Introduction
Guilds occur across different ecosystems and are formed by groups of species that together exploit a common, potentially limited resource (extra guild prey) and show interactions, ranging from cooperation and mutualism to competition and predation. Such complex interactions between different species have been previously reported in a number of aphidophagous guilds (Evans Reference Evans1991; Dixon Reference Dixon2000; Omkar et al. Reference Omkar, Mishra and Pervez2002; Zannou et al. Reference Zannou, Hanna, Moraes and Kreiter2005; Meszaros et al. Reference Meszaros, Tixier, Cheval, Barbar and Kreiter2007; Ware and Majerus Reference Ware and Majerus2008; Sato et al. Reference Sato, Shinya, Yasuda, Kindlmann and Dixon2009; Omkar and Pervez Reference Omkar and Pervez2011). Ferguson and Stiling (Reference Ferguson and Stiling1996) have reported that the interactions between guild members on being released in the field on a common aphid prey, may lead to any of the five situations: (1) they may cause higher mortality to the aphid prey than expected (synergistic interaction, Losey and Denno Reference Losey and Denno1998, Reference Losey and Denno1999), a phenomenon termed as “Predator Facilitation” (Charnov et al. Reference Charnov, Orians and Hyatt1976), (2) both the observed and expected (sum of individual prey mortalities) aphid prey mortalities are equivalent (additive interaction, Chang Reference Chang1996; Straub and Snyder Reference Straub and Snyder2006), (3) observed aphid prey mortality may be less than the expected prey mortality, (4) observed prey mortality may be less than that caused by one predator alone but not the other, and (5) the observed prey mortality may be less than when either predators acts alone; the last three cases being antagonistic interactions (Prasad and Snyder Reference Prasad and Snyder2004; Kajita et al. Reference Kajita, Takano, Yasuda and Evans2006; Majerus et al. Reference Majerus, Strawson and Roy2006; Hodek and Michaud Reference Hodek and Michaud2008).
Additive or synergistic interactions occur when the activity of one predator increases the susceptibility of a shared prey to another predator(s) (Losey and Denno Reference Losey and Denno1998, Reference Losey and Denno1999). However, the nonadditive or antagonistic interactions are mediated either through (i) exploitative competition where a predator reduces the abundance of a shared prey affecting the other predator(s) or (ii) through interference competition where the activity of one predator reduces the access of other predator(s) to the shared prey (Mills Reference Mills2006). The consequences of such interactions among guild predators are important for the biocontrol of economically important pests. It is because of these variations in the predatory guilds that Godfray and Waage (Reference Godfray and Waage1991) have suggested that unlicensed release of natural predators should not be encouraged and prior evaluation of combinations of natural predators for the identification of effective biocontrol agents is essential.
Among the number of factors on which the interactions within guilds are assessed, body size (biomass) of constituent predators is a crucial one that influences success or failure in the guild (Lucas et al. Reference Lucas, Coderre and Brodeur1998; Felix and Soares Reference Felix and Soares2004; Armsby and Tisch Reference Armsby and Tisch2006; Mochizuki et al. Reference Mochizuki, Naka, Hamasaki and Mitsunaga2006). Larger species have a competitive advantage over smaller species during interference competition, where owing to their large size and higher consumption rates (Finlayson et al. Reference Finlayson, Alyokhin, Gross and Porter2010), they physically interfere with their competitors and/or prey upon them (Lawton and Hassell Reference Lawton and Hassell1981; Persson Reference Persson1985; Spiller Reference Spiller1986; Wissinger and McGrady Reference Wissinger and McGrady1993; Sato et al. Reference Sato, Jimbo, Yasuda and Dixon2008). In prey abundant conditions of aphidophagous guilds, larger ladybirds are more effective than the smaller ladybirds but not so under prey scarce conditions (Sloggett Reference Sloggett2008). Prey scarce conditions are, however, better exploited by smaller ladybirds (Dixon Reference Dixon2007). Aphid colonies are often first attacked by a small and then a large species of ladybird, and hence the smaller species starts exploiting the resources before the larger species (Dixon Reference Dixon2007; Sloggett Reference Sloggett2008), indicating them to be more effective during exploitative competition.
While exploring these interactions, earlier studies were restricted to the final guild predation outcomes (additive or nonadditive), and have not evaluated changes in guild conversion efficiencies or guild growth rates, or the body mass change of the guild predators sharing the common prey. Some recent studies revealed that larger ladybirds are more voracious and smaller ones are more efficient at prey use and suggest the use of their combinations in the form of heterospecific guilds for biocontrol purposes (Mishra et al. Reference Mishra, Kumar, Shahid and Singh2011, Reference Mishra, Omkar, Kumar and Pandey2012). Omkar and Pervez (Reference Omkar and Pervez2011) reported synergistic functional response when Coccinella transversalis (Fabricius) (large ladybird) and Propylea dissecta (Mulsant) (small ladybird), were released in tandem.
