Introduction
The Hymenoptera – ants, bees, wasps, and sawflies – have been tremendously successful, increasing their taxonomic and morphological diversity, filling ecospace by assuming a wide range of trophic roles, and saturating communities with great numbers of individuals across the globe. They are one of four hyperdiverse insect orders today, along with the Diptera, Lepidoptera, and Coleoptera. Hymenoptera comprise over 153 000 named species, perhaps almost 10% of all described species of life, and if unnamed species are considered, there might be four or more times that number (Gaston Reference Gaston1991; Sharkey Reference Sharkey2007; Davis et al. Reference Davis, Baldauf and Mayhew2010; Aguiar et al. Reference Aguiar, Deans and Engel2013; Klopfstein et al. Reference Klopfstein, Vilhelmsen, Heraty, Sharkey and Ronquist2013). Today they are major elements of modern terrestrial ecosystems, acting across a broad spectrum of feeding guilds as predators, parasitoids, and hyperparasitoids of other arthropods, scavengers, pollinators, and herbivores consuming plant organs both externally and internally, including pollen and nectar feeding, galling, leaf chewing and mining, and wood and stem boring. Some also tend phytophagous insects in return for fluid excretions, farm fungi for food, and engage in other symbiotic activities. In these ways, they regulate populations of plants and arthropods, affecting their community interactions, reproduction, diversities, and distributions.
Most major modern hymenopteran trophic guilds and lineages to the family level were established in the Mesozoic, but expansion of ecologically key groups below this level – notably of social Hymenoptera (ants, some bees, and some Vespidae), parasitoids (Ichneumonidae), and phytophagous Tenthredinoidea – is first seen in the Eocene, showing the onset of their rise to become the major elements of terrestrial ecosystems that they are today.
Hymenoptera were amongst the first Ypresian (early Eocene) Okanagan Highlands insects collected by Geological Survey of Canada geologists George Mercer Dawson in 1877 (Dawson Reference Dawson1879) and Lawrence Lambe in 1906 (Handlirsch Reference Handlirsch1910) in the decades immediately after British Columbia entered Confederation in 1867. The specimens were sent to Samuel Scudder in Cambridge, Massachusetts, United States of America (e.g., Scudder Reference Scudder1877, Reference Scudder1878, Reference Scudder1879, Reference Scudder1890) and Anton Handlirsch in Vienna, Austria (Handlirsch Reference Handlirsch1910). Much of the interior of British Columbia, however, remained remote and difficult to reach due to rough terrain and dense forests until well into the 20th century, and research on its fossil Hymenoptera after this initial interest was sporadic (e.g., Rice Reference Rice1968), lagging behind work on European Eocene deposits such as Baltic amber and those in the mid-continental United States of America that were easily accessed by railway, such as the Green River (Grande Reference Grande1984) and Florissant (Meyer Reference Meyer2003) Formations. The known Okanagan Highlands Hymenoptera were summarised by Cameron (Reference Cameron1917) as four species of Ichneumonidae, two of Braconidae, and three of Formicidae. Very little was written about them for the next 60 years, and they only began to receive intensive attention in the final decades of the century, primarily sparked by the works of Wilson (Reference Wilson1977a, Reference Wilson1978a, Reference Wilson1978b, Reference Wilson1982), followed by Douglas and Stockey (Reference Douglas and Stockey1996), and in the first reports of insects from the rich deposits at the southernmost Okanagan Highlands locality in Republic, Washington, United States of America by Lewis (Reference Lewis1992), Wehr and Barksdale (Reference Wehr and Barksdale1996), and Wehr (Reference Wehr1998). While some works treated Hymenoptera among insects in general (e.g., above references, and Archibald and Mathewes Reference Archibald and Makarkin2000), relatively a few focussed on them until recently (Rice Reference Rice1968; Dlussky and Rasnitsyn Reference Dlussky and Rasnitsyn1999, Reference Dlussky and Rasnitsyn2003; Pulawski et al. Reference Pulawski, Rasnitsyn, Brothers and Archibald2000; Engel and Archibald Reference Engel and Archibald2003; Archibald et al. Reference Archibald, Bossert, Greenwood and Farrell2006; Archibald and Rasnitsyn Reference Archibald, Morse, Greenwood and Mathewes2015), and knowledge of the order in the Okanagan Highlands has not kept pace with the potential provided by increasingly large collections of their fossils in recent years. Here, we provide an overview of the rich, but understudied, Ypresian fossil Hymenoptera assemblage of the Okanagan Highlands of British Columbia, Canada and Washington, United States of America (Archibald et al. Reference Archibald2010, Reference Archibald, Greenwood and Mathewes2011a), in light of recent collecting, and evaluate its signficance in the modernisation of the order.
Materials and methods
The fossiliferous lacustrine shales of the Okanagan Highlands often consist of fine, easily splitting laminae, which have been found, in cases where tested, usually to consist of sapropel and siliceous laminae of diatomaceous origin (Wilson Reference Wilson1977b; Mustoe Reference Mustoe2005, Reference Mustoe2015; Wolfe and Edlund Reference Wolfe and Edlund2005). This presumably promoted fine-level preservation and increased fidelity of the fossil assemblage to the life assemblage (Archibald and Makarkin Reference Archibald, Johnson, Mathewes and Greenwood2006, based on taphonomic processes modelled at Florissant by McLeroy and Anderson Reference McLeroy and Anderson1996; Harding and Chant Reference Harding and Chant2000; O’Brien et al. Reference O’Brien, Meyer, Reilly, Ross and Maguire2002, Reference O’Brien, Meyer and Harding2008). Amber is also present at some sites such as Hat Creek, where it is usually clear to yellow, in pieces up to 3–4 cm in diameter, found in situ within coal beds.
The Okanagan Highlands (Fig. 1) deposits occur in former lake and swamp basins scattered from west-central British Columbia, Canada across ~1000 km to north-central Washington, United States of America (Archibald et al. Reference Archibald, Greenwood and Mathewes2011a). All major localities bear Hymenoptera fossils (Fig. 1; Table 1). These include the Klondike Mountain Formation exposures at Republic, Washington, United States of America; and in British Columbia, Canada, the Allenby Formation at Princeton, Coldwater Beds at Quilchena, unnamed formations at Falkland, McAbee (often informally called “Tranquille Shales”), Hat Creek, and Horsefly River, and in the Ootsa Lake Group shales at Driftwood Canyon Provincial Park near Smithers. The location near the town of Quesnel in the central Cariboo region of British Columbia where fossil insects were collected by G.M. Dawson in the 1870s (Scudder Reference Scudder1877, Reference Scudder1878: see Ichneumonoidea and ants, below) is currently unknown, but it appears to be part of the Okanagan Highlands series. Dawson also sent Scudder insects from an exposure of the Allenby Formation on a branch of the Similkameen River that is today called the Tulameen. The Tranquille locality (see Handlirsch’s species of Ichneumonidae, below) is on the north shore of Kamloops Lake between Kamloops and McAbee.

Fig. 1 Map of the Okanagan Highlands, British Columbia, Canada and Washington, United States of America, with localities mentioned in the text.
Table 1 Distribution of Okanagan Highlands Hymenoptera families.

Notes: X, new records, new specimens, and previously reported with specimens examined by us (see text). Previously reported only, specimens not seen by us, but confirmed by photographs and/or drawings: RI, Rice (1968); L, Labandeira (2002); DS, Douglas and Stockey (1996) and DS?, questionable records in that work (Cynipidae) or occurrence based on specimens that were not illustrated nor seen by us (Allenby Ichneumonidae).
R, Republic and surrounding localities of the Klondike Mountain Formation; A, Allenby Formation, Princeton and surrounding localities; Q, Quilchena; F, Falkland; M, McAbee; Hc, Hat Creek amber; Hf, Horsefly; D, Driftwood Canyon.
While some genera and species described by Scudder (Reference Scudder1877, Reference Scudder1878, Reference Scudder1879) and Handlirsch (Reference Handlirsch1910) over a century ago are in need of revision, we mostly agree with their determinations of Hymenoptera fossils to the family level (see below). The Okanagan Highlands insect taxon lists of Wehr and Barksdale (Reference Wehr and Barksdale1996) and Wehr (Reference Wehr1998) do not associate specimens with taxa; therefore, as the identities of individual specimens are revised, the status of some listed families has become unclear. As we examined all specimens that we presume they based their lists on, the Hymenoptera portions of those are superseded here. For some families where large numbers of specimens are known, we list exemplars, as noted.
Specimens were borrowed from institutional collections or collected by Archibald and Mathewes, and a few were evaluated from published illustrations as indicated. Our goal here is to provide a family-level overview, with determinations below this in some cases. Several higher groups, including “Symphyta”, “Parasitica”, and “Spheciformes” are generally recognised as paraphyletic and are thus informal, but nevertheless useful in discussion. We will not burden the text by indicating them between quotation marks hereafter. Recent molecular analyses further suggest that groups such as Vespoidea, Crabronidae, and Tenthredinidae might also be paraphyletic (Pilgrim et al. Reference Pilgrim, Von Dohlen and Pitts2008; Song et al. Reference Song, Tang, Wei and Chen2016 and references therein; Branstetter et al. Reference Branstetter, Danforth and Pitts2017; Peters et al. Reference Peters, Krogmann and Mayer2017); we prefer to take a conservative position until consensus is achieved, and so we generally follow the systematic arrangement of Aguiar et al. (Reference Aguiar, Deans and Engel2013) without further comment, except that we recognise Scelionidae as a distinct family (see discussion of McKellar and Engel Reference McKellar and Engel2012) and do not recognise Aulacidae, which appears insufficiently distinguishable from the Gasteruptiidae in the Mesozoic (Townes Reference Townes1950). Furthermore, these molecular studies include dated phylogenies that differ in their estimated times of origin of various groups, and we will not attempt to comment on these, but rather refer to actual fossil occurrences for dating.
Institutional abbreviations for particular specimens examined or cited are: CDM, the Courtenay and District Museum and Archives, Courtenay, British Columbia, Canada; CMN, Canadian Museum of Nature, Ottawa, Ontario, Canada; GSC, Geological Survey of Canada, Ottawa, Ontario, Canada; DMNH, the Denver Museum of Nature and Science, Denver, Colorado, United States of America; KM, Kelowna Museums, Kelowna, British Columbia, Canada; RBCM, Royal British Columbia Museum, Victoria, British Columbia, Canada; PMF, the Princeton and District Museum and Archives, Princeton, British Columbia, Canada; ROM, Royal Ontario Museum, Toronto, Ontario, Canada; SFU, Simon Fraser University, Burnaby, British Columbia, Canada; SR, SRUI, the Stonerose Interpretive Center, Republic, Washington, United States of America; TRU, Thompson Rivers University, Kamloops, British Columbia, Canada; UAPAL, University of Alberta, Edmonton, Alberta, Canada; UWBM, University of Washington, Burke Museum, Seattle, Washington, United States of America. Type and other specimens referred to with these prefixes to their catalogue numbers are housed in these institutions. “SBA-” numbers were collected by Archibald and are housed at Simon Fraser University; “SBA-” specimens from the Driftwood Canyon locality in Driftwood Canyon Provincial Park are the property of BC Parks (British Columbia Ministry of Environment), and are also housed at Simon Fraser University until BC Parks establishes a permanent repository. Many shale specimens include both the “part” and “counterpart”, i.e., both sides of a split piece of shale with a fossil on one side and its mirror image on the other. The part is designated the “a” side and the counterpart the “b” side where this is specified on accession numbers, and this appears where relevant in the text.
