Introduction
The Mediterranean fruit fly or Medfly, Ceratitis capitata (Wiedmann) (Diptera: Tephritidae), is one of the principal pests of Spanish agriculture, mainly affecting the citrus industry. It is a globally widespread, highly polyphagous species (Fimiani Reference Fimiani1989; Fletcher Reference Fletcher1989; Liquido et al. Reference Liquido, Shinoda and Cunningham1991) that exhibits high fecundity (Weems Reference Weems1981; Fletcher Reference Fletcher1989), and is multivoltine (Muñiz and Gil Reference Muñiz and Gil1984). Moreover, it is one of the most pestiferous of species and is a target of quarantine measures in most countries (European and Mediterranean Plant Protection Organization 2012). This underlines the need for special control measures to be undertaken in the production areas affected by this pest.
Biological control measures are becoming increasingly recognised as a major component of Integrated Pest Management programmes, and the use of native species for the control of their natural enemies is gaining interest (Urbaneja and Jacas Reference Urbaneja and Jacas2008). Accordingly, studies have been undertaken (Pérez-Hinarejos and Beitia Reference Pérez-Hinarejos and Beitia2008; Tormos et al. Reference Tormos, Beitia, Böckmann and Asís2009, Reference Tormos, Beitia, Alonso, Asís and Gayubo2010; Böckmann et al. Reference Böckmann, Tormos, Beitia and Fischer2012) to assess the potential of the ectoparasitoid, Spalangia cameroni Perkins (Hymenoptera: Pteromalidae), as a biological control agent for C. capitata.
Spalangia cameroni is an idiobiont and solitary pupal parasitoid of several Diptera families (Muscidae, Sarcophagidae, Tephritidae), and it is sold commercially as a biological control agent against filth flies, including the stable fly, Stomoxys calcitrans (Linnaeus) and the house fly, Musca domestica Linnaeus (Diptera: Muscidae) (Birkemoe et al. Reference Birkemoe, Soleng and Aak2009). Following the discovery in Spain that S. cameroni is a parasitoid of the Medfly (Falcó et al. Reference Falcó, Garzón-Luque, Pérez-Hinarejos, Tarazona, Malagón and Beitia2006), the Valencian Institute for Agricultural Research (IVIA) in Spain has explored the possibility of using S. cameroni as a biological control agent (Tormos et al. Reference Tormos, Beitia, Alonso, Asís and Gayubo2010; Böckmann et al. Reference Böckmann, Tormos, Beitia and Fischer2012). Since it is a native parasitoid, there were no previous studies of its impact upon other, nontarget species, or of its interactions with Medfly under natural conditions. Laboratory data on the fecundity, adult progeny, host-induced mortality, and sex ratio of S. cameroni grown on C. capitata have been reported by Pérez-Hinarejos and Beitia (Reference Pérez-Hinarejos and Beitia2008). Although S. cameroni does not exhibit higher relative fecundity rates than other tephritid-specific larval parasitoids, such as Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae) (Martins et al. Reference Martins, Skouri, Chermiti, Aboussaid, El Messoussi and Oufdou2010), it could complement the action of these other larval parasitoids.
The initial breeding system, used by Falcó et al. (Reference Falcó, Garzón-Luque, Pérez-Hinarejos, Tarazona, Malagón and Beitia2006) for the laboratory experiments referred to above, relied upon living Medfly pupae. This meant that adult flies could emerge from the nonparasitised pupae. It also caused increases in humidity, leading to the growth of fungi; and the technique presented difficulties in separating the parasitoids (Tormos et al. Reference Tormos, Beitia, Alonso, Asís and Gayubo2010). Furthermore, with this methodology, fly pupae had to pass through the adult-fly emergence stage before being removed, and this presented a risk of Medfly outbreaks in the vicinity of the production facility.
In the past, host pupae (Diptera: Muscidae) killed by cold shock had been proven to be unsuitable for rearing Spalangia Latreille species (Morgan et al. Reference Morgan, Smittle and Patterson1986; Roth et al. Reference Roth, Fincher and Summerlin1991; Klunker and Fabritius Reference Klunker and Fabritius1992). However, in a recent study we evaluated the use of pupae (of C. capitata) killed by “cold shock” as hosts for S. cameroni (Tormos et al. Reference Tormos, Beitia, Alonso, Asís and Gayubo2010). This study concluded that parasitism rates obtained with S. cameroni using freeze-killed C. capitata pupae did not differ significantly from those obtained using live pupae.