In the present investigation, the efficacy of size-based 11 heterospecific guilds, formed from the combinations of four locally co-occurring predatory ladybirds (Coleoptera: Coccinellidae), as follows Coccinella septempunctata (Linnaeus), C. transversalis, Cheilomenes sexmaculata (Fabricius), and P. dissecta on the pea aphid, Acyrthosiphon pisum (Harris) (Hemiptera: Aphididae) have been assessed. Of the four ladybirds, the former two are large and the later two are small ones (Mishra et al. Reference Mishra, Kumar, Shahid and Singh2011). The aphid prey forms an equivalent niche for these locally abundant aphidophagous ladybirds that coexist as its predators (Mishra et al. Reference Mishra, Kumar, Shahid and Singh2011).
The inefficiencies of additive models have enforced us to use multiplicative risk model (Soluk and Collins Reference Soluk and Collins1988; Sih et al. Reference Sih, Englund and Wooster1998) in the present study to analyse the “additive” or “nonadditive” effects. Additive models normally generate higher or unrealistic “expected prey mortality” and cannot correct for prey depletion within additive experimental designs, and in such cases “multiplicative risk model” is a more appropriate null model for the prey response variables that are usually measured (e.g., proportion or number of prey killed or surviving; see Sih et al. Reference Sih, Englund and Wooster1998; Mills Reference Mills2006).
The present investigation, therefore, aims to assess (i) size-based predatory interactions (synergistic/additive/antagonistic) within heterospecific guilds, (ii) costs of being large while sharing common aphid prey, in view of the adaptive significance of being large (as eliminating competitors) but its negative aspects are poorly understood (Sato et al. Reference Sato, Jimbo, Yasuda and Dixon2008), (iii) changes within guild conversion efficiencies and guild growth rates, and (iv) finding the suitable heterospecific guild(s) for biocontrol purposes.
Materials and methods
Stock maintenance
Adults of four predaceous ladybird species, as follows C. septempunctata (C7), C. transversalis (Ct), C. sexmaculata (Cs), and P. dissecta (Pd) were collected from agricultural fields around the city of Lucknow, India (26°50′N, 80°54′E), placed in plastic Petri dishes (14.5 × 1.5 cm) and reared under constant abiotic conditions (27 ± 1 °C; 65 ± 5% relative humidity; 14:10 hours light:darkness) in Environmental Test Chambers (ETC, CH-6S, Remi Instruments, India). Adults were fed with ad libitum supply of aphid, A. pisum on broad bean, Vicia faba Linnaeus (Fabaceae) maintained in polyhouse (22 ± 1 °C; 65 ± 5% relative humidity and 14 hours light:10 hours dark photoperiod). Eggs laid were collected every 24 hours and observed for hatching. The newly hatched larvae were isolated and reared till adult emergence in separate Petri dishes with a daily-replenished supply of aphids. Being the most voracious predatory stages, fourth instar larvae (12 hours after moulting) and 10-day-old unmated adult females were used in the experiments. Unmated adult females were used to avoid error due to exceptionally high variations in the reproductive potential of these ladybird species (Omkar Reference Omkar2004; Omkar et al. Reference Omkar, Pervez, Mishra, Srivastava, Singh and Gupta2005)
Experimental design
Prior to experimentation, the fourth instar of the predatory species was starved for 12 hours. At the start of the experiment, the larvae were massed using an electronic balance (Sartorius CP225-D; Sartorious AG, Goettingen, Germany; 0.01 mg precision). A single premassed fourth instar was kept in a plastic Petri dish (14.5 × 1.5 cm) along with 50 mg of aphid at constant abiotic conditions (27 ± 1 °C; 65 ± 5% relative humidity; 14:10 hours light:darkness) in ETC for 24 hours. The larva was then separated from the aphid biomass and both were massed individually. This was repeated 10 times. Similar experiments were also carried out for the remaining predatory species (n = 40). The reduction in aphid biomass in presence of predator(s) was considered to be due to prey mortality.