Ages, names, and spellings of Cretaceous amber deposits follow Rasnitsyn et al. (Reference Rasnitsyn, Bashkuev and Kopylov2016). We follow Smith et al. (Reference Smith, Singer and Carroll2003, Reference Smith, Singer and Carroll.2004) in considering the Green River Formation insects as deposited at sites ranging through the second half of the Ypresian, i.e., contemporaneous with those of the Okanagan Highlands; Lenz et al. (Reference Lenz, Wilde, Mertz and Riegel2015) in considering the lacustrine shale at Messel, Germany, to be latest Ypresian into the Lutetian; Kodrul (Reference Kodrul1999) in considering Sakhalin amber to be Lutetian (early middle Eocene); Dilcher (Reference Dilcher1973) and Dockery (Reference Dockery1996) in considering the Cockfield Formation (formerly clays of the Wilcox Formation) to be Bartonian (late middle Eocene); and Perkovsky et al. (Reference Perkovsky, Rasnitsyn, Vlaskin and Taraschuk2007) in considering Baltic amber as Priabonian (late Eocene).
Family Pamphiliidae (Symphyta: Pamphilioidea)

Fig. 2 Symphyta: Siricidae (A), Cephidae (B), Pamphiliidae (C), Cimbicidae (D–M). A, RBCM.EH2015.004.0001.001A, holotype of Ypresiosirex orthosemos Archibald and Rasnitsyn, McAbee. B, TRU F-1545, holotype of Cuspilongus cachecreekensis Archibald and Rasnitsyn, McAbee. C, UWBM 77532, holotype forewing of Ulteramus republicensis Archibald and Rasnitsyn, Republic. D, SBA-2990, McAbee. E, TRU F-848, McAbee. F, RBCM.EH2007.002.0001, Allenby Formation. G, TRU F-771, McAbee. H, TRU F-1565b, McAbee. I, TRU F-1563, McAbee. J, TRU F-1182, McAbee. K, TRU F-1564a, McAbee. L, UWBM-54840, Republic. M, SR 92-18-08, Republic. N, SBA-2418, McAbee. D–N, to same scale.
Specimens. Republic: UWBM 77532, holotype, Ulteramus republicensis Archibald and Rasnitsyn.
Remarks. Today the Pamphiliidae has 291 described species in the subfamilies Pamphiliinae, Cephalciinae, and Juralydinae, distributed in temperate and boreal Eurasia and North America (Taeger et al. Reference Taeger, Blank and Liston2010; Aguiar et al. Reference Aguiar, Deans and Engel2013). Their larvae spin silk, living either singly or in groups, and feed on foliage of the Pinaceae (Cephalciinae) or on angiosperm leaves, which they roll (Pamphiliinae) (Goulet Reference Goulet1993). Their fossil record extends to the late Jurassic with the subfamily Juralydinae, previously thought to be extinct, but which was recently expanded to include an extant genus (Wang et al. Reference Wang, Rasnitsyn, Shih and Ren2014b, Reference Wang, Rasnitsyn, Li, Shih, Sharkey and Dong2016). Ulteramus republicensis appears to belong to one of the two other subfamilies, previously unknown before the early Oligocene (summary: Archibald and Rasnitsyn Reference Archibald, Morse, Greenwood and Mathewes2015).
Family Cephidae (Symphyta: Cephoidea)
Specimens. Horsefly River: Cephinae species A (Archibald and Rasnitsyn Reference Archibald, Morse, Greenwood and Mathewes2015): RBCM.EH2015.005.0001.001 and RBCM.EH2015.005.0001.002; McAbee: Cuspilongus cachecreekensis Archibald and Rasnitsyn, holotype (Cuspilonginae): TRU F-1545 (part) and TRU F-1546 (counterpart).
Remarks. The family has 160 described modern species (Aguiar et al. Reference Aguiar, Deans and Engel2013), almost all of which are members of the Cephinae. They are predominantly temperate/boreal Holarctic, with a few species ranging into lower latitudes (summary: Archibald and Rasnitsyn Reference Archibald, Morse, Greenwood and Mathewes2015). Their larvae mostly feed inside grass stems or within the twigs of woody plants (Goulet Reference Goulet1993). The fossil record of the Cephidae is sparse: outside of the Okanagan Highlands, there are three species of unassigned subfamily affinity from the early Cretaceous of Asia (one at Baissa and two from Obeschchayushchiy), one from Mongolia (Bon-Tsagaan) belonging to the Cuspilonginae along with the McAbee species (Kopylov and Rasnitsyn Reference Kopylov and Rasnitsyn2016), and two more in the Priabonian (one each in Florissant shale and Baltic amber), both belonging to the Cephinae; the two species described by Heer (Reference Heer1847) in the Miocene of Oeningin, Germany are ants (Formicidae) (Archibald and Rasnitsyn Reference Archibald, Morse, Greenwood and Mathewes2015).
Family Siricidae (Symphyta: Siricoidea)
Specimens. McAbee: RBCM.EH2015.004.0001.001A&B, holotype, Ypresiosirex orthosemos Archibald and Rasnitsyn, in subfamily Siricinae (as defined therein). Republic: SRUI 99-97-08.1.
Remarks. Siricidae has 111 described modern species (Aguiar et al. Reference Aguiar, Deans and Engel2013). They are primarily Holarctic, with a few species that are native to lower latitudes, and some introduced in the Southern Hemisphere (review: Archibald and Rasnitsyn Reference Archibald, Morse, Greenwood and Mathewes2015). Their larvae bore through wood feeding on fungus, which they grow in galleries, killing the tree (Schiff et al. Reference Schiff, Goulet, Smith, Boudreault, Wilson and Scheffler2012). A group of siricids (sensu lato) assigned to currently loosely defined extinct subfamilies or to none, extend from the Pliensbachian (early Jurassic) (Rasnitsyn Reference Rasnitsyn1968) to the Albian (latest early Cretaceous) (Gromov et al. Reference Gromov, Dmitriev, Zherikhin, Lebedev, Ponomarenko, Rasnitsyn and Sukaczewa1993). Siricidae sensu stricto (=Siricinae, sensu Rasnitsyn Reference Rasnitsyn1968, i.e., which includes those currently assigned to the Tremicidae: see also Wedmann Reference Wedmann1998; Wedmann et al. Reference Wedmann, Pouillon and Nel2014; Archibald and Rasnitsyn Reference Archibald, Morse, Greenwood and Mathewes2015) has a modest fossil record beginning in the Albian.
Family Tenthredinidae (Symphyta: Tenthredinoidea)

Fig. 3 Symphyta: Tenthredinidae (A–M, O–R) and Tenthredinoidea incertae sedis N. A, SBA-82A, McAbee. B, UWBM 7522A, McAbee. C, UWBM 77545, McAbee. D, TRU F-1581A, McAbee. E, TRU F-1548, McAbee. F, TRU F-1569, McAbee. G, TRU F-1567, McAbee. H, SBA-192, McAbee. I, TRU F-803, McAbee. J, TRU F-1568, McAbee. K, SBA-227A, McAbee. L, SBA-248B, McAbee. M, TRU F-1573a, McAbee. N, PMF.2016.0824.002, Allenby Formation. O, SRUI 99-90-50, Republic. P, SBA-5421, Horsefly River. Q, SBA-5151, Horsefly River. R, SFU Q-5147A, Quilchena. All to same scale, except for N.
Specimens. Driftwood Canyon: SBA-3155, SBA-5134. Horsefly: SBA-5151, SBA-5421, SBA-5525, SBA-5559. McAbee, many, e.g., TRU F-803, TRU F-1548, TRU F-1567, TRU F-1568, TRU F-1569, TRU F-1570, TRU F-1571, TRU F-1572, TRU F-1573, TRU F-1581, SBA-2000, SBA-2374, SBA-2618, SBA-2794, UWBM 7522A, UWBM 77545, UWBM PB-4087; the following subfamilies identified at McAbee (Archibald Reference Archibald2007): Tenthredininae: SBA-192, SBA-227, SBA-329, SBA-375, SBA-536, SBA-2185, SBA-2497, SBA-2524, SBA-2959; Allantinae: SBA-82, SBA-244, SBA-248, SBA-2186, SBA-2388; Blennocampinae: SBA-2828; Nematinae: SBA-108, SBA-2831, SBA-1966, SBA-2559. Quilchena: SFU Q-5147. Republic: SRUI 99-90-50, SR 05-03-02, SR 99-90-80.2.
Previous records. Allenby Formation: GSC 22688 Eriocampa tulemeenensis Rice (Allantinae). Horsefly River: GSC 22689 Pseudosiobla campbelli Rice (Allantinae), both described by Rice (Reference Rice1968). Douglas and Stockey (Reference Douglas and Stockey1996) reported specimens UAPAL 4500, 4548, and 4545 from Horsefly River as tenthredinids; however, we agree with Nel (Reference Nel2004) that these are too poorly preserved to confidently assign to a family.
Remarks. The Tenthredinidae is today the largest family of phytophagous Hymenoptera, with 5500 described species that primarily inhabit Holarctic regions as far north as plant growth, although a small number is found in the Southern Hemisphere (Goulet Reference Goulet1993; Smith Reference Smith2003; Aguiar et al. Reference Aguiar, Deans and Engel2013). They mostly feed externally on leaves, but some are stem or twig borers, or leaf miners. Palaeathalia layangensis Zhang from the Laiyang Formation (roughly correlated with the Barremian-Aptian Yixian Fm) in northeast China (Zhang Reference Zhang1985) confidently belongs to the family, and there are undescribed undoubted tenthredinid species from the Aptian of Mongolia and the Ola Formation of northeast Russia, dated Santonian or possibly early Campanian (A.P.R., personal observation) (see further discussion, below). They are described from the Paleocene of Menat, and then found in various Okanagan Highlands localities (above) and in the Priabonian at Florissant and in Baltic amber and younger deposits (reviewed by Vilhelmsen and Engel Reference Vilhelmsen and Engel2012).
Family Cimbicidae (Symphyta: Tenthredinoidea)
Specimens. McAbee: TRU F-771, TRU F-848, TRU F-1182, TRU F-1563, TRU F-1564, TRU F-1565, TRU F-1566, SBA-530, SBA-2990; Cimbicinae: SBA-1975, SBA-1139; Coryninae or Pachylostictinae: SBA-2418 (subfamilies as in Archibald Reference Archibald2007). Allenby: RBCM.EH2007.002.0001. Republic: SR 92-18-08, UWBM-54840.
