Kladivko Reference Kladivko2001). The loss of canopy cover increased soil surface temperature and decreased soil moisture, creating unfavourable conditions for many of the larger carabid species and their prey (Brust Reference Brust1990). Carabid abundance and diversity would depend largely on the quality of ground cover (Brust Reference Brust1990; Cárcamo et al. Reference Cárcamo, Niemalä and Spence1995).
Tillage can also physically harm insects. Moldboard ploughing inverts the soil and exposes insect larvae to predators and to more extreme environmental conditions (Thiele Reference Thiele1977). Susceptibility of carabid larvae to tillage depends on overwintering depth in the soil (Hartke et al. Reference Hartke, Drummond and Liebman1998). The absence of tillage (NT) generally favours abundance and diversity of carabid beetles, partly because of the stable physical conditions in NT compared with tilled systems (House Reference House1989; Hance et al. Reference Hance, Grégoire-Wibo and Lebrun1990; Stinner and House Reference Stinner and House1990; Kromp Reference Kromp1999). Effects of tillage on carabids will also vary according to local conditions (Holland and Luff Reference Holland and Luff2000).
The type and sequence of crops grown over time influence invertebrates through the effects of planting dates, length of crop season, crop canopy architecture, pest control measures, and type and amount of crop residues (Curt and Truelove Reference Curt and Truelove1986; Anderson Reference Anderson2008). Narrow-row crops, crops with rapid canopy closure, and long-season crops favour abundance and diversity of insect predators by providing a moderate microclimate and diversified sources of food and shelter (Booij and Noorlander Reference Booij and Noorlander1992). Cover crops serve this same purpose, particularly in wide-row crops with relatively slow canopy closure. Accordingly, a red clover (Trifolium pratense Linnaeus; Fabaceae) cover crop increased the abundance and activity of granivorous insects in broccoli (Brassica oleracea var. botrytis Linnaeus; Brassicaceae)-squash (Cucurbita maxima Duchesne; Cucurbitaceae) cropping systems (Gallandt et al. Reference Gallandt, Molloy, Lynch and Drummond2005). Post-dispersal seed predation of giant foxtail (Setaria faberi Herrmann; Poaceae) increased twofold when a red clover cover crop was grown with wheat (Triticum aestivum Linnaeus; Poaceae) (Davis and Liebman Reference Davis and Liebman2003).
Pesticides have both direct and indirect effects on carabid populations. Insecticides generally have a direct impact on insect populations but carabids may avoid some of these negative effects because of their nocturnal habit (Çilgi and Jepson Reference Çilgi and Jepson1992). Herbicides will indirectly affect carabid populations by destroying shelter, particularly by suppressing vegetation between crop rows, and eliminating a potential food source by curtailing weed seed production (Brust Reference Brust1990; Zhang Reference Zhang1993; Holland and Luff Reference Holland and Luff2000; Taylor et al. Reference Taylor, Maxwell and Boik2006). The effect of pesticides could be more important for spring breeder species with adult phases during the spray period (Huusela-Veistola, Reference Huusela-Vesitola1996).
To our knowledge, very little is known on the effects of crop rotation and tillage on organisms of functional importance, such as carabid beetles, inhabiting cropping systems under cool humid climatic conditions. A study, initiated in 1987 at La Pocatière, Québec, Canada examined the potential of using conservation tillage in cereal-based crop rotations. A corn test crop, grown in 2006 at the end of the second crop rotation phase (Légère et al. Reference Légère, Stevenson and Vanasse2011b), offered the opportunity to measure cumulative and residual treatment effects on various components of agro-ecosystem biodiversity. Here, we report the effects of crop rotation and tillage on carabid species community structure, activity density and diversity after 18 years of treatment. We hypothesised that carabid activity density and diversity would be greater as tillage was reduced, more so following the crop rotation than the monoculture, and that carabid community structure would vary according to tillage and crop rotation.
Material and methods
The study, initiated in 1987, was conducted at the Centre de Développement Bioalimentaire du Québec at La Pocatière, Québec, Canada (47° 21′ N, 70° 02′ W). The experiment was conducted on a Kamouraska clay: a fine, mixed, frigid Typic Humaquept with 10% sand, 30% silt, and 60% clay in the surface horizon (pH = 6.1; organic matter = 50 g/kg; Mehlich 3 extractable nutrients: 149 kg P/ha and 558 kg K/ha). Treatments were set up in a split plot design with crop sequence as the main factor, tillage as the subfactor, and four replicates. The crop sequence factor compared a cereal monoculture treatment with a crop rotation treatment alternating a cereal crop with a broadleaf crop. The tillage factor included three treatments: moldboard plough (MP) in the fall (tillage depth: 15–18 cm), followed by spring secondary tillage; chisel plough (CP) in the fall (tillage depth: 12–15 cm), followed by spring secondary tillage; and no-till (NT). Experimental units (subplots) were 5 m (width) by 30 m (length), and were separated from one another by 10 m on the length axis and 3 m on the width axis. These buffer zones were left uncropped and mowed as needed.