To evaluate the combined predatory potential of ladybird species, guilds were formed on the basis of the size of the ladybird predators (segregated according to mean body mass of predators; C7 = 17.7±1.99b mg; Ct = 15.3±0.90b mg; Cs =8.8±0.91a mg; and Pd = 7.2±0.81a mg; F = 16.25; P < 0.0001; df = 3, 39, based on one-way ANOVA followed by Tukey's post hoc comparison of means and superscript “a” and “b” mean statistically significant differences). These were S + S (Cs + Pd), L + S (C7 + Cs, C7 + Pd, Ct + Cs, Ct + Pd), L + L (C7 + Ct), 2L + S (C7 + Ct + Pd, C7 + Ct + Cs), L + 2S (C7 + Cs + Pd, Ct + Cs + Pd), and 2L + 2S (C7 + Ct + Cs + Pd) (L = large; S = small). Within these guilds, combinations of two, three, and four heterospecific fourth instar were placed in plastic Petri dishes (14.5 × 1.5 cm) having one individual of each predatory species along with aphid mass equivalent to 100 mg (for two predators), 150 mg (for three predators), and 200 mg (for four predators) of A. pisum, and maintained at the above mentioned standard abiotic conditions for 24 hours. Prior to experimentation, the relative prey–predator proportion was standardised and this proportion remained unaltered to prevent intraguild predation. Also, the size of experimental arena was kept constant to promote the maximum possible predatory interactions between the guild species. The larvae were massed individually prior and post experiment. Each treatment was replicated 10 times.
Similar experiments were also repeated with heterospecific unmated 10-day-old adult females (C7 = 27.8 ± 1.32c mg; Ct = 21.3 ± 1.32b mg; Cs = 14.4 ± 1.61a mg; and Pd = 11.5±0.50a mg; F = 33.43; P < 0.0001; df = 3, 39), individually (n = 40) and within combinations (n = 110). During the selection of aphid size, intermediate instars of A. pisum were taken to allow best development and survival for both larger and smaller ladybird predators (Roger et al. Reference Roger, Coderre and Boivin2000).
To assess the natural reduction in aphid biomass, if any, in the absence of predators, all four standard biomass, as follows 50, 100, 150, and 200 mg of aphids, were placed in Petri dishes and kept under similar conditions for 24 hours, remassed and considered as controls. The average loss of biomass, if any, based on five replicates per standard aphid biomass, was used to normalise the data on consumption prior to calculating the various parameters (Tables 1 and 2).
Table 1 Observed and expected prey mortalities (%) showing overall predatory interactions using multiplicative risk model in fourth instar guilds.
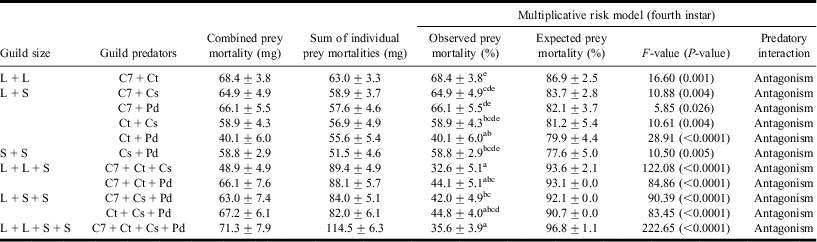
Values are mean ± SE; F-values significant at P < 0.05; df = 1, 19.
L, S, C7, Ct, Cs, and Pd represent large, small, Coccinella septempunctata, Coccinella transversalis, Cheilomenes sexmaculata, and Propylea dissecta, respectively.
a,b,c,d,eLowercase letters represent comparison of means within guild predators based on Tukey's post hoc comparison of means.
Table 2 Observed and expected prey mortalities (%) showing overall predatory interactions using multiplicative risk model in adult female guilds.
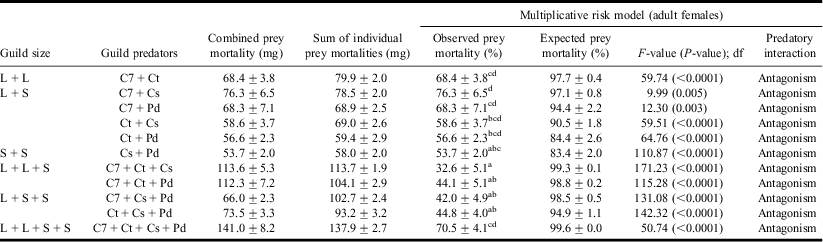
Values are mean ± SE; F-values significant at P < 0.05; df = 1, 19.
L, S, C7, Ct, Cs, and Pd represent Large, Small, Coccinella septempunctata, Coccinella transversalis, Cheilomenes sexmaculata, and Propylea dissecta, respectively.
a,b,c,dLowercase letters represent comparison of means within guild predators based on Tukey's post hoc comparison of means.
Statistical analysis
We aimed to decipher through statistical analysis of data (1) size-based predatory interactions within guilds while sharing common aphid prey, (2) the changes in guild conversion efficiencies, guild growth rates, and individual predator biomass within each guild. All data obtained in the study were checked for normal distribution using Kolmogorov–Smirnoff test for normality and Bartlett's test for homogeneity of variances prior to being subjected to further analysis.