Remarks. Cimbicids range through much of the Holarctic, in the Western Hemisphere as far south as the United States of America, except for a subfamily native to Brazil, Argentina, and Paraguay (Smith Reference Smith1988). They comprise 182 described modern species of sometimes-large sawflies, whose larvae feed on the leaves of a variety of dicot angiosperms (Goulet Reference Goulet1993; Taeger et al. Reference Taeger, Blank and Liston2010; Aguiar et al. Reference Aguiar, Deans and Engel2013). Their earliest recorded occurrence is Cenocimbex menatensis Nel from the Paleocene of Menat, France (Nel Reference Nel2004). Eopachylosticta byrami Cockerell from the Green River Formation is roughly contemporaneous with the Okanagan Highlands, and all other published occurrences are younger, in the Priabonian of Florissant and a variety of Miocene localities (Taeger et al. Reference Taeger, Blank and Liston2010).
Family incertae sedis (Symphyta: Tenthredinoidea)
Specimen. Allenby: PMF.2016.0824.002
Remarks. This caterpillar belongs to the Tenthredinidae, Cimbicidae, or Diprionidae, based on the numerous (six or seven) annulets per segment (other larval Symphyta have at most four annulets), but cannot be assigned to any of these on the characters preserved.
Family Megaspilidae (Apocrita: Parasitica: Ceraphronoidea)

Fig. 4 Parasitica: Trigonalidae (A, C), Megaspilidae (B), Diapriidae (D–K). A, SFU Q-5086, Quilchena. B, SBA-HC-9, Hat Creek amber. C, SBA-2993, Falkland. D, SBA-3124, Driftwood Canyon. E, SBA-3478, Driftwood Canyon. F, SBA-4814, Driftwood Canyon. G, SBA-5723, Driftwood Canyon. H, SBA-5718, Driftwood Canyon. I, SBA-3697, Driftwood Canyon. J, SBA-228, McAbee. K, SRUI 99-97-09, Republic. Scales differ, except D–K to same scale.
Specimens. Hat Creek amber. SBA-HC-9.
Remarks. The Megaspilidae has 299 described modern species (Aguiar et al. Reference Aguiar, Deans and Engel2013). They are small, cosmopolitan parasitoids and hyperparasitoids whose hosts are little known, although some are reported to attack Coccoidea, Neuroptera, Diptera, or Boreidae (Mecoptera), and some are hyperparasitoids of aphids through braconid wasps (Masner Reference Masner1993a). Megaspilids are rare as fossils before Okanagan Highlands time: undescribed species recorded in Burmese and Vendean Cretaceous amber (compiled by Rasnitsyn et al. Reference Rasnitsyn, Bashkuev and Kopylov2016, supplementary information table 1), two described species in Santonian Taimyr amber (one specimen each) and one in Campanian Canadian amber (two specimens); after Okanagan Highlands time they become more numerous, with eight species from Priabonian Baltic amber and two from Burdigalian shale of Spain (reviewed by McKellar and Engel Reference McKellar and Engel2011, Reference McKellar and Engel2012).
Family Trigonalidae (Apocrita: Parasitica: Trigonaloidea)
Figure 4A, C.
Specimens. Falkland: SBA-2993. Quilchena: SFU Q-5086
Remarks. The Trigonalidae is a small group of 92 rare modern species (Aguiar et al. Reference Aguiar, Deans and Engel2013). They are cosmopolitan, but predominantly tropical and subtropical, inhabiting a range of environments, but mostly montane forests (Carmean Reference Carmean1991; Weinstein and Austin Reference Weinstein and Austin1991; Carmean and Kimsey Reference Carmean and Kimsey1998). They may have complex life histories that include sequential hosts: symphytan or lepidopteran caterpillars consume their tiny eggs deposited on leaves, and they then become hyperparasitoids within their conspecifics or other parasitoids (e.g., Ichneumonidae or Tachinidae (Diptera)). They may even change hosts again to a predatory wasp that consumes the caterpillar+parasitoids (or +hyperparasitoids); the final instar feeds externally (Weinstein and Austin Reference Weinstein and Austin1991). These records are the oldest confident fossils of the family, as all currently reported fossils need confirmation (Cretaceous and Paleogene: e.g., Nel et al. Reference Nel, Perrichot and Néraudeau2003; Poinar Reference Poinar2005), except Trigonalys bischoffi Statz from the Aquitanian of Rott, which undoubtedly belongs to it. The majority of Cretaceous records compiled by Rasnitsyn et al. (Reference Rasnitsyn, Bashkuev and Kopylov2016, supplementary information table 1), actually refer to the misinterpreted extinct family Maimetshidae, except for Albiogonalys Nel et al., which most probably belongs to another extinct family, the Praeaulacidae (A.P.R., personal observation).
Family Ichneumonidae (Apocrita: Parasitica: Ichneumonoidea)

Fig. 5 Parasitica: Ichneumonidae. A, TRU F-1575, McAbee. B, SBA-2820A, McAbee. C, SFU Q-0018, Quilchena. D, PMF.2017.0134.001, Allenby Formation. E, SBA-140, McAbee. F, SBA-5845, Horsefly River. G, SBA-389A, McAbee. H, SBA-2097B, McAbee. I, SFU Q-5252, Quilchena. J, SBA-388B, McAbee. K, SR 87-61-10, Republic. L, SBA-605, McAbee. M–W, all from Driftwood Canyon: M, SBA-5013; N, SBA-3920; O, SBA-4474; P, SBA-4665; Q, SBA-5079; R, SBA-5255A; S, SBA-5303; T, SBA-5719; U, SBA-5248A; V, SBA-4425; W, SBA-4505. X, SRUI 99-85-75B; Y, SR 05-11-18; Z, SR 06-01-29A; AA, SR 01-08-18B; BB, SR 06-62-08, Republic. A–L and X–BB to same scale; M–W, to same scale.
Specimens. Driftwood Canyon: SBA-3719, SBA-3920, SBA-4367, SBA-4425, SBA-4469, SBA-4474, SBA-4484, SBA-4505, SBA-4540, SBA-4665, SBA-4857, SBA-5013, SBA-5025, SBA-5079, SBA-5248, SBA-5249, SBA-5255, SBA-5269, SBA-5303, SBA-5719, SBA-5724. Horsefly River: SBA-5845. McAbee: SBA-250, SBA-254, SBA-387, SBA-388, SBA-389, SBA-470, SBA-632, SBA-652, SBA-1801, SBA-2272, SBA-2560, SBA-3002, TRU F-1575. Quilchena: SFU Q-0018, SFU Q-5252. Allenby: PMF.2017.0134.001. Republic: UWBM-77457, SRUI 99-85-75, SR 01-08-18, SR 05-11-18, SR 06-01-29, SR 06-08-06, SR 06-62-08, SR 09-18-03, SR 09-18-05, SR 87-61-10.
Previous records. Samuel Scudder (Reference Scudder1877) described Pimpla saxea Scudder, P. decessa Scudder, and P. senecta Scudder from the shales at Quesnel, and Handlirsch (Reference Handlirsch1910) described Xylonomus lambei Handlirsch from the Tranquille River. Wings of the following were figured by Archibald (Reference Archibald2007), all from McAbee: SBA-110, SBA-138, SBA-140, SBA-146, SBA-349, SBA-378, SBA-394, SBA-412, SBA-563, SBA-605, SBA-638, SBA-668, SBA-684, SBA-767, SBA-1927, SBA-1988, SBA-2023, SBA-2096, SBA-2097, SBA-2106, SBA-2107, SBA-2109, SBA-2110, SBA-2155, SBA-2156, SBA-2200, SBA-2214, SBA-2258, SBA-2263, SBA-2416, SBA-2417, SBA-2428, SBA-2433, SBA-2444, SBA-2499, SBA-2551, SBA-2558, SBA-2710, SBA-2783, SBA-2820, SBA-2839, SBA-2848, SBA-2859, SBA-2953, SBA-2975, SBA-3001. Douglas and Stockey (Reference Douglas and Stockey1996) report UAPAL 4599 and UWBM 57112 as ichneumonids from the Allenby Formation, but they are not illustrated and we have not seen them.
Remarks. There are 24 025 described modern species of Ichneumonidae (Aguiar et al. Reference Aguiar, Deans and Engel2013), distributed across the globe. Although there is debate whether their species richness lies in mid-latitudes (Janzen Reference Janzen1981; Quicke Reference Quicke2012; Veijalainen et al. Reference Veijalainen, Wahlberg, Broad, Erwin, Longino and Sääksjärvi2012), there is some evidence supporting this notion (see Discussion section). They are parasitoids and hyperparasitoids, overwhelmingly of the larvae or pupae of holometabolous insects (most commonly Symphyta and Lepidoptera) and in some cases of the adults or eggs of Chelicerata, either internally or externally (Gauld Reference Gauld1988). They may feed upon immobilised, paralysed prey (idiobionts) or allow their host to remain active (koinobionts), in close synchrony with them, often exerting control over their development. As endoparasites they employ sophisticated chemical control of their hosts’ immune response. They may attack prey that are exposed or concealed within plant tissue. They first appear in the early Cretaceous, but remain a small group, becoming diverse and numerous only in the Eocene (see Discussion, below) (Grimaldi et al. Reference Grimaldi, Shedrinsky and Wampler2000; Rasnitsyn Reference Rasnitsyn2002; Zherikhin Reference Zherikhin2002; Kopylov Reference Kopylov2010; Kopylov et al. Reference Kopylov, Brothers and Rasnitsyn2010; McKellar et al. Reference McKellar, Kopylov and Engel2013). Ichneumonids are among the most numerous insects found at McAbee after March flies (Diptera, Bibionidae, Plecia Wiedemann species) and Auchenorrhyncha (Hemiptera) (Archibald et al. Reference Archibald2010), and are anecdotally so throughout the Okanagan Highlands. Exemplars of their many specimens are listed here.
Family Braconidae (Apocrita: Parasitica: Ichneumonoidea)

Fig. 6 Parasitica: Braconidae. A, TRU F-237, McAbee. B, SR 11-31-01, Republic. C, TRU F-1090, McAbee. D, SFU Q-5088, Quilchena. E, SBA-756, Horsefly River. F, SBA-3698, Driftwood Canyon. G, SBA-3466, Driftwood Canyon. H, SBA-4613, Driftwood Canyon. I, SBA-3004, Driftwood Canyon. J, SBA-3567, Driftwood Canyon. K, SBA-4403, Driftwood Canyon. L, SBA-3732, Driftwood Canyon. M, SR 06-59-03A, Republic. A–D, K, and M to same scale; E–F and I–J to same scale; G–H and L to same scale.
Specimens. Driftwood Canyon: SBA-3004, SBA-3466, SBA-3567, SBA-3688, SBA-3698, SBA-3732, SBA-4477, SBA-4403, SBA-4567, SBA-4613. Horsefly River: SBA-756. McAbee: TRU F-1090, TRU F-237; Quilchena: SFU Q-5088. Republic: SR 06-59-03, SR 11-31-01.
Previous records. Scudder (Reference Scudder1879) reported several specimens (Geological Survey of Canada numbers GSC 69 and 78) as Bracon Fabricius species from an Allenby Formation exposure on the Tulameen River (“north fork of the Similkameen”: see Introduction). Scudder (Reference Scudder1877) also described Calyptites antediluvianum Scudder from the shales at Quesnel as belonging to the Braconidae, but this insect was later considered to be an ant by Wheeler (Reference Wheeler1911), and as having an unresolved family position by Bolton (Reference Bolton2003).