Cropping history: 1987–2005
From 1988 to 1994, the two crop sequence treatments consisted of a spring barley (Hordeum vulgare Linnaeus; Poaceae) monoculture (M), and a 2-year cereal–forage rotation (R) (spring barley–red clover). All plots were planted to wheat (T. aestivum Linnaeus; Poaceae) in 1995–1996, and to barley in 1997. In 1998–2005, the spring barley monoculture was compared with a cereal–oilseed rotation (barley–canola [Brassica napus Linnaeus; Brassicaceae]–wheat–soybean [Glycine max (Linnaeus) Merrill; Fabaceae]). By 2005, the 4-year rotation had been repeated twice.
Tilled (MP and CP) plots were ploughed at the end of October every other year in the cereal–forage rotation (1987–1994) and every year thereafter. Spring secondary tillage consisted of two passes of a rigid-tooth finishing harrow. Management practices such as cultivar selection, seeding rates and dates, fertilisation, herbicide selection, and agrochemical application rates and timing followed provincial recommendations and best management practices. No insecticides were used throughout the study.
Field operations: 2006
Tilled plots (MP and CP) were harrowed on 30 May 2006. Glyphosate resistant ‘Baxxos’ corn was seeded at 74 000 plants/ha on 1 June 2006 in 76 cm rows. Fertilisation was set at approximately half of the recommended level to allow the expression of residual treatment effects in corn. Fertiliser was broadcast just prior to seeding (130 kg/ha of 14–10–10 N–P2O5–K2O), followed by a post-seeding broadcast application 10 days later (165 kg/ha of 27–0–0 + 25 kg/ha of 0–20–20 N–P2O5–K2O). Glyphosate was applied at 900 g acid equivalent/ha on 21 June 2006 in NT plots and on 5 July 2006 in MP and CP plots.
Average temperature (15.2°C) and total precipitation (411 mm) for May–September 2006 were similar to long-term means (15.2°C and 396 mm for temperature and precipitation, respectively, for the previous 18 years). However, some departures from mean monthly precipitation were observed, with May (+29%) and June (+52%) above average, and July–September (−23%, −22%, −15%) below the 18-year monthly averages (Table 1).
Table 1 Mean monthly temperature and total precipitation for the 2006 growing season compared to averages for the 1988-2006 study period at La Pocatière, Québec, Canada.

Data collection: 2006
Carabid beetle populations were estimated from propylene glycol pitfall traps once per month, five times during the season (May–September), a sampling intensity and frequency similar to that used by Menalled et al. (Reference Menalled, Smith, Dauer and Fox2007). At each sampling event, three pitfall traps were positioned at 7.5, 15.0, and 22.5 m in the centre of the plots and were collected 7 days later. Pitfall traps consisted of a 500 mL plastic container buried in the ground with its rim at the soil surface level so as to catch carabids moving about the plot. Insects were collected from the traps and kept in ethyl alcohol (70%) until their identification to the species level. Carabid species identification, food preference (i.e., predator, omnivore, or granivore), species status (i.e., exotic or native), and seasonal activity (i.e., spring or autumn breeders) were determined according to Lindroth (Reference Lindroth1961–1969), Larochelle (Reference Larochelle1990), and Larochelle and Larivière (Reference Larochelle and Larivière2003). Voucher specimens were deposited at the Laboratoire de diagnostic en phytoprotection (Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec, Québec, Canada). Hereafter, pitfall trap data will be referred to as activity density (sensu Bourassa et al. Reference Bourassa, Cárcamo, Spence, Blackshaw and Floate2010) to account for the relative effects of carabid density and mobility.
The small size of the plots relative to the mobility of many carabid species may have influenced some of our results. However, pitfall traps were separated by at least 16 m (lengthwise) and 8 m (crosswise) between treatments, which is close to the range of separation distance of 15.0–17.5 m suggested by Kujawa et al. (Reference Kujawa, Sobczyk and Kajak2006) for field crops. Also, our trapping intensity was comparable if not superior to that reported in the literature pertaining to experimental plot studies of cropping systems. Our sampling pressure corresponded to one trap per 50 m2 compared to one trap: per 25 m2 (Harvey et al. Reference Harvey, van der Putten, Turin, Wagenaar and Bezemer2008), per 62 m2 (Cárcamo et al. Reference Cárcamo, Niemalä and Spence1995), per 263 m2 (Bourassa et al. Reference Bourassa, Cárcamo, Spence, Blackshaw and Floate2010), per 378 m2 (O'Rourke et al. Reference O'Rourke, Heggenstaller, Liebman and Rice2006), and 465 m2 (Ellsbury et al. Reference Ellsbury, Powell, Forcella, Woodson, Clay and Riedell1998). Treatment effects observed in our data were confirmed statistically, in spite of this potential plot size-mobility limitation.