Multiplicative risk model and predatory interactions
The expected/observed aphid prey mortality within 24 hours by each guild combination was assessed by “multiplicative risk model” (using equations (1), (2), (3), and (4)), to correct for prey depletion within additive experimental designs (see Soluk and Collins Reference Soluk and Collins1988; Sih et al. Reference Sih, Englund and Wooster1998).
1. Proportion of aphid prey mortality by predator A or B or C or D alone (Pa or Pb or Pc or Pd)
 $$ \quad= {\frac{{\displaystyle{\rm{Aphid}}\ {\rm{prey}}\ {\rm{mortality}}\ {\rm{(mg)}}\ {\rm{by}}\ \atop \displaystyle{\rm{predator}}\,{\rm{A}}\ {\rm{or}}\ {\rm{B}}\ {\rm{or}}\ {\rm{C}}\ {\rm{or}}\ {\rm{D}}\ {\rm{alone}}}}{{\displaystyle{\rm{Aphid}}\ {\rm{prey}}\ {\rm{(mg)}}\ {\rm{provided}}\ {\rm{to}}\ \atop \displaystyle{\rm{predator}}\ {\rm{A}}\ {\rm{or}}\ {\rm{B}}\ {\rm{or}}\ {\rm{C}}\ {\rm{or}}\ {\rm{D}}\,{\rm{alone}}}}$$
$$ \quad= {\frac{{\displaystyle{\rm{Aphid}}\ {\rm{prey}}\ {\rm{mortality}}\ {\rm{(mg)}}\ {\rm{by}}\ \atop \displaystyle{\rm{predator}}\,{\rm{A}}\ {\rm{or}}\ {\rm{B}}\ {\rm{or}}\ {\rm{C}}\ {\rm{or}}\ {\rm{D}}\ {\rm{alone}}}}{{\displaystyle{\rm{Aphid}}\ {\rm{prey}}\ {\rm{(mg)}}\ {\rm{provided}}\ {\rm{to}}\ \atop \displaystyle{\rm{predator}}\ {\rm{A}}\ {\rm{or}}\ {\rm{B}}\ {\rm{or}}\ {\rm{C}}\ {\rm{or}}\ {\rm{D}}\,{\rm{alone}}}}$$
(For larval guilds, Pa (C7) = 0.65 ± 0.04; Pb (Ct)=0.61 ± 0.06; Pc (Cs) = 0.53 ± 0.07; Pd (Pd) = 0.50 ± 0.08);
(For adult guilds, Pa (C7) = 0.89 ± 0.04; Pb (Ct) = 0.70 ± 0.04; Pc (Cs) = 0.68 ± 0.03; Pd (Pd) = 0.48 ± 0.03)
2. Expected proportion of aphid prey mortality
= 1−[(1−Pa)(1−Pb)]Two; 1−[(1−Pa)(1−Pb)(1−Pc)]Three; 1−[(1−Pa)(1−Pb)(1−Pc)(1−Pd)]Four;
where Pa, Pb, Pc, and Pd are proportion of aphid prey mortality by predator A, B, C, and D alone, respectively.
3. Observed proportion of aphid prey mortality (within guild)
4. Expected/observed aphid prey mortality (%)
= expected/observed proportion of aphid prey mortality × 100
Variation in expected and observed aphid prey mortality within each guild was analysed using one-way ANOVA. Before to ANOVA, all per cent data were subjected to arcsine square root transformation.
For assessing the influence of guild size (or predator diversity) on predatory interactions, the data were subjected to two-way ANCOVA (general linear model) with predatory stage (larval and adult) and guild size (S + S, L + S, L + L, 2L + S, L + 2S, and 2L + 2S) as independent factors, observed prey mortality as dependent factor and guild biomass (combined biomass of the predator species constituting that guild) as a covariate (general linear model) followed by Tukey's post hoc comparison of means.
Further, for assessing the effect of increasing guild size (predator diversity) on observed prey mortality, the data were subjected to Pearson's correlation analysis.
Conversion efficiencies, growth rates and individual predator biomass within each guild
The conversion efficiencies and growth rates within each guild (fourth instar and adult females) were calculated using following formulae:
1. Conversion efficiency (modified after Dixon 2000)
2. Growth rate (day−1) (modified after Waldbauer 1968; Ramdev and Rao 1979)
Variations in conversion efficiencies, growth rates and biomass change of predators within guilds and when placed individually were analysed using one way ANOVA, followed by Tukey's post hoc comparison of means.