Remarks. Braconids are generally considered the second-most diverse family of Hymenoptera after the Ichneumonidae, with 19 205 described modern species, distributed across the globe (Wahl and Sharkey Reference Wahl and Sharkey1993; Aguiar et al. Reference Aguiar, Deans and Engel2013). They are most often endoparasitic idiobionts, and, unlike ichneumonids, many attack nymphal Hemimetabola, are not known to prey upon Araneae (spiders), and are less frequently hyperparasitoids (Gauld Reference Gauld1988; Wahl and Sharkey Reference Wahl and Sharkey1993). They have a small Cretaceous fossil record beginning in the Berriassian of Mongolia, and are first seen as diverse and numerous in the Priabonian (Rasnitsyn Reference Rasnitsyn2002; Perrichot et al. Reference Perrichot, Nel and Quicke2009; Ortega-Blanco et al. Reference Ortega-Blanco, Delclòs and Engel2011a; Belokobylskij Reference Barreda, Cúneo, Wilf, Currano, Scasso and Brinkhuis2012; McKellar and Engel Reference McKellar and Engel2012; Li et al. Reference Li, Kopylov, Shih and Ren2017) (further detail: see Discussion, below).
Family Monomachidae (Apocrita: Parasitica: Diaprioidea)
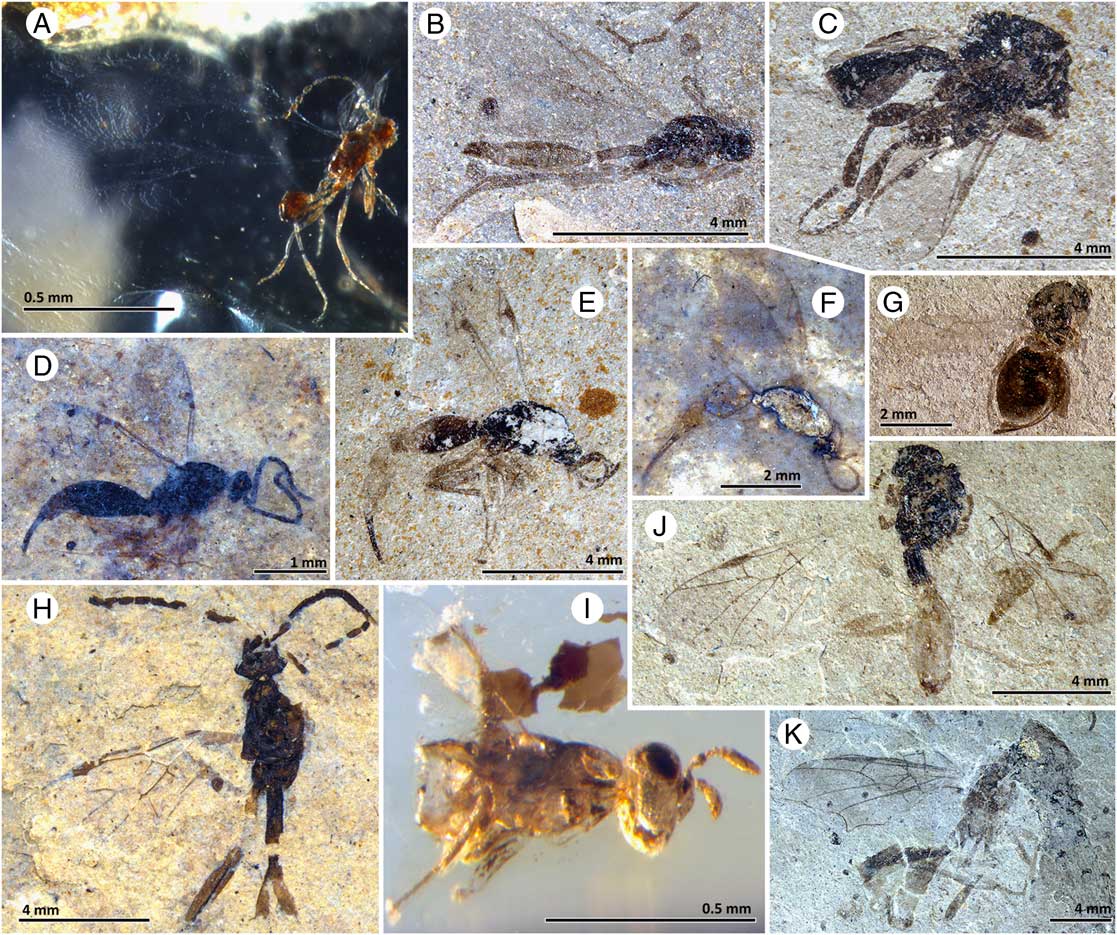
Fig. 7 Parasitica: Mymarommatoidea family incertae sedis (A), Monomachidae (B), Peradeniidae (C), Proctotrupidae (D–F), Figitidae (G), Roproniidae sensu lato. (H), Chalcidoidea family incertae sedis. (I), Heloridae (J), near Roproniidae (K). A, SBA-HC-10.1, Hat Creek amber. B, TRU F-1549, McAbee. C, SBA-2855, McAbee. D, SBA-4102, Driftwood Canyon. E, TRU F-1550, McAbee. F, SBA-3960, Driftwood Canyon. G, SBA-507, McAbee. H, SR 04-08-05, Republic. I, SBA-HC-7, Hat Creek amber. J, TRU F-1057, McAbee. K, TRU F-1552, McAbee. Scales differ throughout.
Specimens. McAbee: TRU F-1549.
Remarks. Monomachidae are rare today, with 30 species, mostly inhabiting the New World tropics extending north into tropical Mexico, but also in New Guinea and Australia (Masner Reference Masner1993b; Johnson and Musetti Reference Johnson and Musetti2012; Aguiar et al. Reference Aguiar, Deans and Engel2013). Their natural history is poorly known, but a few species are known to be parasitoids of Stratiomyidae (Diptera). This is the only confirmed fossil record of the family beyond a tentative re-identification (by Rasnitsyn Reference Rasnitsyn1990a) of one of the paratypes of Westratia nana Jell and Duncan, an early Cretaceous fossil from the Koonwarra fossil beds in Australia (Jell and Duncan Reference Jell and Duncan1986, fig. 66F).
Family Diapriidae sensu lato (Apocrita: Parasitica: Diaprioidea)
Figure 4D–K.
Specimens. Driftwood Canyon: SBA-3124, SBA-3478, SBA-3685, SBA-3697, SBA-4660, SBA-4812, SBA-4814, SBA-5718, SBA-5723, SBA-5902. Horsefly River: SBA-760, SBA-5179, SBA-5585. McAbee: SBA-228, SBA-720. Republic: SRUI 99-75-03, and SRUI 99-97-09.
Remarks. The Diapriidae sensu lato (the Diapriidae and Ismaridae of Sharkey et al. Reference Sharkey, Carpenter and Vilhelmsen2012) are generally small wasps that are distributed globally. They are mostly endoparasitic in Diptera, but are also known to feed upon some other groups. Their adults are most common in moist, shaded habitats in forests, near water, or in soil (Masner Reference Masner1993b). As small to very small wasps, they are mostly known as fossils from ambers (see appendix 1 of Perrichot and Nel Reference Perrichot and Nel2008 and Engel et al. Reference Engel, Ortega-Blanco, Soriano, Grimaldi and Delclòs2013b).
Sharkey et al. (Reference Sharkey, Carpenter and Vilhelmsen2012) recognised the subfamily Ismarinae as a separate family, with a single genus and 29 species (Aguiar et al. Reference Aguiar, Deans and Engel2013). It has a small fossil record of a few species restricted to the Cretaceous, beginning in Aptian Chosi amber of Japan (Skidmore Reference Skidmore1999; Perrichot and Nel Reference Perrichot and Nel2008, appendix 1; Engel et al. Reference Engel, Ortega-Blanco, Soriano, Grimaldi and Delclòs2013b). The remaining Diapriidae sensu stricto has 2048 described modern species (Aguiar et al. Reference Aguiar, Deans and Engel2013). The earliest undoubted occurrence of Diapriidae sensu stricto is in Albian French amber (Perrichot and Nel Reference Perrichot and Nel2008), although a stem diapriid was described as an ant from the Aptian of Koonwarra, Australia (Cretacoformica explicata Jell and Duncan) and putative Diapriidae were described from the Berriasian of England (Rasnitsyn et al. Reference Rasnitsyn, Jarzembowski and Ross1998). Diapriids sensu lato of unknown affinities have been reported from Cenomanian Burmese amber, and other ambers through Florissant shale (Perrichot and Nel Reference Perrichot and Nel2008; Rasnitsyn et al. Reference Rasnitsyn, Bashkuev and Kopylov2016). Until recently, when a number of belytine diapriids were reported from Lutetian Kishenehn Formation of Montana (Greenwalt et al. Reference Greenwalt, Rose, Siljestrom, Goreva, Constenius and Wingerath2015), Diapriidae sensu lato were thought rare until the Priabonian, and diverse and abundant from that time on (Zherikhin Reference Zherikhin2002; Perrichot and Nel Reference Perrichot and Nel2008).
We tentatively assign SBA-3124, SBA-4814, SBA-5723, and possibly SBA-5718 to the Diapriidae sensu stricto (sensu Sharkey et al. Reference Sharkey, Carpenter and Vilhelmsen2012). We consider all of the remaining as Diapriidae sensu lato (Diapriidae, but possibly also Ismaridae; i.e., Diaprioidea except Monomachidae, Maamingidae, and Spathiopterygidae). Of those, SBA-228, SBA-720, SBA-760, SBA-3478, SBA-3697, SRUI 99-97-09, SRUI 99-75-03, and SBA-4812 are distinct from all modern and almost all fossil members by their short second metasomal segment. This condition is found in the similar Cretaceous wasps Iberopria Engel et al. from Albian Spanish (Álava) amber and Cretacoformica Jell and Duncan from the early Cretaceous of Australia. Iberopria was placed in the Diapriidae sensu lato as a stem-group member based on this morphology (Engel et al. Reference Engel, Ortega-Blanco, Soriano, Grimaldi and Delclòs2013b), and the enigmatic Cretacoformica also shares this (considered a member of various groups including the Diapriidae: reviewed by Perrichot and Nel Reference Perrichot and Nel2008). SBA-3685, SBA-660, SBA-5902, SBA-5179, and SBA-5585 are wings only.
Family incertae sedis (Apocrita: Parasitica: Mymarommatoidea)
Specimens. Hat Creek: SBA-HC-10.1 and SBA-HC-01.2, two specimens as syninclusions in a piece of amber. A precise family-level identification is prevented by imperfect preservation, particularly of the head and wings.
Remarks. The Mymarommatoidea and its fossil record were treated in detail by Gibson et al. (Reference Gibson, Read and Huber2007), Engel and Grimaldi (Reference Engel and Grimaldi2007), and Ortega-Blanco et al. (Reference Ortega-Blanco, Peñalver, Delclòs and Engel2011b). The fossil record of these extremely tiny wasps – some as small as 0.3 mm in length – is entirely in amber. The superfamily consists of the extant Mymarommatidae, with a fossil record from Albian Spanish amber through Miocene Sicilian amber, and two extinct, early Cretaceous families: the Alvarommatidae, with one species from Albian Spanish amber, and the Gallorommatidae, with five species from Cenomanian Taimyr, Burmese, and French Bezonnais ambers. They may be parasites of insect eggs, but their natural history is essentially unknown. They have 10 described extant species distributed widely across the globe, but these minute microhymenoptera are assumed to be greatly undercollected and understudied.