Statistical analyses
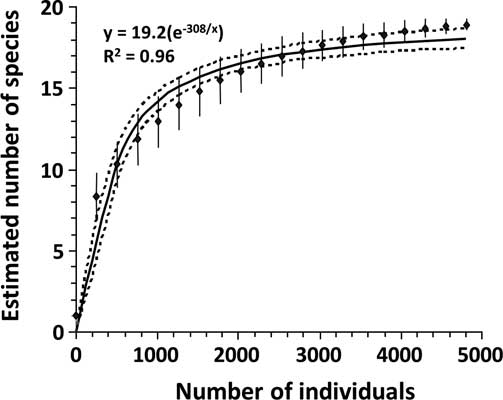
An individual-based rarefaction curve was computed, using data pooled over the entire sampling period (Gotelli and Colwell Reference Gotelli and Colwell2001; Buddle et al. Reference Buddle, Beguin and Bolduc2005). Estimates were obtained from EcoSim 7.0 (Gotelli and Entsminger Reference Gotelli and Entsminger2001) by random sampling of the dataset 1000 times. A negative exponential curve was fitted to the rarefaction curve, and the asymptote (y max) determined.
An analysis of variance for the activity density data of the five most abundant species was conducted separately for each date, using the GLIMMIX procedure of Statistical Analysis Systems (2005). Crop sequence, tillage, and date were considered as fixed effects, and replicate as a random effect. The analysis used a negative binomial probability distribution and log link function model specifications. The Shannon diversity index H′ and evenness E were calculated using the formula in Magurran (Reference Magurran2004). An analysis of variance was conducted for total carabid activity density, species richness, evenness, and diversity data, using the MIXED procedure of Statistical Analysis Systems (2005). Crop sequence, tillage and date were considered as fixed effects, and replicate as a random effect.
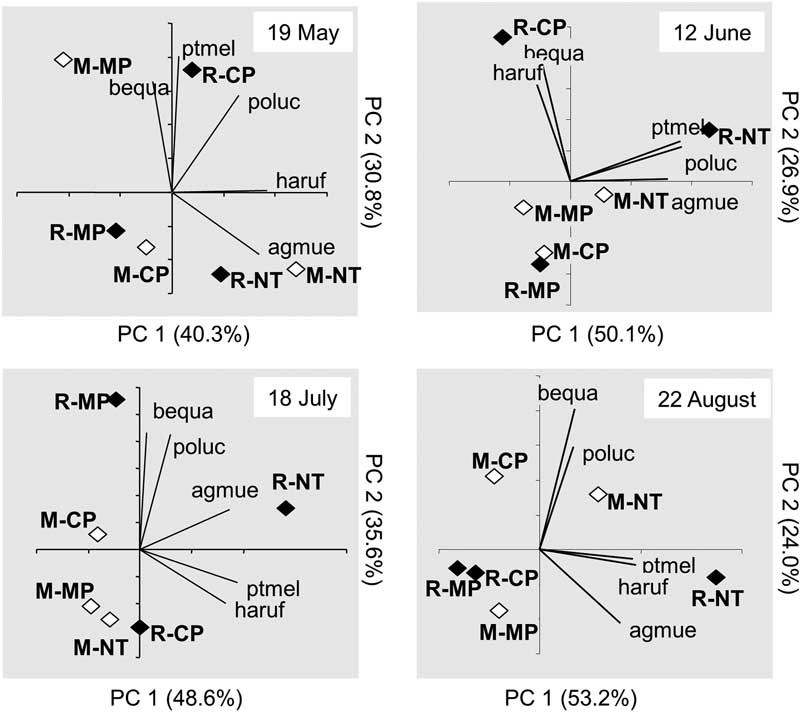
Multivariate analyses were conducted using the PRINQUAL procedure of Statistical Analysis Systems (2004) with a monotonic transformation. Data from the five most abundant species were analysed; these species were present in at least four out of the five sampling periods. The results were summarised in a biplot, with the mean principal component scores for treatments plotted as points in the ordination space. Eigenvectors (correlation between the transformed and original data) for the response variables were plotted as points at the end of vectors projecting from the origin into various positions in the ordination space. The lengths of the vectors indicated the strength of these correlations. The coincidence of carabid species vectors and treatments in the ordination space suggested associations/preferences between carabid species and treatments.
Results
The rarefaction curve suggested that sampling intensity had been sufficient to characterise carabid species richness at this site. Catching only a tenth (n = 500) of the total individuals (n = 5000) collected was sufficient to account for half of the estimated maximum carabid species richness at the site (19.2 species) (Fig. 1). Actual number of catches in each tillage treatment ranged from near twice to six times that number (n = 500; Appendix 1).
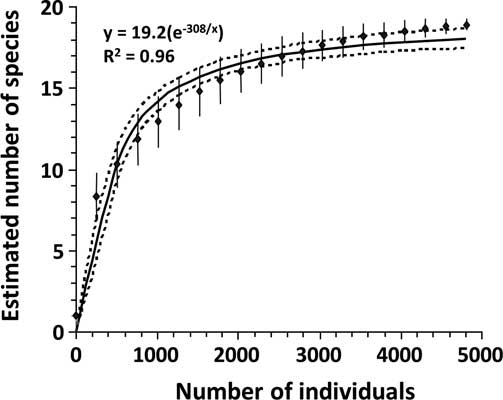
Fig. 1 Rarefied estimates (diamonds) of species richness with standard deviation (vertical line), and fitted curve (solid line) with 95% confidence interval (dotted lines) for carabid beetles sampled in corn at La Pocatière Québec, Canada.