All statistical analyses were performed using MINITAB 16 (Minitab Inc., State College, Pennsylvania, United States of America).
Results
Multiplicative risk model and predatory interactions
Statistical tests revealed that both larval and adult guilds revealed antagonistic effects, i.e. within each guild, observed prey mortality was less than the expected prey mortality (Tables 1 and 2).
Results of two-way ANCOVA further revealed that within both larval and adult guilds, the observed prey mortality was influenced by the guild size (F ANCOVA (stage × guild size) = 4.22; P = 0.001; df = 5, 219). Also, within the guilds of similar size, guild biomass (F ANCOVA (covariate) =16.60; P < 0.0001; df = 1, 219) significantly affected the observed prey mortality.
Further, observed prey mortality was found to have an inverse linear relationship with the guild size in both larval (r = −0.507, P < 0.0001) and adult (r = −0.759, P < 0.0001) guilds. With increasing guild size or the predator richness, reduction in aphid prey mortality was observed. Larval guild of C7 + Ct and C7 + Cs adult guild resulted in maximum prey mortality.
Conversion efficiencies, growth rates and individual predator biomass within each guild
Results of one-way ANOVA revealed lower conversion efficiencies (Table 3) and growth rates (Table 4) within larval and adult guilds while sharing common aphid prey.
Table 3 Conversion efficiencies in heterospecific fourth instar and adult female guilds.
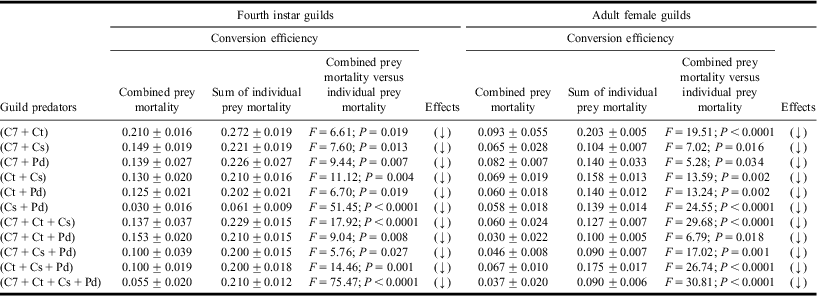
Values are mean ± SE; F-values significant at P < 0.05; df = 1, 19.
C7, Ct, Cs, and Pd represent Coccinella septempunctata, Coccinella transversalis, Cheilomenes sexmaculata, and Propylea dissecta, respectively.
(↑), (→), and (↓) represent gain, no change, or loss in conversion efficiencies, respectively, based on Tukey's post hoc comparison of means.
Table 4 Growth rates in heterospecific fourth instar and adult female guilds.
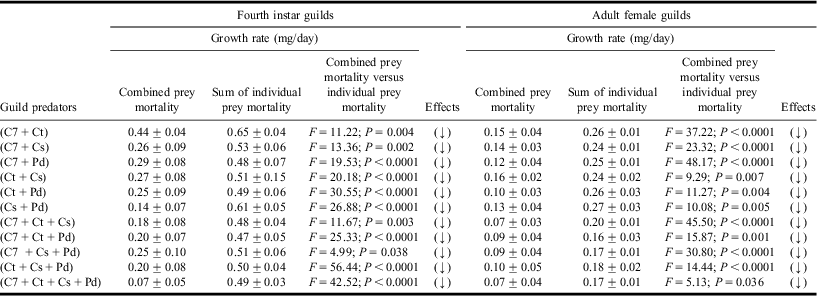
Values are mean ± SE; F-values significant at P < 0.05; df = 1, 19.
C7, Ct, Cs and Pd represent Coccinella septempunctata, Coccinella transversalis, Cheilomenes sexmaculata, and Propylea dissecta, respectively.
(↑), (→) and (↓) represent gain, no change, or loss in growth rates, respectively, based on Tukey's post hoc comparison of means.
Within larval and adult guilds the larger ladybirds (C. septempunctata and C. transversalis) reported decline whereas the smaller ladybirds (C. sexmaculata and P. dissecta) reported no changes in their body mass (Figs 1, 2).