The objectives of this study were to examine, within the context of small rearing colonies (between 5000 and 10,000 parasitoid individuals), the extent to which the use of freeze-killed C. capitata pupae can affect: (i) parasitoid adult progeny (total number of adult offspring produced per female); (ii) sex ratio; (iii) realised fecundity (total number of eggs laid by a female); and (iv) levels of superparasitism (supernumerary eggs).
Materials and methods
Study centre and insectsFootnote 1
Spalangia cameroni and C. capitata were obtained from laboratory colonies housed at the Valencian Institute of Agrarian Research (Instituto Valenciano de Investigaciones Agrarias (IVIA), Valencia, Spain). The C. capitata colony, named IVIA 2002, was established in 2002 by collecting infested fruits from various locations in the province of Valencia (San Andrés et al. Reference San Andrés, Ortego and Castañera2007; Monzó et al. Reference Monzó, Sabater-Muñoz, Urbaneja and Castañera2010). The S. cameroni colony was established in 2003, with specimens obtained from Medfly taken from apples in a town (Bétera) in Valencia province (Spain) (Falcó et al. Reference Falcó, Verdú and Beitia2004). Since 2010, S. cameroni have been maintained at the IVIA by culture on freeze-killed Medfly pupae, as described by Tormos et al. (Reference Tormos, Beitia, Alonso, Asís and Gayubo2010). Pupae are freeze-killed at −20°C for 60 minutes and these freeze-killed pupae can then be stored for up to 30 days at +5°C to rear S. cameroni. However, for the second experiment reported in this work, a culture of the parasitoid on living Medfly pupae was initiated specifically, and maintained for the duration of this study.
All host Medfly pupae (whether freeze-killed or living) that were used for the culture of S. cameroni were 3–5 days old, in order to minimise the confounding effects of host age (King Reference King1998). To further minimise the effects of host size upon sex ratio (King and King Reference King and King1994), only pupae of similar size (l=3.95–4.68 mm (
![]() $\bar{x}$
±SE=4.37±0.01, n=52), maximum w=2.1–2.49 mm (
$\bar{x}$
±SE=4.37±0.01, n=52), maximum w=2.1–2.49 mm (
![]() $\bar{x}$
±SE=2.25±0.02, n=42) and colour (brown) were used.
$\bar{x}$
±SE=2.25±0.02, n=42) and colour (brown) were used.
Experimental design
Two experiments were conducted to assess the efficiency of freeze-killed Medfly pupae as hosts for S. cameroni. In Experiment 1, parasitism of freeze-killed Medfly pupae was compared to parasitism of living Medfly pupae, using S. cameroni that had been reared on freeze-killed pupae. In Experiment 2, parasitism of freeze-killed and living Medfly pupae was again compared, but in this case parasitoids had been reared either on living pupae or on freeze-killed pupae. Experiment 2 was performed to take account of experimental error attributable to the method by which the parasitoids had been reared.
The experiments were performed in a climate cabinet (Sanyo MLR 350; Sartorius, Barcelona, Spain), maintained at a temperature of 24.5±0.5°C and at 60±10% relative humidity, and under a 16:8 hours (light/dark) cycle. Both experiments were performed within translucent plastic boxes (20×15×10 cm), which were treated as experimental units.
In Experiment 1, each experimental unit contained one female and one male S. cameroni and also held a Petri dish (diameter: 60 mm) with 10 freeze-killed or living Medfly pupae, together with water, and honey to provide supplementary food. The pupae were replaced daily until the death of the female wasp. The parasitoids included in the experiment were newly emerged from freeze-killed C. capitata puparia.
There were two different experimental groups: in one group (female mean longevity (±SE): 25.3±0.47 days), pupae obtained daily from the experimental units were dissected in order to determine the realised fecundity, including the supernumerary eggs (more than one egg/pupa); and in the other group (female mean longevity (±SE): 25.9±0.52 days), pupae were kept until the emergence of adult parasitoids, in order to determine the adult progeny (also % emerging adults/exposed pupae) and the sex ratio. For each experimental group, three replicates (blocks or sets) were undertaken, with five repetitions per replicate; thus, in total, 15 repetitions (experimental units) were performed (Table 1). The results of two-way analysis of variance (ANOVA), considering the block effect as a random factor, indicated that the block effect did not provide any additional variability. For this reason, we treated it as if it was one replicate comprising 15 repetitions. This methodology was used for both freeze-killed and living pupae.