Of the 19 carabid species found in the traps during the season, only four species were found at all five sampling dates (Appendix 1). Twelve species were observed in May, 11 species in June and August, but only seven species in July, and four species in September. Catches were low for most species, except for Harpalus rufipes (Appendix 1). Only five species averaged more than 1.0 individual per trap per plot on at least one sampling date. Harpalus rufipes accounted for 71% of all catches during the season whereas P. melanarius, Agonum muelleri (Herbst), and P. lucublandus accounted for 14%, 9%, and 3% of the total, respectively (Appendix 1). The other 15 species made up ∼3% of total catches. Eight species were granivorous, seven species were predators, and four species omnivorous (Appendix 1). Harpalus rufipes accounted for nearly all of the omnivorous catch. The other four dominant species were all predators. Three times as many carabids were collected from traps in NT (60.2%) than either tilled (MP: 17.9%; CP: 21.7%) treatments (Appendix 1). This trend was observed for many species, regardless of feeding habit.
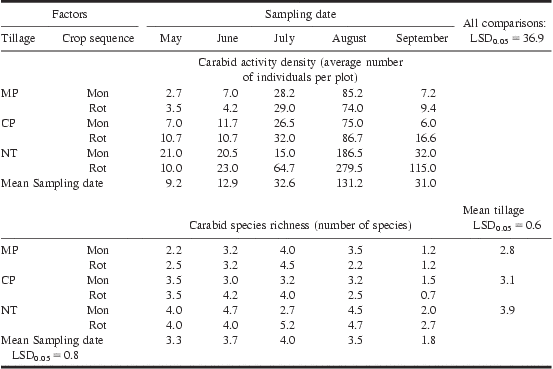
Crop sequence and tillage effects on total carabid activity density varied according to date (P = 0.04). On average, carabid activity density prior to corn seeding (May) was similar to post-seeding (June, July) activity density, but was greater in August than in other months (131 versus 21 carabids) (Table 2). In August and September, carabid activity density was greater in NT than in tilled systems, more so in the rotation than in the monoculture (Table 2). In August, 280 and 187 carabid beetles were observed in the NT rotation and NT monoculture, respectively, compared with an average of 80 carabids in other treatments.
Table 2 Response of carabid activity density to tillage, crop sequence, and sampling date, and of carabid species richness (number of carabid species) to tillage, crop sequence (not significant), and sampling date.

MP: moldboard plough; CP: chisel plough; NT: no-till; Mon: monoculture; Rot: rotation.
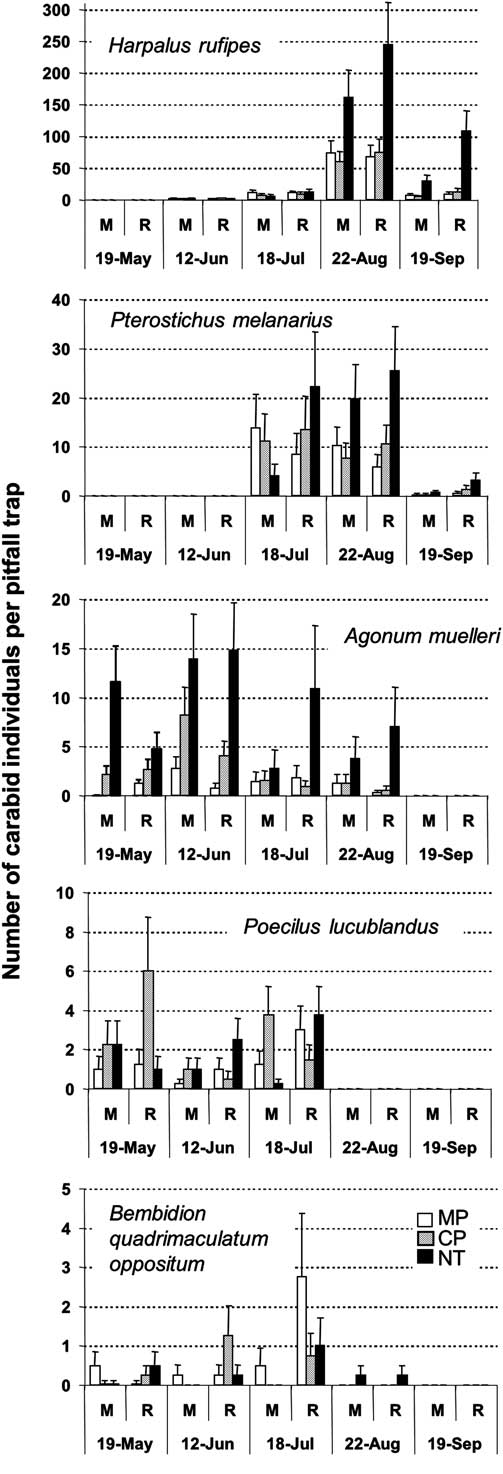
Peak activity density of dominant species occurred at different times during the season. Activity density for H. rufipes was greater in NT than in tilled treatments in August and September (P ≤ 0.001), and greater in the rotation than in the monoculture in September (P = 0.051) (Fig. 2). Pterostichus melanarius was observed in the second half of the season, mainly in July–August, and was more abundant for NT than the tilled plots in August (P = 0.021). Catches were low in September but activity density was greater in the rotation than in the monoculture (P = 0.031). Agonum muelleri was observed between May and August and was more abundant in NT than in tilled treatments (P ≤ 0.016). Poecilus lucublandus and Bembidion quadrimaculatum oppositum Say were observed in the first half of the season (Fig. 2). In May, activity density of P. lucublandus was greater in CP than in MP and NT for the rotation (P = 0.05); no tillage effect was observed for the monoculture (P = 0.558). Bembidion quadrimaculatum oppositum was present in most treatments from May through July but was not affected by any treatments.