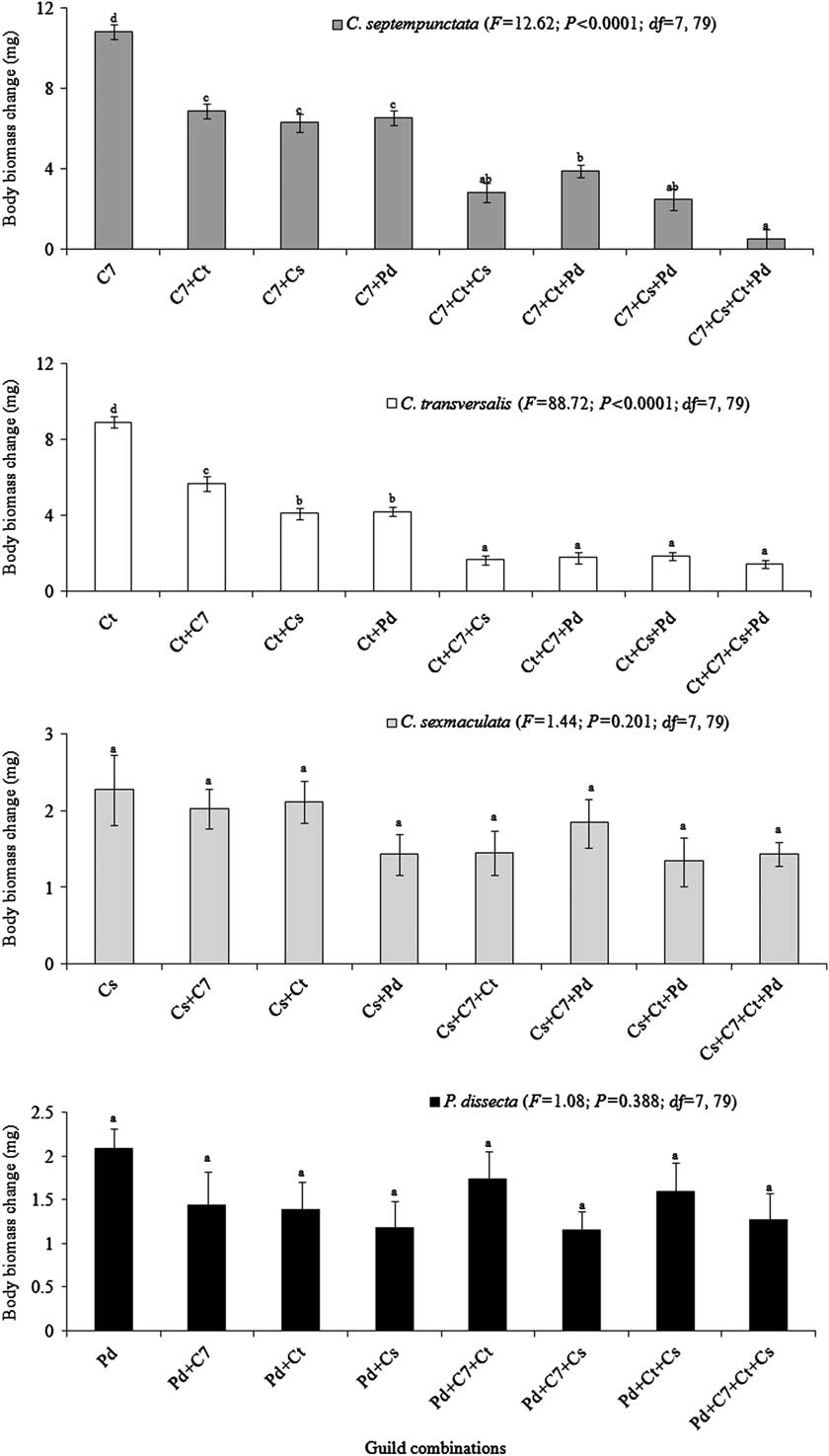
Fig. 1 Body mass change (mg) by individual Coccinellidae while sharing the common prey population (combined predation) in fourth instar guilds. C7, Ct, Cs, and Pd represent Coccinella septempunctata, Coccinella transversalis, Cheilomenes sexmaculata, and Propylea dissecta, respectively. F-value significant P < 0.05.