Table 1. Experimental protocol to elucidate the effects of freeze-killed Medfly pupae upon adult progeny, realised fecundity, superparasitism, and sex ratio in a laboratory rearing of Spalangia cameroni.
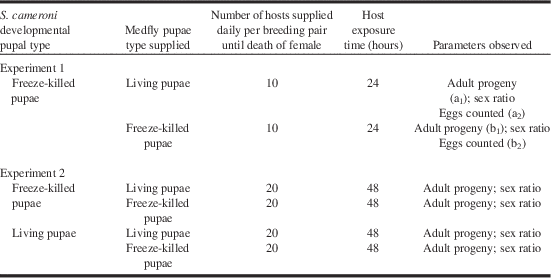
Three replicates were studied, with five repetitions per replicate: that is, 15 repetitions per experimental parameter. The value for adult progeny was obtained from the total number of adult offspring produced (a1 and b1 indicate pupae used to obtain adults). Values for realised fecundity (number of eggs laid) and superparasitism (supernumerary eggs: more than one egg/host) were obtained by dissecting the host pupae (a2 and b2 indicate pupae used to observe realised fecundity and superparasitism).
In Experiment 2, determinations were made only of adult progeny (also % emerging adults/exposed pupae) and of sex ratio. In each experimental unit, 20 Medfly pupae in a Petri dish were offered to a breeding pair of parasitoids; pupae were replaced every 48 hours. Water and honey were provided as supplementary food, until the death of the female. These wasps came from either freeze-killed or living Medfly pupae.
Four scenarios were analysed: (1) living pupae exposed to parasitoids reared on living pupae; (2) living pupae exposed to parasitoids reared on freeze-killed pupae; (3) freeze-killed pupae exposed to parasitoids reared on living pupae; and (4) freeze-killed pupae exposed to parasitoids reared on freeze-killed pupae. The values for female mean longevity (±SE) for the four scenarios were, respectively: 24.9±0.51 days, 25.7±0.53 days, 26.1±0.49 days, and 25.5±0.54 days. As in the case of Experiment 1, three replicates with five experimental units (repetitions) per replicate and, thus, a total of 15 repetitions (experimental units) were undertaken for each experimental condition (Table 1). The results of two-way ANOVA, considering the block effect as a random factor, indicated that the block effect did not provide any additional variability. For this reason, we treated it as if it was one replicate comprising 15 repetitions.
Statistical analysis
Analysis of variance (one-way (fixed factor: type of pupae: live/freeze-killed pupae) (Experiment 1) and two-way ANOVA (fixed factors: (a) type of pupae: live/freeze killed pupae; (b) parasitoid source: parasitoids reared on living/freeze killed pupae) (Experiment 2)), a linear regression (Experiment 1) and χ² analysis (Experiments 1 and 2) were used to establish the relationships between the different responses being measured (realised fecundity (one-way ANOVA), adult progeny (one-way and two-way ANOVA), sex ratio (χ² analysis) and superparasitism (one-way ANOVA)). All variables were normally distributed and were not transformed before analyses. In addition, the distributions of residuals were approximately normal. Values are reported as means±SE. Analyses were performed using the SPSS statistical software package (IBM, Spain; v15.0; critical P-value used, 0.05).
Results
Experiment 1: parasitism of living versus freeze-killed Medfly pupae by Spalangia cameroni that had been laboratory-reared on freeze-killed Medfly pupae
In this experiment (Tables 2, 3), the adult progeny were found not to differ significantly between living and freeze-killed hosts and there was no significant effect upon the numbers of females and males produced. There was therefore also no significant difference in the sex ratio according to whether living or freeze-killed pupae were used. The sex ratio, using either living or freeze-killed pupae, was significantly biased towards females (living (18.8% ♂♂, 81.2% ♀♀), χ²=70.031, df 1, P<0.001; freeze-killed (20.8% ♂♂, 79.2% ♀♀), χ²=65.873, df 1, P<0.001).
Table 2. Lifetime production of progeny, realised fecundity and superparasitism (mean±SE) for females (n=15) for Spalangia cameroni provided with living or freeze-killed hosts.
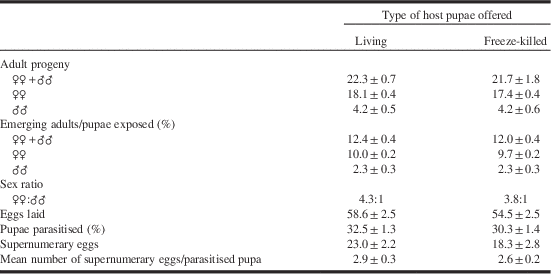
The S. cameroni had been reared upon freeze-killed pupae.