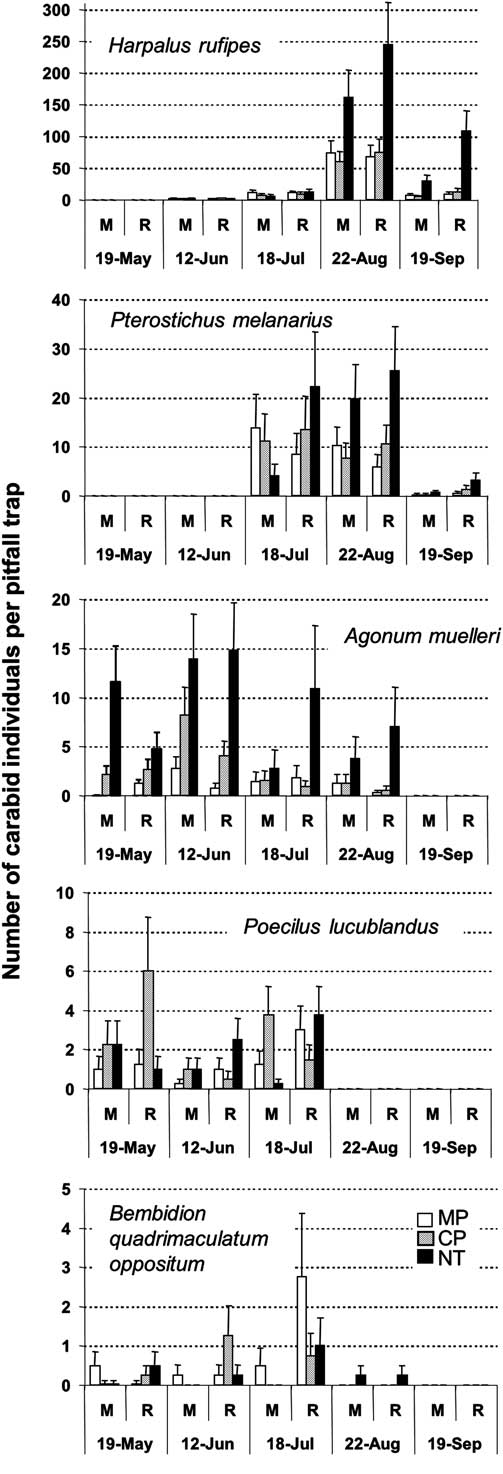
Fig. 2 Crop sequence and tillage effects on mean number of carabid per trap (with standard error bars) for five carabid beetle species observed at five sampling dates. Abbreviations: M, monoculture; R, rotation; MP, moldboard plough; CP, chisel plough; NT, no-till.
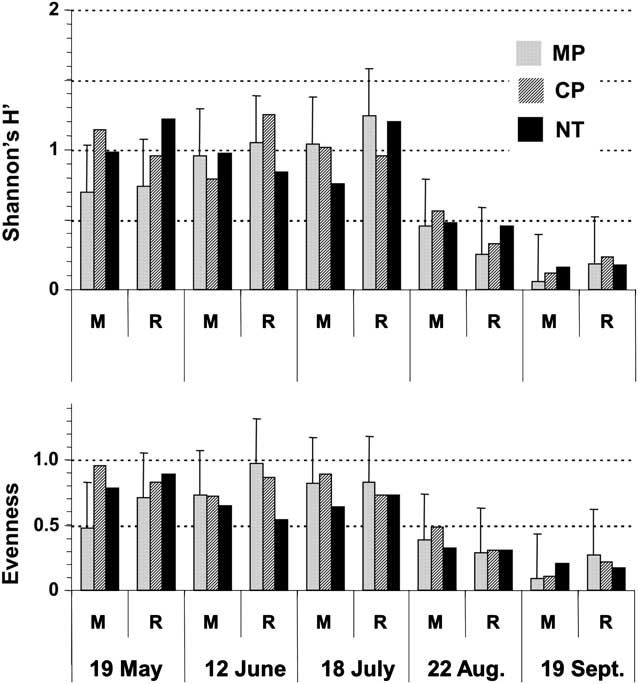
Carabid beetle diversity (H′), evenness (E) and species richness were affected by sampling date (P < 0.001) (Fig. 3). Carabid beetle diversity (H′) values were wide ranging (H′ = 0.1–1.3) and decreased over time, mainly because of the decrease for evenness in August and September (Fig. 3), and that of species richness (number of species) in September (Table 2). By August, evenness values had decreased nearly by half, corresponding with the clear dominance by H. rufipes (Fig. 2). On average, species richness in September (1.8 species) was half that observed in previous months (3.5 species) (Table 2). Also by September, the total number of carabid species observed across all traps had dropped down to 4 species, compared with 7–12 species on previous dates (Appendix 1). Species richness for the NT treatment (3.9 species) was greater than in tilled (MP: 2.8 species; CP: 3.1 species) treatments (P = 0.003).