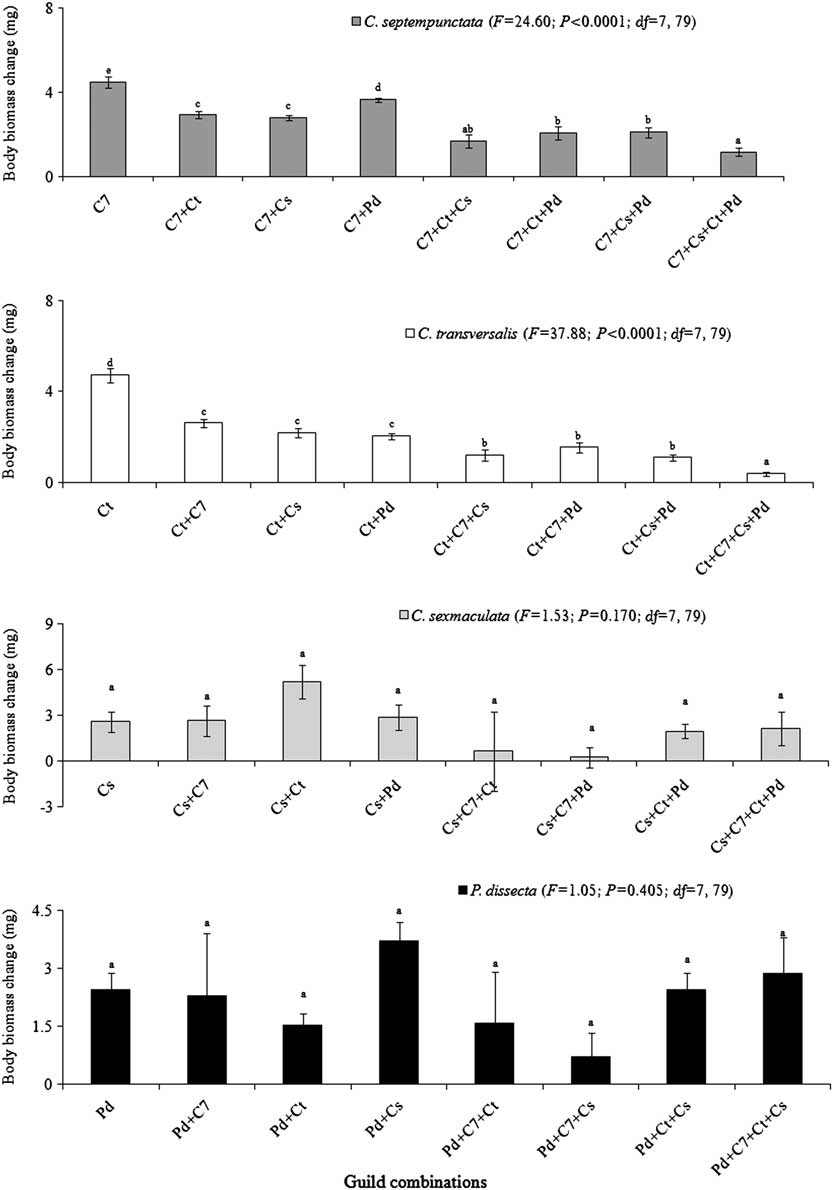
Fig. 2 Body mass change (mg) by individual Coccinellidae while sharing the common prey population (combined predation) in adult female guilds. C7, Ct, Cs, and Pd represent Coccinella septempunctata, Coccinella transversalis, Cheilomenes sexmaculata, and Propylea dissecta, respectively. F-value significant P < 0.05.
Discussion
In the present study, both the larval and adult guilds with multiple predators have shown antagonistic effects. Decreased aphid prey mortality with increase in predator richness within the guild probably indicates the existence of predator–predator interactions, either through interference competition and/or the exploitative competition (Michelakis Reference Michelakis1973; Hassell et al. Reference Hassell, Lawton and Beddington1976; Eveleigh and Chant Reference Eveleigh and Chant1982). In the presence of multiple predators, it is believed that the area searched by individual predators decreases (Pandey et al. Reference Pandey, Kumar, Singh, Shanker and Tripathi1984) and they probably consume less aphid prey than their usual predation rates (Muller and Godfray Reference Muller and Godfray1999; Amarasekare Reference Amarasekare2000; Noia et al. Reference Noia, Borges and Soares2008; Hodek et al. Reference Hodek, van Emden and Honek2012). Results indicating antagonistic effects on use of multiple predators have been reported in many earlier studies (Snyder and Ives Reference Snyder and Ives2001; Eubanks et al. Reference Eubanks, Blackwell, Parish, Delamar and HullSanders2002; Kaplan and Eubanks Reference Kaplan and Eubanks2002; Denno and Finke Reference Denno and Finke2006).
The recorded antagonistic effects and the influence of guild size and/or guild biomass on the observed prey mortality within the larval and adult guilds further reveal that the responses of ladybird predators under experimental conditions are associated with the difference in their body size.
Larger ladybirds have the capacity to feed on large aphids at both low and high densities, but need high quantity of small aphids to sustain themselves (Dixon and Hemptinne Reference Dixon and Hemptinne2001; Dixon Reference Dixon2007; Sloggett Reference Sloggett2008; Sloggett et al. Reference Sloggett, Haynes and Obrycki2009). Despite the presence of intermediate aphid instars in the experimental arena (equally suitable for both the large and small ladybirds) of the present study, larger ladybirds have shown reduction in their usual body mass when kept with either larger or smaller ladybird predators. This might be (i) due to the consumption of less aphid prey, and/or (ii) the consumption of aphid prey less efficiently, owing to their lower conversion efficiencies and growth rates (Mishra et al. Reference Mishra, Omkar, Kumar and Pandey2012).