Table 3. Mean number (±SE) of progeny per Spalangia cameroni breeding pair.
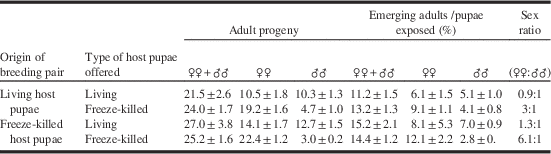
Breeding pairs had been reared on living or freeze-killed Medfly pupae. They were provided every 48 hours, until the death of the female, with 20 living or freeze-killed pupae.
No significant differences were observed with regard either to realised fecundity or to the incidence of supernumerary eggs (superparasitism) between the two experimental conditions (i.e., freeze-killed or living pupae). Nevertheless, a linear regression analysis revealed a significant positive relationship between realised fecundity and superparasitism, both for living pupae (F=55.857, df 1, 13, P<0.001; R 2=0.811) and for freeze-killed pupae (F=95.957, df 1, 13, P<0.001; R 2=0.881). The ratio of emerging adults to exposed pupae (expressed as %) ranged between 12% and 13% (Table 2) in both experimental groups.
These results demonstrate clearly that freeze-killed pupae and living pupae can be used equally well for rearing S. cameroni.
Experiment 2: parasitism of living versus freeze-killed Medfly pupae by Spalangia cameroni that had been laboratory-reared either on freeze-killed or on living Medfly pupae
In this experiment, the proportion of females obtained was higher with the use of freeze-killed pupae than when living pupae were parasitised (two-way ANOVA: F(1,19)=12.452, P=0.026). Regardless of how the parasitoids had been reared, there were also significant sex-ratio differences between the use of freeze-killed pupae (of the individuals analysed, 19% were males and 81% were females) and living pupae (of the individuals analysed, 47% were males and 53% were females) (χ²=66.715, df 1, P<0.0001). The sex ratio using freeze-killed pupae was biased significantly towards females (χ²=46.681, df 1, P<0.001), whereas when living pupae were used the sex ratio was only slightly biased towards females, and this was not statistically significant (107/255=0.42) (χ²=3.496, df 1, P=0.075). As shown in Table 3, however, the two-way ANOVA did not reveal any significant effect of the factors (parasitoids reared on living pupae versus parasitoids reared on freeze-killed pupae) upon the proportion of females, nor of the factors [living pupae versus freeze-killed pupae; parasitoids reared on living pupae versus parasitoids reared on freeze-killed pupae) upon total adult progeny. Furthermore, the two-way ANOVA did not reveal any significant interaction between the factors (living pupae versus freeze-killed pupae; parasitoids reared on living pupae versus parasitoids reared on freeze-killed pupae) either upon total adult progeny, or upon the proportion of females. The ratio of emerging adults to exposed pupae (expressed as %) ranged between 11% and 15%, in all four experimental scenarios (Table 3).
According to these results, the effects of the hosts being alive or freeze-killed were similar to those observed in Experiment 1, and there was no effect of the mode of rearing of the adults upon their adult progeny.
Discussion and conclusions
In a previous study (Tormos et al. Reference Tormos, Beitia, Alonso, Asís and Gayubo2010), we began to evaluate the possibility of using C. capitata pupae killed by cold shock in a laboratory-scale S. cameroni breeding programme, analogous to the successful use of cold-shock-killed M. domestica pupae to rear this parasitoid, reported by Geden and Kaufman (Reference Geden and Kaufman2007), Kaufman and Geden (Reference Kaufman and Geden2009), and Ogawa et al. (Reference Ogawa, Ito, Fukuda, Tebayashi and Arakawa2012). Freeze-killed C. capitata pupae have also recently been used in laboratory assays with Pachycrepoideus vindemmiae (Rondani) (Hymenoptera: Pteromalidae) (Wyckhuys et al. Reference Wyckhuys, López Acosta, García and Jiménez2011). Cold shock (defined as exposure to −20°C for 60 minutes) results in 100% host death, and lengthens the storage life of Diptera pupae (which is otherwise only about 2 or 3 days) to as long as 30 days (maintained at between +4°C and +5°C). This reduces production costs while maintaining the degree of host quality required for parasitoid development (Floate Reference Floate2002; Geden and Kaufman Reference Geden and Kaufman2007; Tormos et al. Reference Tormos, Beitia, Alonso, Asís and Gayubo2010). Therefore, in this work we have analysed the effects of using freeze-killed C. capitata pupae on the following parameters in S. cameroni: realised fecundity, adult progeny, sex ratio, and superparasitism. The values we have obtained are in agreement with previous findings relating to superparasitism (Böckmann et al. Reference Böckmann, Tormos, Beitia and Fischer2012; Tormos et al. Reference Tormos, Asís, Sabater-Muñoz, Baños, Gayubo and Beitia2012), natural history (Tormos et al. Reference Tormos, Beitia, Böckmann and Asís2009), and breeding-substrate modification (Tormos et al. Reference Tormos, Beitia, Alonso, Asís and Gayubo2010).