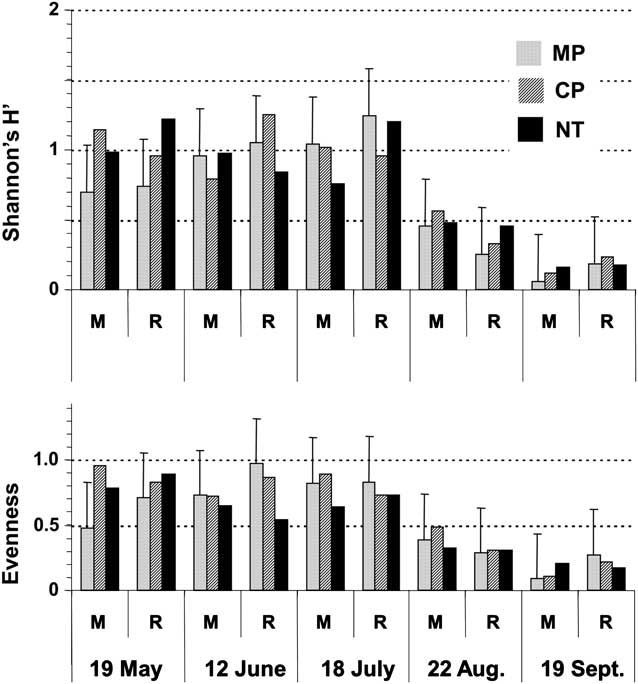
Fig. 3 Effects of crop sequence and tillage on diversity (H′) and evenness of carabid beetle communities measured at various dates during the season. Bars are for LSD0.05 values. Abbreviations: M, monoculture; R, rotation; MP, moldboard plough; CP, chisel plough; NT, no-till.
The cumulative variance for the first and second principal components of the multivariate analysis accounted for 71–84% of the total variance (Fig. 4). Treatments separated according to tillage and crop sequence on all dates, but treatment grouping and species associations varied from date to date. From June to August, the R-NT treatment occurred at the right end of the ordination space near the first principal component axis. The other treatment combinations often were either clustered close to the origin of the biplot or positioned further away to the upper or lower left portions of the ordination space. Harpalus rufipes, P. melanarius and A. muelleri often were associated with R-NT and R-CP treatments (Fig. 4). The other carabid species had no consistent association with any of the tillage or crop sequence treatments.
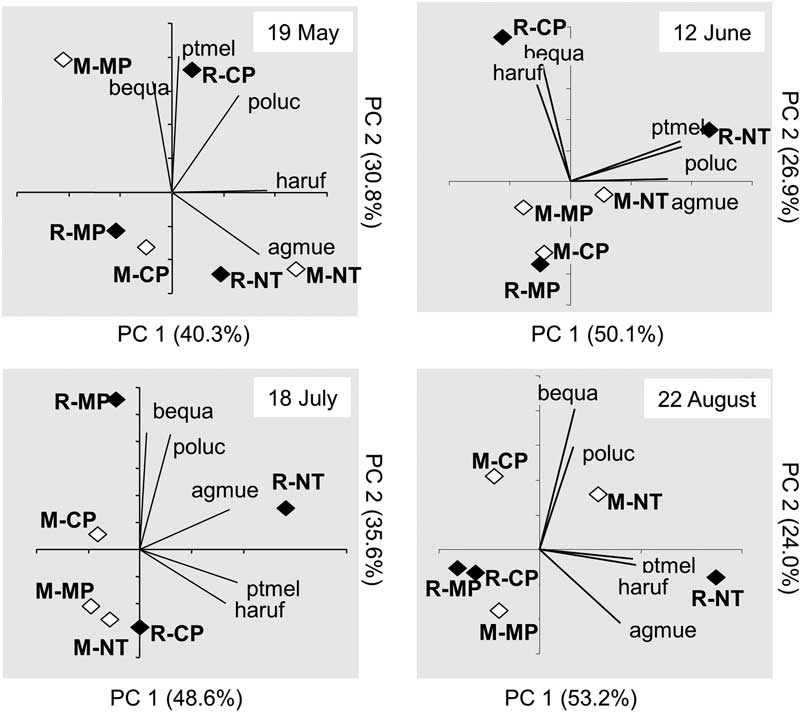
Fig. 4 Biplots illustrating results from the principal component analysis conducted for carabid beetle communities at various sampling dates. Abbreviations for carabid species: agmue, Agonum muelleri; bequa, Bembidion quadrimaculatum oppositum; haruf, Harpalus rufipes; poluc, Poecilus lucublandus; ptmel, Pterostichus melanarius; treatment abbreviations: M, monoculture; R, rotation; MP, moldboard plough; CP, chisel plough; NT, no-till.