On the contrary, small ladybirds are more efficient in exploiting small aphids under both low and high aphid densities due to their lower food requirements; hence, they show a competitive advantage over larger ones during exploitative competition (Obrycki et al. Reference Obrycki, Giles and Ormord1998; Evans Reference Evans2004). Eventually, with their reduced energy requirements and higher conversion efficiencies (Mishra et al. Reference Mishra, Kumar, Shahid and Singh2011, Reference Mishra, Omkar, Kumar and Pandey2012), they may compensate for reduced prey consumption by further enhancing their prey exploitation and conversion efficiencies, resulting in attainment of their usual body mass, as reported by Schuder et al. (Reference Schuder, Hommes and Larnik2004) in the larvae of smaller ladybird, Adalia bipunctata (Linnaeus) under reduced prey availability.
The reduction in guild conversion efficiencies and guild growth rates also strengthens the presence of predator–predator interactions among the constituent guild predators. Studies have shown that species-specific morphological and behavioural tendencies like large body size, strong larval spines, chemical protection, rapid larval development, great nutritional plasticity, and high aggressiveness (Labrie et al. Reference Labrie, Lucas and Coderre2006; Pervez and Omkar Reference Pervez and Omkar2006; Sato et al. Reference Sato, Jimbo, Yasuda and Dixon2008) play a vital role in influencing predation, and in many cases, even lead to displacement of many native predatory species (Dixon Reference Dixon2000; Ware and Majerus Reference Ware and Majerus2008; Gardiner et al. Reference Gardiner, O'Neal and Landis2011; Grez et al. Reference Grez, Viera and Soares2012). Also, during such interactions, species-specific toxins or alkaloids (Hautier et al. Reference Hautier, Gilles, Callier, de Biseau and Gregoire2011) adversely affect the foraging behaviour of co-guild predators (Agarwala et al. Reference Agarwala, Yasuda and Kajita2003; Wilder and Rypstra Reference Wilder and Rypstra2004; Magalhães et al. Reference Magalhães, Tudorache, Montseratt, van Maanen, Sabelis and Janssen2005; Nakashima et al. Reference Nakashima, Birkett, Pye and Powell2006; Montserrat et al. Reference Montserrat, Bas, Magalhães, Sabelis, de Roos and Janssen2007; Rypstra et al. Reference Rypstra, Schmidt, Reif, DeVito and Persons2007).
Among the experimental guilds, the observed prey mortalities were relatively higher in two-predator guilds, probably due to low predator–predator interactions. Within these two-predator combinations, the highest prey mortalities were recorded in those guilds (C7 + Ct within larval and C7 + Cs within adult guilds) where C. septempunctata was one of the predators. This may be due to its large size and exceptionally high voracity than the other ladybird species. These and similar other intrinsic properties of C. septempunctata are also associated with its dominance in most habitats of Palaearctic and Nearctic regions (Omkar and Pervez Reference Omkar and Pervez2002; Hodek and Michaud Reference Hodek and Michaud2008).
The results indicate that larger ladybirds have higher voracities (Finlayson et al. Reference Finlayson, Alyokhin, Gross and Porter2010; Mishra et al. Reference Mishra, Kumar, Shahid and Singh2011), and smaller ladybirds have higher conversion efficiencies (Mishra et al. Reference Mishra, Omkar, Kumar and Pandey2012), and both have competitive advantages over each other during interference and exploitative competitions, respectively. Yet, the extraguild aphid prey is at lower risk of being preyed when both are released in tandem under laboratory conditions. Also, in the same field, despite exploitative or interference competitions, both these large and smaller ladybirds co-exist. This may be attributed to their tendency to feed on larger and smaller aphid instars, respectively (Sloggett Reference Sloggett2008), their tendency to occupy different spatial positions (Omkar and Mishra Reference Omkar and Mishra2003; Lucas et al. Reference Lucas, Labrecque and Coderre2004; Janssen et al. Reference Janssen, Sabelis, Magalhaes, Montserrat and van der Hammen2007) or they are benefitted intrinsically (Omkar et al. Reference Omkar, Pervez, Mishra, Srivastava, Singh and Gupta2005; Hodek and Michaud Reference Hodek and Michaud2008). However, to validate and strengthen the findings, laboratories and field studies are still needed.
Acknowledgements
The authors are thankful to the Government of Uttar Pradesh, India for providing funds in the form of Centre of Excellence in Biocontrol of Insect Pests and also to Head, Department of Zoology, University of Lucknow for laboratory facilities and encouragements.