Our results show that there are no significant differences in the key indicator that we have analysed (adult progeny) between parasitoids reared on either freeze-killed or living Medfly pupae. However, using freeze-killed pupae the sex ratio favoured females (as shown in Experiment 2); a slight variability in sex ratio was observed when living pupae were used, but this was not statistically significant. In the context of these findings, the argument of behavioural ecology that behaviour is adaptive becomes relevant. Thus, a shift in realised fecundity and in sex ratio could arise in response to the quality of the host. For example, in the present case, the use of freeze-killed pupae is clearly advantageous. Currently, there are companies in South America and Europe, such as Perkins Ltda (perkinsltda.com.co) and Muscidia (www.muscidia.fr) that commercially produce S. cameroni for fly control in livestock. In this respect, the results obtained in this study would allow improvements to be made in the mass-rearing of S. cameroni on C. capitata.
In order to use S. cameroni in a biological control programme for fruit flies, the parasitoid must first be mass-produced in rearing facilities. Although the simultaneous production of large numbers of parasitoids and hosts is not problematic, the natural history of C. capitata means that high production rates cannot be sustained during periods or seasons when the parasitoid is not being released, except when the pest is used in Sterile Insect Technique programmes (see Tormos et al. Reference Tormos, Beitia, Alonso, Asís and Gayubo2010). However, maintaining low-output rearing programmes for much of the year may mean that it is difficult to increase output rapidly when parasitoid releases are required, given that it usually takes several generations to obtain an adequate number of natural enemies for effective use in the field. This fact, coupled with other drawbacks resulting from the presence of viable hosts in breeding cages (notably increased moisture, fungal growth, and difficulties in separating the parasitoids), makes the use of viable host pupae more difficult and expensive in mass rearing programmes.
Clearly, both the laboratory-scale production and the mass-rearing of S. cameroni can be improved by using freeze-killed C. capitata pupae, and this system can provide the parasitised host material that is required in order to perform the field trials that are necessary for the real effectiveness of parasitoid releases to be assessed. Indeed, this parasitoid-breeding system, lacking as it does the risk of introducing adult flies, may facilitate the adoption of a strategy involving the release of parasitised pupae in crops before emergence of parasitoids. Such releases might be done with both inundative and inoculative goals in view. Furthermore, the manipulation of parasitised pupae is easier and less damaging to the parasitoids than the handling of adult parasitoids for release, or even than keeping them ready for release in small cages (Cancino and Montoya Reference Cancino and Montoya2006).
Finally, it is noteworthy that measures of biotic potential, such as realised fecundity, number of parasitised pupae and adult progeny, are slightly higher when freeze-killed pupae of M. domestica are used as hosts for S. cameroni than when freeze-killed pupae of C. capitata are used. Moreover, when M. domestica is used as the host, the percentage of aborted parasitoids is lower (however, although about 35% of parasitoids abort when C. capitata is used as the host, the adult-progeny value is still quite high). Superparasitism is higher with M. domestica as host (F.B. and J.T., personal observation), whereas the sex ratio is almost the same for both hosts. In spite of all these considerations, however, it is preferable to rear S. cameroni using C. capitata as the host, on grounds of economy and convenience.
We conclude that S. cameroni may be a promising candidate for use in the biological control of Medfly and other flies that are considered to be pests in Spain. Further studies must now be carried out under field conditions, however, to determine the effectiveness of this parasitoid against these pests.
Acknowledgements
The authors are indebted to Kevin Floate (Lethbridge Research Centre, Canada) and Bethia King (Northern Illinois University, United States of America) for their observations and critical reading of the manuscript. This study was carried out in the laboratories of the Departamento de Protección Vegetal, I.V.I.A. (Valencia, Spain). Financial support for this paper was provided from the Junta de Castilla y León, project SA064A08.