Discussion
The carabid fauna observed in this study was typical of that found in open habitats in Québec (Larochelle and Larivière Reference Larochelle and Larivière2003). However, the total number of species captured appeared relatively low in comparison with another single year study, which reported 29 carabid species in barley fields in Alberta, Canada (Cárcamo Reference Cárcamo1995) compared with a total of 19 species in our study. Mercado Cárdenas and Buddle (Reference Mercado Cárdenas and Buddle2009) observed 23 and 30 species in corn fields in southern Québec. The number of ground and tiger beetles species observed in borders adjacent to corn fields in the Lanaudière region, Québec, was approximately three times greater than that recorded in our plots (Maisonhaute et al. Reference Maisonhaute, Peres-Neto and Lucas2010). Bourassa et al. (Reference Bourassa, Cárcamo, Spence, Blackshaw and Floate2010) recorded twice as many carabid species in experimental corn plots in western Canada. The reason for species scarcity in our study is unclear but may be related to the fact that sampling occurred only one week per month. However, Menalled et al. (Reference Menalled, Smith, Dauer and Fox2007) collected 33 species using a similar sampling scheme in a cropping systems study in Michigan, United States of America. The geographical location of the experimental site with its inherent agro-environmental limitations could maybe explain our results. La Pocatière is located at the north-eastern edge of the St-Laurent lowlands ecoregion where the climate is variable and characterised by temperature extremes and extended periods of wet or dry weather (Gullett and Skinner Reference Gullett and Skinner1992; Ecological Stratification Working Group 1994).
A lower number of carabid species was observed in July compared with May, June, and August. This may have been due to the reduced vegetation cover in the inter-row, following herbicide applications in late June–early July, which may have contributed to reduce spring breeder activity, not yet compensated by that of autumn breeders. In addition, the above-normal June precipitation may have delayed autumn breeder activity. The carabid fauna in our study was clearly dominated by only a few species. Carabid communities in hedgerows at La Pocatière were also dominated by only three species (Desbiens Reference Desbiens2010). Similarly, carabid communities in corn in southern Québec were dominated by two or three species (Mercado Cárdenas and Buddle Reference Mercado Cárdenas and Buddle2009). Four species accounted for 97% of total catches compared with 80% in the Northern Great Plains (Ellsbury et al. Reference Ellsbury, Powell, Forcella, Woodson, Clay and Riedell1998). In two studies of Western Canada farming systems, five carabid species accounted for 57% of total catches (Melnychuk et al. Reference Melnychuk, Olfert, Youngs and Gilliott2003), and eight species for 90% of total catches (Cárcamo et al. Reference Cárcamo, Niemalä and Spence1995).
Carabid diversity was low and varied only according to time. Contrary to our hypothesis and to results from mid-west cropping systems (Menalled et al. Reference Menalled, Smith, Dauer and Fox2007), crop sequence and tillage did not affect Shannon's diversity and evenness indices. Carabid diversity and evenness remained stable from May through July, but decreased to very low values in August and September, corresponding with the dominance of H. rufipes. This species accounted for 87% and 97% of the total carabid catches in August and September, respectively. However, tillage did affect carabid richness, particularly later in the season, with carabid species being more numerous in NT than in tilled systems. Most carabid species from this assemblage may have a similar colonising capacity such that multiple species could successfully occupy the same niche. In this study, it was not possible to determine which agro-environmental characteristic (e.g. habitat characteristics, Hatten et al. Reference Hatten, Bosque-Pérez, Johnson-Maynard and Eigenbrode2007) was more favourable to any particular carabid species.
Carabid community structure and activity density varied according to tillage, crop sequence, and time. Crop sequence affected carabid assemblage but to a lesser extent than tillage. Carabids may have been attracted to the more abundant and diversified food sources, resulting from 18 years of various crop types and cropping practices applied in the crop rotation treatment. For example, wheat was occasionally grown in the rotation treatment (1995, 1996, 2000, 2001, 2004, 2005), and may have harboured wheat midge populations, such as reported previously for this location by Mongrain et al. (Reference Mongrain, Couture, Dubuc and Comeau1997). The presence of midges could have positively influenced some of the omnivorous and predator species listed in Appendix 1. Indeed, many carabid species, including the dominant H. rufipes, occasionally had greater activity density in the crop rotation than in the monoculture treatments, although the statistical significance of this trend was often marginal.