Family Proctotrupidae (Apocrita: Parasitica: Proctotrupoidea)
Figure 7D–F.
Specimens. Driftwood Canyon: SBA-3960, SBA-4102, SBA-4452, SBA-4874, SBA-4903 (tentatively in the Proctotrupidae). Horsefly River: SBA-5868. McAbee: SBA-485 (tentatively), SBA-2902, TRU F-1550, TRU F-1551. Republic: SRUI 09-99-91.
Remarks. Proctotrupids are cosmopolitan today, but most diverse in the Holarctic, with 403 described species (Aguiar et al. Reference Aguiar, Deans and Engel2013). Adults are found in damp, shaded habitats, e.g., forests, marshes, near water, or in soil. They are mostly endoparasitoids of Coleoptera, but also of Diptera (Mycetophilidae, Sciaridae), Lepidoptera (Oecophoridae), and centipedes (Chilopoda: Lithobiidae) (Masner Reference Masner1993b; Kolyada and Perkovsky Reference Kolyada and Perkovsky2011). Their earliest occurrence is in the early Cretaceous, when their fossils are more numerous than at any time later; their previously reported Cenozoic record begins in the Priabonian of Baltic and Rovno ambers, Florissant, and the Bembridge Marls (Kolyada and Mostovski Reference Kolyada and Mostovski2007; Kolyada Reference Kolyada2009; Kolyada and Perkovsky Reference Kolyada and Perkovsky2011; Antropov et al. Reference Belokobylskij2014).
Family Heloridae (Apocrita: Parasitica: Proctotrupoidea)
Specimens. McAbee: TRU F-1057.
Remarks. Heloridae has 12 rare modern species, distributed around the world, but mostly in the Holarctic and apparently absent from the lowland tropics (Masner Reference Masner1993b; Achterberg Reference Achterberg2006; Aguiar et al. Reference Aguiar, Deans and Engel2013). They are solitary endoparasitoids of Chrysopidae. The previously known fossil record of the family includes 15 species from the late middle Jurassic to the early Cretaceous of Asia (Shih et al. Reference Shih, Feng and Ren2011; Shi et al. Reference Shi, Zhao, Shih and Ren2013, Reference Shi, Zhao, Shih and Ren2014).
Family Peradeniidae (Apocrita: Parasitica: Proctotrupoidea)
Specimens. McAbee: SBA-2855.
Remarks. Peradeniidae is a little-known family with two rare modern species in one genus known only from Tasmania and Victoria, Australia, and whose ecology and hosts are unknown (Naumann and Masner Reference Naumann and Masner1985; Masner Reference Masner1993b). One specimen of Peradenia galerita Johnson et al. from Priabonian Baltic amber is the only other known fossil of the family (Johnson et al. Reference Johnson, Musetti and Janzen2001).
Family Roproniidae sensu lato (Apocrita: Parasitica: Proctotrupoidea)
Specimens. Republic: SR 04-08-05.
Remarks. The distinction between Roproniidae sensu stricto (20 extant species, Holarctic and Oriental: Aguiar et al. Reference Aguiar, Deans and Engel2013) and Proctorenyxidae (three species, eastern Palearctic: Kim et al. Reference Kim, Lelej, Park and Lee2016) needs confirmation in our opinion, and here we treat these together as Roproniidae sensu lato. These are parasitoids with little-known hosts, except in one case reared from the cocoons of Symphyta (Masner Reference Masner1993b). Its fossil record (all Roproniidae sensu stricto) includes two species from the middle Jurassic of China and undescribed specimens from the middle Jurassic of Mongolia, early Cretaceous (Neocomian to Aptian) of Transbaikalia and Mongolia, the mid-late Cretaceous of the Russian Far East near Magadan (Rasnitsyn Reference Rasnitsyn1990b), and five species from the middle to late Jurassic of China (review: Zhang and Zhang Reference Zhang and Zhang2000).
Family incertae sedis (Apocrita: Parasitica: Proctotrupoidea)
Specimens. McAbee: TRU F-1552.
Remarks. TRU F-1552 is evidently near Roproniidae sensu lato, however, it does not belong to that group, as the hind wing possesses a closed cell that distinguishes it from those and is more like the condition found in the Monomachidae.
Family Cynipidae (?) (Apocrita: Parasitica: Cynipoidea)
Previous records. Horsefly: UAPL 4556 (Douglas and Stockey Reference Douglas and Stockey1996). Quilchena: UAPL 4581 (Douglas and Stockey Reference Douglas and Stockey1996), galls (Archibald and Mathewes Reference Archibald and Makarkin2000).
Remarks. Cynipids have 1412 modern species, distributed across the world (Aguiar et al. Reference Aguiar, Deans and Engel2013). They are the only phytophagous Cynipoidea, their larvae feeding in galls that they induce or as inquilines in those of others (Ritchie Reference Ritchie1993). Cynipoids are rare in the fossil record; there is an equivocal cynipid in Turonian New Jersey amber, and they are first established in Campanian Canadian amber, and known again in the Priabonian of Florissant and Baltic amber and in younger deposits (Grimaldi et al. Reference Grimaldi, Shedrinsky and Wampler2000; Grimaldi and Engel Reference Grimaldi and Engel2005; Liu et al. Reference Liu, Engel and Grimaldi2007; and see Ronquist et al. Reference Ronquist, Nieves-Aldrey, Buffington, Liu, Liljeblad and Nylander2015). We consider all of the Okanagan Highlands records to be tentative: the drawings of Douglas and Stockey (Reference Douglas and Stockey1996) of the wings from Horsefly River and Quilchena are consistent with Cynipidae, but some doubt remains, and the galls reported by Archibald and Mathewes (Reference Archibald and Makarkin2000) are equivocal.
Family Figitidae (Apocrita: Parasitica: Cynipoidea)
Specimens. McAbee: SBA-507, SBA-24. The specimen SBA-24 lacks wings, but the body as preserved indicates that, while not conspecific with SBA-507, they appear closely related.
Remarks. The Figitidae has 1571 modern species (Aguiar et al. Reference Aguiar, Deans and Engel2013). They are cosmopolitan, primarily parasitoids of Diptera, but also of lacewings (Neuroptera: Hemerobiidae and Chrysopidae), and some are hyperparasitoids of Braconidae or Chalcidoidea within Aphididae and Psyllidae (Ritchie Reference Ritchie1993; Liu et al. Reference Liu, Engel and Grimaldi2007). Their known fossil record begins in the late Cretaceous of Turonian New Jersey amber, Santonian Taimyr amber of Russia, and Campanian Canadian amber. In the Cenozoic, they are reported in the Priabonian of Baltic amber and Florissant shale, and in younger deposits (Liu et al. Reference Liu, Engel and Grimaldi2007; and see Buffington et al. Reference Buffington, Brady, Morita and Van Noort2012).
Family incertae sedis (Apocrita: Parasitica: Chalcidoidea)
Specimens. Driftwood Canyon: SBA-3082, SBA-4524, SBA-5720. Hat Creek amber: SBA-HC-7. McAbee: RBCM.EH.2004.001.1972.
Remarks. Chalcidoids are cosmopolitan, with some 20 997 described modern species distributed in habitats from equatorial forests to the northernmost tundra, and from deserts to wetlands (Gibson Reference Gibson1993; Aguiar et al. Reference Aguiar, Deans and Engel2013). They are small wasps, which attack a wide number of insect orders and sometimes Arachnida (Araneae and Acari), mostly as parasitoids or hyperparasitoids and rarely as predators; a few are phytophagous, gall formers or seed eaters, and may be inquilines in the galls of other species (Gibson Reference Gibson1993). The oldest record of the superfamily is in earliest Cretaceous Mongolian shale (Rasnitsyn et al. Reference Rasnitsyn, Basibuyuk and Quicke2004), and they have been reported in Albian through Campanian Cretaceous ambers (Rasnitsyn et al. Reference Rasnitsyn, Bashkuev and Kopylov2016, supplementary information table 1), but are never diverse until the Priabonian in Baltic amber (Heraty and Darling Reference Heraty and Darling2009).
Family Chrysididae (Apocrita: Aculeata: Chrysidoidea)
Specimens. McAbee: SBA-225 (Chrysidinae).
Remarks. The Chrysididae is a moderately large family of 2500 modern described species (Aguiar et al. Reference Aguiar, Deans and Engel2013). They are cosmopolitan, with a greatest diversity in temperate deserts of both hemispheres (Finnamore and Brothers Reference Finnamore and Brothers1993). Their larvae are parasitoids of insect eggs and larvae, or cleptoparasites. They are known from the Cretaceous in Barremian Lebanese amber, late Barremian-early Aptian Turga shale, early Albian Álava amber, Cenomanian Burmese amber (age: Shi et al. Reference Shi, Grimaldi and Harlow2012), Turonian New Jersey amber, Santonian Taimyr amber, Campanian Canadian amber (summarised by Rasnitsyn et al. Reference Rasnitsyn, Bashkuev and Kopylov2016, supplementary information table 1); and then not until the Priabonian (e.g., Cockerell Reference Cockerell1907) and younger (e.g., Engel Reference Engel2006). The Chrysidinae, its largest subfamily, are usually brightly metallic coloured cleptoparasites in the nests of bees and wasps (Finnamore and Brothers Reference Finnamore and Brothers1993).
Family Pompilidae (Apocrita: Aculeata: Vespoidea)

Fig. 8 Aculeata: Scoliidae (A, B), Pompilidae (C), aculeates of unknown affinity (D, E). A, SR 96-04-03, Republic. B, SR 09-13-01, Republic. C, SR 14-001-002, Republic. D, TRU F-1556, McAbee. E, TRU F-1557, McAbee. Scales differ throughout. TRU F-1557, McAbee. Scales differ throughout.
Specimen. Republic: SR 14-001-002.
Previous records. McAbee: CMN100040 (Douglas and Stockey Reference Douglas and Stockey1996).
Remarks. The Pompilidae has 4855 modern species (Aguiar et al. Reference Aguiar, Deans and Engel2013). They are a cosmopolitan, but predominantly tropical family (Brothers and Finnamore Reference Brothers and Finnamore1993). Each larva consumes a single paralysed spider (seldom other Arachnida) usually in a constructed cell, sometimes in another’s cell or in a cavity, rarely on an active spider. Their fossils were not previously established older than the numerous species described from the Priabonian of Baltic and Rovno ambers and Florissant shale, and subsequently in the Oligocene and Miocene of Europe (reviewed by Engel and Grimaldi Reference Engel and Grimaldi2006; Rodriguez et al. Reference Rodriguez, Waichert, von Dohlen, Poinar and Pitts2016). Rodriguez et al. (Reference Rodriguez, Waichert, von Dohlen, Poinar and Pitts2016) removed the Cretaceous Bryopompilus interfector Engel and Grimaldi from Pompilidae and placed it in the family Bryopompilidae, although mistakenly claiming to establish this as a new family despite Engel and Grimaldi (Reference Engel and Grimaldi2006) having established the tribe Bryopompilini for it. A.P.R. recently restudied the type, and we agree that the fossil represents a family of its own (Bryopompilidae Engel and Grimaldi), not closely related to Pompilidae. We agree with Douglas and Stockey’s (Reference Douglas and Stockey1996) determination of CMN100040 as a pompilid from examination of the figures provided.
Family Scoliidae (Apocrita: Aculeata: Vespoidea)
Figure 8A–B.
Specimens. Republic: SR 09-13-01, SR 96-04-03 (both Archaeoscoliinae).
Previous records. Allenby: UAPL 4524 (Douglas and Stockey Reference Douglas and Stockey1996) (here treated as belonging to the Archaeoscoliinae).
Remarks. The Scoliidae has 560 described modern species (Aguiar et al. Reference Aguiar, Deans and Engel2013) with a cosmopolitan, predominantly tropical distribution. Their larvae are ectoparasitoids of soil-dwelling Coleoptera larvae, usually Scarabaeoidea (Brothers and Finnamore Reference Brothers and Finnamore1993). Their fossils have been found throughout the Cretaceous, beginning in the Barremian of Spain (Rasnitsyn Reference Rasnitsyn1993; Rasnitsyn and Martínez-Delclòs Reference Rasnitsyn and Martínez-Delclòs1999; Zhang et al. Reference Zhang, Rasnitsyn and Zhang2002, Reference Zhang, Zhang, Rasnitsyn and Jarzembowski2015). In the Cenozoic, they first appear in the Lutetian at Messel, Germany, and then the Priabonian at Florissant and the Bembridge Marls (Lutz Reference Lutz1990; Rasnitsyn Reference Rasnitsyn1993; Antropov et al. Reference Belokobylskij2014). The extinct subfamily Archaeoscoliinae is known from the Barremian of Spain, Aptian of Bon Tsagaan, Turonian of northern Kazakhstan, Campanian of northeast Siberia, and the Priabonian of Florissant (Rasnitsyn Reference Rasnitsyn1993; Zhang et al. Reference Zhang, Rasnitsyn and Zhang2002).
Family Vespidae (Apocrita: Aculeata: Vespoidea)

Fig. 9 Aculeata: Vespidae. A, SBA-3070, Driftwood Canyon. B, SR 06-21-03, Republic. C, SFU Q-5897, Quilchena. D, TRU F-1553, McAbee. E, TRU F-1021, McAbee. F, SBA-1094, McAbee. G, SR 99-07-20A, Republic. H, SFU Q-0037, Quilchena. All to same scale.
Specimens. Driftwood Canyon: SBA-3070, SBA-4897. McAbee: SBA-1094, TRU F-1020/1021, SBA-593, TRU F-1553. Quilchena: SFU Q-5879. Republic: SR 05-03-03, SR 06-21-03, SR 02-01-12, SR 99-07-20, SR 06-01-01.
Previous records. Allenby Formation, Blakeburn Mine: ROM 31319 (Wilson 1977a). Quilchena: SFU Q-0037 (Archibald and Mathewes Reference Archibald and Makarkin2000).
Remarks. The Vespidae has 4932 species today (Aguiar et al. Reference Aguiar, Deans and Engel2013), with a cosmopolitan, but predominantly tropical distribution. Most species are solitary, but many are social, from semi-social to the highly organised eusocial societies of the subfamilies Stenogastrinae (hover wasps), Vespinae (hornets and yellowjackets), and Polistinae (paper wasps) (Brothers and Finnamore Reference Brothers and Finnamore1993). Larvae of solitary species feed on those of other insects, rarely on pollen and nectar, which are deposited in a cell constructed by the adult female; those of social species are continuously fed on masticated insects provided by the adults, or rarely on their glandular secretions. A few species are cleptoparasites in nests of social species (Brothers and Finnamore Reference Brothers and Finnamore1993).
They first appear in the Cretaceous, where they are known from Asia (Valanginian Baissa, late Barremian-early Aptian Turga shale, Aptian Bon-Tsagaan, Cenomanian Burmese amber, Turonian Kzyl-Zhar), Africa (Turonian Orapa shale of Botswana), and North America (Turonian New Jersey amber), all belonging to non-social taxa; however, a nest from Utah indicates the presence of social Vespidae in the Cretaceous (Carpenter and Rasnitsyn Reference Carpenter and Rasnitsyn1990; Wenzel Reference Wenzel1990; Brothers Reference Brothers1992; Carpenter Reference Carpenter2000; Perrard et al. Reference Perrard, Grimaldi and Carpenter2017). Similar nests have been described from the Coniacean of Central Asia (Nesov Reference Nesov1985, Reference Nesov1995), which, however, were not necessarily made by social wasps.
The body fossils of eusocial vespids are first confidently known from a vespine and a polistine from the Paleocene of Menat, France, found with non-social vespids (Piton Reference Piton1940; Nel and Auvray Reference Nel and Auvray2006). Vespidae (social and non-social) are then known in the Ypresian from the Okanagan Highlands (Allenby Formation and Quilchena), the Green River Formation (United States of America) and the Tadushi Formation (Rasnitsyn Reference Rasnitsyn1980: the Tadushi called Zerkal’naya); the Lutetian of Messel, Germany; the Priabonian of Florissant, the Bembridge Marls (United Kingdom), and Baltic amber; and various Oligocene and Miocene localities (Burnham Reference Burnham1978; Wilson Reference Wilson1978a; Lutz Reference Lutz1990; Archibald and Mathewes Reference Archibald and Makarkin2000; Meyer Reference Meyer2003; Poinar Reference Poinar2005; Nel and Auvray Reference Nel and Auvray2006; Antropov et al. Reference Belokobylskij2014). In all, 14 of the Okanagan Highlands specimens are confidently members of the Vespidae, 12 of which we assign to the Vespinae and/or Polistinae, i.e., were eusocial. The remaining two (SBA-1094, SR 05-03-03) likely belong to the non-eusocial Eumeninae.
Family Formicidae (Apocrita: Aculeata: Vespoidea)

Fig. 10 Aculeata: Formicidae. A, SFU Q-0409, Quilchena. B, SFU Q-0007, Quilchena. C, SFU Q-0400A, Quilchena. D, SFU Q-0002, Quilchena. E, PMF.2016.0001.001, Allenby Formation. F, SFU Q-0015, Quilchena. G, SBA-5781, Driftwood Canyon. H, NH998015004, Dolichoderinae, Hat Creek amber. I, SBAHC-4, Myrmicinae, Leptothorax species (Poinar et al. 1999) or possibly Tetramorium species (Radchenko and Dlussky 2015), Hat Creek amber. J, SBA-HC-3, Formicinae incertae sedis (not Dolichoderinae, Technomyrmex as stated by Poinar et al. 1999), Hat Creek amber. K, SBA-HC-1, Dolichoderinae, Dolichoderus species, Hat Creek amber. L, NH998014008, Dolichoderinae, Hat Creek amber. M, Myrmeciinae, Myrmeciites (?) tabanifluviensis Archibald et al., 2003.2.10.CDM034, Horsefly River. N, 2003.2.9.CDM.033a, Myrmeciinae, Myrmeciites incertae sedis, Falkland. A–G, M–N to same scale; H–L, to same scale.

Fig. 11 Aculeata: Formicidae, McAbee. A, TRU F-1574, Myrmeciinae, likely Myrmeciites (?) goliath Archibald et al. B, Myrmeciinae, TRU F-260. C, SBA-331A. D, TRU F-1554. E, TRU F-789, Myrmeciinae. F, SBA-5926. G, TRU F-990. H, SBA-109A. I, SBA-460. All to same scale.

Fig. 12 Aculeata: Formicidae, Republic. A, SR 07-03-09, Myrmeciinae. B, SR 07-05-06A, Myrmeciinae. C, SR 11-58-10. D, SR 08-35-06. E, SR 05-03-14. F, SRUI 99-84-78. G, SR 88-11-02, holotype, Camponotites kraussei Dlussky and Rasnitsyn. H, SR 10-41-12. I, SR 03-02-01. J, SR 99-14-08. K, SR 06-01-03. L, SR 94-05-07, holotype, Klondikia whiteae Dlussky and Rasnitsyn. M, DMNH-27804. N, SR 04-01-01. O, DMNH-27805. P, SRUI 99-92-24. All to same scale.
Specimens. Ants are known from all major sites. Driftwood Canyon: SBA-5781. Horsefly River: SBA-5849, SBA-5866. Hat Creek: SBA-HC-1, SBA-HC-2, SBA-HC-3, SBA-HC-6, KM NH998015004, KM NH998015008, KM NH998015010, KM NH998015011. McAbee: SBA-109, SBA-331, SBA-460, SBA-1137, SBA-5926, TRU F-260, TRU F-789, TRU F-990, TRU F-1554, TRU F-1574, TRU F-1576, TRU F-1577, TRU F-1578, TRU F-1579, TRU F-1580. Quilchena: SFU Q-0001, SFU Q-0002, SFU Q-0003, SFU Q-0005, SFU Q-0006, SFU Q-0010, SFU Q-0012, SFU Q-0013, SFU Q-0015, SFU Q-0019, SFU Q-0258, SFU Q-0366, SFU Q-0412, SFU Q-0453, SFU Q-0456, SFU Q-0485, SFU Q-0492, SFU Q-0510, SFU Q-0517, SFU Q-5880, SFU Q-5881, SFU Q-5882. Allenby Formation: PMF.2016.0001.001. Republic: DMNH-27804, DMNH-27805, SR 00-02-01, SR 03-02-01, SR 04-01-01, SR 05-03-09, SR 05-03-14, SR 05-03-17, SR 05-03-22, SR 06-01-03, SR 07-03-09, SR 07-05-06, SR 08-35-06, SR 09-11-02, SR 10-41-12, SR 11-02-01, SR 11-58-09, SR 11-58-10, SR 99-14-08, SR 99-82-54, SRUI 07-03-01, SRUI 08-03-03, SRUI 99-75-55, SRUI 99-82-54, SRUI 99-84-27, SRUI 99-84-78, SRUI 99-85-52, SRUI 99-90-08, SRUI 99-92-24.