Carabid assemblage and response to treatments varied over time, in accordance with the biological characteristics of the species. Early season carabid assemblage was composed mainly of three spring breeders with relatively low abundance. Agonum muelleri, which accounted for 9% of total carabid counts, was clearly associated with NT, likely because of its preference for residue cover (Larochelle and Larivière Reference Larochelle and Larivière2003), which provides overwintering shelter (Thiele Reference Thiele1977). The greater activity density of P. lucublandus in rotation–CP relative to other treatments in May, prior to secondary tillage, may have been due to its deep dwelling (≥ 25 cm) wintering habit (Larochelle and Larivière Reference Larochelle and Larivière2003), likely allowing it to escape the physical harm from the CP. In spite of no statistical differences, activity density data for the small-bodied (2.4 mm), diurnal B. quadrimaculatum oppositum would suggest a greater affinity for tilled (R-CP in June; R-MP in July) than NT treatments. It also prefers the warmer, bare soil conditions of tilled treatments (Cárcamo et al. Reference Cárcamo, Niemalä and Spence1995).
The autumn breeder, H. rufipes, accounted for 71% of total catches. Its overall dominance at this site is not surprising as the species accounts for 50%–70% of carabids in agricultural fields in the north-east region (Québec, Maritime provinces, northeastern United States) (Zhang Reference Zhang1993). The success of this species is attributed to its dispersal capacity (Wratten and Thomas Reference Wratten and Thomas1997), and its omnivorous but occasionally highly granivorous diet (Larochelle Reference Larochelle1990; Zhang Reference Zhang1993; Lövei and Sunderland Reference Lövei and Sunderland1996). Harpalus rufipes would feed in equal proportion on animal and plant material (Larochelle Reference Larochelle1990). Both H. rufipes and P. melanarius, another autumn breeder, were most abundant in mid-summer and had a clear preference for NT. These species overwinter in the larval stage at depths of 15–20 cm (Larochelle and Larivière Reference Larochelle and Larivière2003), thus near the bottom of the furrow slice, where they are likely more susceptible to physical harm from deep tillage implements. Accordingly, in a study conducted in Belgium, the dominance of H. rufipes in MP compared with CP and NT treatments was attributed to the shallow depth of the plough treatment (Baguette and Hance Reference Baguette and Hance1997). The dominance of H. rufipes and P. melanarius in our NT plots could be related partly to their predatory feeding habit. Indeed, earthworms were at least twice more abundant in NT than in tilled plots (Eriksen-Hamel et al. Reference Eriksen-Hamel, Speratti, Whalen, Légère and Madramootoo2009). Slugs, also a preferred food (Larochelle Reference Larochelle1990), were abundant at this site in 2006 (O. Lalonde, personal observation), likely as a result of the very wet spring; unfortunately, their identification and abundance relative to tillage treatments were not determined.
Because of their abundance, carabids could play a significant role in NT ecosystem function (Menalled et al. Reference Menalled, Smith, Dauer and Fox2007). The partly granivorous feeding habit of the dominant H. rufipes in NT would suggest a strong effect on weed seedbanks, either by reducing size, or affecting weed species composition, or both (Zhang et al. Reference Zhang, Drummond, Liebman and Hartke1997; Davis et al. Reference Davis, Dixon and Liebman2003; Westerman et al. Reference Westerman, Wes, Kropff and Van der Werf2003). However, weed seedbank data from our study would indicate that the overall effects of seed predation were proportionally small compared with the seed rain differences between tillage treatments (Légère et al. Reference Légère, Stevenson and Benoit2011a). Still, seed feeding preferences such as that of larval instars of H. rufipes for small weed seeds may have affected weed population dynamics and species shifts in weed communities (Hartke et al. Reference Hartke, Drummond and Liebman1998; Menalled et al. Reference Menalled, Smith, Dauer and Fox2007).
Although species composition varied during the season, H. rufipes was clearly the dominant species, particularly in NT treatments. As suggested in a landscape study of beetle communities in the Montréal region of Québec, this introduced species plays an important role in agricultural fields (Mercado Cárdenas and Buddle Reference Mercado Cárdenas and Buddle2009). Functional benefits of this carabid would largely be due to its granivorous feeding habit (Hartke et al. Reference Hartke, Drummond and Liebman1998; Gallandt et al. Reference Gallandt, Molloy, Lynch and Drummond2005). The abundance of carabids could thus contribute to the sustainability of NT systems. Their granivorous habit can provide some level of biological weed control, which can definitely be an asset to NT systems in much need of multiple forms of weed control.
This study characterised the carabid fauna of eastern Québec cereal-based cropping systems. Tillage clearly influenced carabid abundance and community structure, because of its effects on agro-environmental parameters. The affinity of carabids for NT and crop rotation treatments was likely due to improved food and shelter conditions compared with that in other treatments. This confirmed the importance of crop residue and vegetation cover for these organisms in cropping systems.
Acknowledgements
We would like to express our sincere gratitude to the staff of the Centre Bioalimentaire du Québec, and to the 2006 team of summer students from the Laboratoire de diagnostic en phytoprotection (Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec, Québec) for their excellent technical collaboration. We would also like to sincerely thank Dr. Geneviève Labrie (CÉROM: Centre de recherche sur les grains Inc.) and Dr. Owen Olfert (AAFC Saskatoon) for their comments and excellent suggestions on earlier drafts of this manuscript.
Appendix 1
Adult feeding preference, average number of carabid beetle per trap per plot according to sampling date, and total number of carabid beetle catches, according to tillage treatment.

GE: generalist/omnivorous; GR: granivorous; PR: predator.