Previous records. Scudder (Reference Scudder1877) described a series of ants from Quesnel, which he named Formica arcana Scudder, Aphaenogaster longaeva Scudder, and Hypoclinea obliterata Scudder (these determinations below family level are in need of revision). In the same paper, he described Calyptites antediluvianum Scudder, also from Quesnel, as a member of the Braconidae, but it was later considered to be an ant (Wheeler Reference Wheeler1911), and subsequently as belonging to an undetermined family (Bolton Reference Bolton2003). Douglas and Stockey (Reference Douglas and Stockey1996) illustrated SR 88-11-12 from Republic (subsequently named Camponotites kraussei Dlussky and Rasnitsyn, see below), UAPAL 4557 and UAPAL 4558 from Horsefly River, UAPAL 4610 and UAPAL 4604 from Quilchena (all of which we agree are ants, judging from their figures), and further listed (which we have not seen) UAPAL 4542 from Horsefly River and UAPAL 4616 and UAPAL 4582 from Quilchena. Archibald and Mathewes (Reference Archibald and Makarkin2000) reported, but did not describe, ants from Quilchena, illustrating three (SFU Q-0409, SFU Q-0007, SFU Q-0400), listing a further eight (SFU Q-0008, SFU Q-0011, SFU Q-0014, SFU Q-0019, SFU Q-0021, SFU Q-0410, SFU Q-0271) and mentioning that there are numerous others. Dlussky and Rasnitsyn (Reference Dlussky and Rasnitsyn1999) described the formicine Camponotites kraussei Dlussky and Rasnitsyn (holotype: UWBM-78047, part, SR 88-11-02, counterpart; Camponotites Steinbach a form genus), and subsequently (2003) Klondikia whiteae Dlussky and Rasnitsyn (holotype: SR 94-04-24, part, SR 94-05-07, counterpart; subfamily indet.), both from Republic. A myrmicine in Hat Creek amber (SBA-HC-4) was illustrated by Poinar et al. (Reference Poinar, Archibald and Brown1999), which they called Leptothorax Mayr, but which Radchenko and Dlussky (Reference Radchenko and Dlussky2015) thought might be a species of Tetramorium Mayr. They also illustrated an ant that they determined as Technomyrmex Mayr (SBA-HC-5), and mentioned the presence of Dolichoderus Lund (not figured). Archibald et al. (Reference Archibald, Bossert, Greenwood and Farrell2006) described the Myrmeciinae of the Okanagan Highlands, naming Ypresiomyrma orbiculata Archibald et al. (holotype: TRU F-749, part, TRU F-750, counterpart), Ypresiomyrma bartletti Archibald et al. (holotype: GSC 127632a,b, part and counterpart), Avitomyrmex elongatus Archibald et al. (holotype: 2003.2.8CDMM032, part only), Avitomyrmex mastax Archibald et al. (holotype: TRU F-850, paratype: TRU F-929), Avitomyrmex systenus Archibald et al. (holotype: 2003.2.11CDM035, part only; paratype: TRU F-989, part only; additional specimen: TRU F-825, part only. TRU F-825 tentatively assigned to this species), Macabeemyrma ovata Archibald et al. (holotype: TRU F-844, part, TRU F-856, counterpart), and Myrmeciites herculeanus Archibald et al. (holotype: TRU F-974, part only; Myrmeciites Archibald et al., a form genus), all from McAbee; placing two species tentatively in the Myrmeciinae, Myrmeciites (?) tabanifluviensis Archibald et al. (holotype: 2003.2.10CDM034, part only) from Horsefly River, and Myrmeciites (?) goliath Archibald et al. (holotype: TRU F-999, part, TRU F-1000, counterpart) from McAbee; and treating two further myrmeciines as Myrmeciites incertae sedis: a male from Falkland (2003.2.9CDM033a, b, part and counterpart) and a female (worker or queen) from Republic (SR 05-03-01). Archibald (Reference Archibald2007) illustrated the forewings of Dolichoderinae (SBA-367), Formicinae (SBA-390, SBA-2292) and Myrmeciinae (SBA-2832, SBA-2111 possibly a myrmeciine), and an ant not determined to subfamily (SBA-2996) at McAbee. Here, we recognise Propalosoma gutierrezae Dlussky and Rasnitsyn from Republic (holotype: part: SR 93-08-04, counterpart: UWBM 77524) as a myrmeciine ant.
Remarks. Ants comprise 12 199 described modern species (Aguiar et al. Reference Aguiar, Deans and Engel2013). They are cosmopolitan, but predominantly tropical, highly social, and occupy a wide variety of habitats and niches: as mutualists tending aphids and other Sternorrhyncha, Auchenorrhyncha, the caterpillars of Lycaenidae (Lepidoptera), and some other insects for their exudates; as scavengers of dead arthropods; as active predators; as herbivores; and as fungivores (Brothers and Finnamore Reference Brothers and Finnamore1993; Pierce et al. Reference Pierce, Braby, Heath, Lohman, Mathew, Rand and Travassos2002; Wilson and Hölldobler Reference Wilson and Hölldobler2005).
If Armania Dlussky species and their relatives are considered as within the Formicidae, the fossil record of ants extends to the Albian (Dlussky Reference Dlussky1999). This group has just over a dozen species, which are abundant as individuals from the outset at Khetana into the late Cretaceous (Dlussky Reference Dlussky1999; Rasnitsyn Reference Rasnitsyn2002; Zherikhin Reference Zherikhin2002, review of Engel and Grimaldi Reference Engel and Grimaldi2005). Their status as ants has been the subject of debate (Armaniinae within the Formicidae, e.g., Bolton Reference Bolton2003; Armaniidae, e.g., Grimaldi and Engel Reference Grimaldi and Engel2005; treated as “stem ants” along with the Sphecomyrminae by Ward Reference Ward2014).
Uncontroversial ant fossils appear in Albian-Cenomanian French and Cenomanian Burmese amber (Nel et al. Reference Nel, de Ploëg, Millet, Menier and Waller2004; Perrichot Reference Perrichot2015). The 19 species of the primitive, extinct Sphecomyrminae are widespread throughout the Cretaceous from the early Cretaceous at Baikura amber (northern Siberia – distinct from nearby “Taimyr amber”) through latest Albian – earliest Cenomanian French amber (Perrichot Reference Perrichot2015), earliest Cenomanian Burmese amber (Dlussky Reference Dlussky1996; Engel and Grimaldi Reference Engel and Grimaldi2005), Turonian New Jersey amber (Wilson et al. Reference Wilson, Brown and Carpenter1967; Grimaldi et al. Reference Grimaldi, Agosti and Carpenter1997; Engel and Grimaldi Reference Engel and Grimaldi2005), the Turonian of Kazakhstan (Dlussky Reference Dlussky1983), Santonian Taimyr amber (Dlussky Reference Dlussky1987), and in Campanian Canadian amber (Wilson Reference Wilson1985; McKellar and Engel Reference McKellar and Engel2012). None of these primitive ants (or close ant relatives) is known to have survived into the Cenozoic.
After crown-group ants are first seen in mid-Cretaceous amber, they are known from a small number of individuals reported through the remainder of the Cretaceous, never diverse and always rare. Of these, four species have unknown or extinct subfamily affinities, and fewer than 10 are assigned to modern subfamilies (Dlussky et al. Reference Dlussky, Brothers and Rasnitsyn2004; Engel and Grimaldi Reference Engel and Grimaldi2005; Perrichot et al. Reference Perrichot, Lacau, Néraudeau and Nel2008a, Reference Perrichot, Nel, Néraudeau, Lacau and Guyot2008b; McKellar and Engel Reference McKellar and Engel2012; LaPolla et al. Reference LaPolla, Dlussky and Perrichot2013; McKellar et al. Reference McKellar, Kopylov and Engel2013; Perrichot Reference Perrichot2015; and references therein). The early Cenozoic history of ants is reviewed below in the Discussion section.
Spheciformes (Apocrita: Aculeata: Apoidea)
The Spheciformes is a paraphyletic group composed of those apoids that are not bees. The Okanagan Highlands spheciform wasp assemblage presents an unusual challenge in that many specimens are indistinct at the family level, even some that are quite clearly preserved – a surprising situation. We can say, however, that a feature of these is that they almost doubtlessly not only reveal the presence, but also an abundance and diversity of Angarosphecidae, an extinct group not known to persist into the Cenozoic before the description of Eosphecium naumanni Brothers and Archibald (Pulawski et al. Reference Pulawski, Rasnitsyn, Brothers and Archibald2000) in the Okanagan Highlands at Quilchena.
Family Angarosphecidae (Apocrita: Aculeata: Apoidea: Spheciformes)

Fig. 13 Aculeata: Angarosphecidae and possible Angarosphecidae (see text). A, SR 02-25-02, Republic. B, TRU F-1560, McAbee. C, UWBM PB-3823, McAbee. D, UWBM 57297, Allenby Formation. E, TRU F-1561, McAbee. F, SFU Q-0423, Holotype, Eosphecium naumanni Brothers and Archibald, Quilchena. G, SRUI 99-82-01, Republic. H, UWBM 57128, Allenby Formation. I, SR 10-18-27, Republic. J, UWBM-74301, Republic. K, SR 05-03-11A, Republic. All to same scale.
Specimens. Eosphecium Pulawski and Rasnitsyn species: Driftwood Canyon: SBA-2988. McAbee: UWBM PB-3823/4104. Republic: SR 05-03-11, UWBM 74301. Eosphecium species or near: McAbee: TRU F-1560. Republic: SR 02-25-02, SRUI 99-82-01. Almost certainly Angarosphecidae: McAbee: UWBM PB-3909. Allenby Formation (One Mile Creek): UWBM 57297, UWBM 57128. Possibly Angarosphecidae: McAbee: TRU F-1561, TRU F-1562. Republic: SR 10-18-27, SR 12-004-004, SR 92-02-23.
Previous records. Quilchena: SFU Q-0423 Eosphecium naumanni Brothers and Archibald (Pulawski et al. Reference Pulawski, Rasnitsyn, Brothers and Archibald2000).
Remarks. The Angarosphecidae as currently understood represents an extinct group that is supposedly basal in Apoidea and perhaps paraphyletic with regard to the rest. They are among the most abundant Hymenoptera found in the early Cretaceous (Rasnitsyn et al. Reference Rasnitsyn, Pulawski and Martínez-Delclòs1999). Their fossil record begins in the early Berriassian of Dorset, United Kingdom; and then they are found in the Hauterivian–Valanginian Purbeck Limestones (United Kingdom); the Valanginian of Transbaikalia; the early Barremian of Spain; Barremian or Aptian of Shandong, China; the Aptian of Russia, Mongolia, and Brazil (reviewed by Rasnitsyn et al. Reference Rasnitsyn, Jarzembowski and Ross1998, and see Pulawski et al. Reference Pulawski, Rasnitsyn, Brothers and Archibald2000; Rasnitsyn and Ansorge Reference Rasnitsyn and Ansorge2000). After a lengthy hiatus, the family is reported again with a single species in the Ypresian, described from Quilchena in the Okanagan Highlands (Pulawski et al. Reference Pulawski, Rasnitsyn, Brothers and Archibald2000).
With the wide morphological range of the wasps that we associate with that family here (confidently or tentatively to various degrees), the group becomes more difficult to define with clearly diagnostic character states, in particular any that easily separate its species from those of the Ampulicidae and Crabronidae. They remain more or less distinct from the Sphecidae, Heterogynaidae, and the apiform families. As a working definition, we associate those wasps listed above with the Angarosphecidae based on a rather straight alignment of the forewing vein sections of RS+M and M combined with the presence of notauli (and isolated wings whose morphology is close to those of more complete specimens that possess notauli), and we group them by likelihood of being closely related to Eosphecium and allied Cretaceous wasps. We will address this issue in detail in ongoing research.
Family Sphecidae sensu stricto (Apocrita: Aculeata: Apoidea: Spheciformes)
Figure 14A–B.

Fig. 14 Aculeata: Spheciformes (A–B) and Apiformes (C–I). A, TRU F-258, Sphecidae sensu stricto, McAbee. B, SR 14-001-001, Sphecidae sensu stricto, Republic. C, SR 04-08-06, Halictidae, Republic. D, SFU Q-0424, Halictidae, Halictus savenyei Engel and Archibald, Quilchena. E, SR 06-22-01, Republic. F, TRU F-1555, Halictidae, McAbee. G, TRU F-263, Apidae, McAbee. Megachilidae leaf cutting damage (oval cuts) (H and I): H, SR 94-05-31, Republic, on a Prunus leaf. I, SBA-5195, Horsefly River. Scales differ, except (C–G) to same scale.
Specimens. McAbee: TRU F-692, TRU F-258. Republic: SR 14-001-001.
Remarks. Today, the Sphecidae (sensu stricto) are cosmopolitan, medium to large wasps, with 724 described species, which have a wide range of behaviours from parasitoid-like to primitively social nest building or crevice dwelling (Finnamore and Michener Reference Finnamore and Michener1993; Aguiar et al. Reference Aguiar, Deans and Engel2013). They provision their young with prey that includes spiders, orthopteroids, and Lepidoptera larvae.
No previously known fossil Sphecidae (sensu stricto) is older than the Priabonian, where they are reported from Florissant and the Bembridge Marls: Hoplisidea kohliana Cockerell (transferred to the Sphecidae by Menke and Rasnitsyn Reference Menke and Rasnitsyn1987) and Protosceliphron brevior (Cockerell) (revised by Antropov et al. Reference Belokobylskij2014). We associate the fossils listed above confidently with the Sphecidae sensu stricto.
Family incertae sedis (Apocrita: Aculeata: Apoidea: Spheciformes)
Specimens. Driftwood Canyon: SBA-4905; SBA-3505; SBA-3550; SBA-4390. Hat Creek amber: SBA-HC-8. McAbee: UWBM PB-2747. Republic: SR 09-11-06.
Remarks. These specimens might belong to the Angarosphecidae, Sphecidae, or Crabronidae.
Apiformes (Apocrita: Aculeata: Apoidea)
Modern bees (Apiformes or Anthophila) have 19 844 described, almost entirely pollinivorous, solitary, and semi-social to eusocial species that range across the globe outside of the polar regions (Michener Reference Michener2007; Aguiar et al. Reference Aguiar, Deans and Engel2013). Most dig or construct external nests, which are complex in some species.
The oldest reported putative bee, Melittosphex burmensis Poinar and Danforth, from Cenomanian Burmese amber, comprises the extinct family Melittosphecidae (Poinar and Danforth Reference Poinar and Danforth2006). It is now, however, considered to be an apoid wasp in an unresolved position near bees and crabronids (Michener Reference Michener2007; Ohl and Engel Reference Ohl and Engel2007; Danforth and Poinar Reference Danforth and Poinar2011). An advanced apid was described from putative Maastrichtian New Jersey amber (Michener and Grimaldi Reference Michener and Grimaldi1988; Engel Reference Engel2000); however, the amber piece that contains it was found unlabelled in a drawer, leaving its provenance in doubt, and other insects found as syninclusions are consistent with it being of much younger Cenozoic age, which we find most reasonable (discussion: Rasnitsyn and Michener Reference Rasnitsyn and Michener1991; Grimaldi Reference Grimaldi1999; Zherikhin Reference Zherikhin2002). An ichnofossil from Uruguay was described as a Cretaceous bee nest (Roselli Reference Roselli1939); however, Zeuner and Manning (Reference Zeuner and Manning1976) concluded that while its structure does bear some resemblance to that of bee cells, it may not be Cretaceous. The Cenozoic record of bees is reviewed in the Discussion section, below.
Family Apidae (Apocrita: Aculeata: Apoidea: Apiformes)
Specimen. McAbee: TRU F-263.
Remarks. The Apidae is one of the most speciose families of bees today, with 5749 described species widespread across the globe, and includes most eusocial bees (e.g., the honey bee, Apis mellifera Linnaeus), as well as many less social and solitary species (Michener Reference Michener2007; Aguiar et al. Reference Aguiar, Deans and Engel2013). The fossil record of the family is reviewed in the Discussion section, below.
Family Megachilidae (Apocrita: Aculeata: Apoidea: Apiformes)
Figure 14H–I.
Specimen. Horsefly River: SBA-5195, a leaf with megachilid cutting (Fig. 14I).
Previous records. Leaf-cutting damage was reported from Republic by Lewis (Reference Lewis1994): UWBM-57529a, b on a Prunus Linnaeus (Rosaceae) leaf, one side subsequently designated SR 94-05-31 (Fig. 14H); and Labandeira (Reference Labandeira2002): UWBM 95726 on an Ulmus Linnaeus (Ulmaceae) leaf. Labandeira (Reference Labandeira2002, and see Wedmann et al. Reference Wedmann, Wappler and Engel2009) reported such damage on a Ginkgo Linnaeus (Ginkgoaceae) leaf from McAbee (UWBM 77597).
Remarks. The Megachilidae is a large family of bees today, with 4096 described species, inhabiting all continents except Antarctica (Gonzalez et al. Reference Gonzalez, Griswold, Praz and Danforth2012; Aguiar et al. Reference Aguiar, Deans and Engel2013). They may use the abandoned nests of other insects or construct their own, either openly, in cavities, or digging tunnels in soil, which they may line with a variety of materials, sometimes leaf pieces cut in arcs from angiosperm leaves (but see an instance of Ginkgo, above) (Finnamore and Michener Reference Finnamore and Michener1993; Engel Reference Engel1999).
The earliest body fossil of a member of the Megachilidae is from the Paleocene of Menat (Nel and Petrulevičius Reference Nel and Petrulevičius2003), and others subsequently in the Eocene from the Priabonian in Baltic and Rovno ambers and at Florissant (see review by Engel and Perkovsky Reference Engel and Perkovsky2006). The earliest occurrences of their distinctive leaf damage are in the Okanagan Highlands and the Green River Formation (Republic: Lewis Reference Lewis1994; Labandeira Reference Labandeira2002; McAbee: Wedmann et al. Reference Wedmann, Wappler and Engel2009; Green River Formation: Labandeira Reference Labandeira2002), and then from the Lutetian of Argentina (Sarzetti et al. Reference Sarzetti, Labandeira and Genise2008) and Eckfelt Maar in Germany (Wappler and Engel Reference Wappler and Engel2003), the Bartonian Cockfield (Wilcox) Formation of Kentucky and Tennessee, United States of America (Berry Reference Berry1931; Brooks Reference Brooks1955), the Priabonian of Florissant (Cockerell Reference Cockerell1910), and in younger deposits (reviewed: Wappler and Engel Reference Wappler and Engel2003; Wedmann et al. Reference Wedmann, Wappler and Engel2009).
Family Halictidae (?) (Apocrita: Aculeata: Apoidea: Apiformes)
Figure 14C–D, F
Specimens. McAbee: TRU F-1555; Republic: SR 04-08-06.
Previous records. Quilchena: SFU Q-0424, Halictus? savenyei Engel and Archibald.
Remarks. Sweat bees, the Halictidae, have 4327 described species today (Aguiar et al. Reference Aguiar, Deans and Engel2013), spread over much of the globe. They include many eusocial species and a wide variety of social to solitary, cleptoparasitic, and socially parasitic species among the remainder, and nest by burrowing in soil and sometimes rotting wood (Michener Reference Michener2007; Danforth et al. Reference Danforth, Cardinal, Praz, Almeida and Michez2013).
The oldest fossil ascribed to the Halictidae is Halictus? savenyei from the Okanagan Highlands locality at Quilchena (Engel and Archibald Reference Engel and Archibald2003) and the rest of the fossil record is Priabonian (Baltic amber, one species; Florissant, six species) and younger (reviewed by De Meulemeester et al. Reference De Meulemeester, Michez, Aytekin and Danforth2012). Although Halictus? savenyei was described as a halictid, possibly a species of Halictus Latreille, here we consider this and the other Okanagan Highlands specimens to be likely, but unconfirmed members of the family, and so the Halictidae is tentatively present there.
Family incertae sedis (Apocrita: Aculeata: Apoidea: Apiformes)
Specimens. Driftwood Canyon: SBA-4534. McAbee: SBA-2994. Republic: SR 06-22-01.
Vespoidea or Spheciformes family incertae sedis (Apocrita: Aculeata: Apoidea)
Figure 8D–E.
Specimens. McAbee: UWBM PB-4332, TRU F-1556ab, TRU F-1557, TRU F-1558, TRU F-1559. Republic: SR 08-33-04, SRUI 09-95-31.1.
Remarks. The wing venation of these seven wasps is similar to that of Paleorhopalosoma menatensis Nel et al. from the Paleocene of Menat (Nel et al. Reference Nel, Azar and Hevret2010). However, we question the attribution of P. menatensis to Rhopalosomatidae, as its wing venation strongly differs from the very characteristic venation of other Rhopalosomatidae and is similar to that of, e.g., Pompilopterus corpus Rasnitsyn et al. from the Barremian of the United Kingdom (Rasnitsyn et al. Reference Rasnitsyn, Jarzembowski and Ross1998). The female tarsi are only slightly widened in P. menatensis, unlike the condition found in unquestionable Rhopalosomatidae. In addition, the Okanagan Highlands fossils, which have a great similarity to P. menatensis and are probably related to it, show a comparatively long pronotum with a straight hind margin (specimen TRU F-1557: Fig. 8E) and a triangular area comparable with a propodeal enclosure (TRU F-1559). This suggests that a more logical attribution of P. menatensis as well as these Okanagan Highlands fossils is to the Spheciformes, possibly near or in the Angarosphecidae. SR 08-33-04 is known from an isolated wing with similar venation, except that cell 1 mcu is somewhat longer and 3 rm is slightly shorter; it may be closely related to the above species. Finally, specimen UWBM PB-4332 has a somewhat similar general appearance. Its propodeum bears a structure suggesting a propodeal enclosure, and cell 2 rm has an odd form, with the only analogue being in Pompilopterus wimbledoni Rasnitsyn et al. (Reference Rasnitsyn, Jarzembowski and Ross1998) from the Berriassian of the United Kingdom. It may be a member of the above assemblage or closely related.
Discussion
We find a minimum of 25 named families, or at least 30 including those tentatively assigned (Cynipidae and Halictidae) and those that are distinct at the family level but not currently associated with named families (e.g., Chalcidoidea and Mymarommatoidea family incertae sedis), and surely a number of others (e.g., see in the Apoidea) in collections of Okanagan Highlands Hymenoptera examined and confirmed in literature reviewed (Table 1).
McAbee (19 named families, maybe more than 24) and Republic (14 named families, maybe more than 16) show the greatest family diversities. Driftwood Canyon has a moderate family representation (nine named families, maybe more than 10), as do the localities at Horsefly River (seven named families, maybe more than eight), Quilchena (seven named families, maybe more than nine), and in the Allenby Formation (eight named families, maybe more than eight), about half or less of the family diversities of McAbee and Republic. The Falkland locality (four families) is much more difficult to access than the others, and its insects have been infrequently collected. Hat Creek amber has hardly been examined to date (three named families, maybe more than five), and, as expected, contains the smallest identified specimens, e.g., Mymarommatoidea. These numbers among shale sites likely reflect differential collecting intensities to some degree, but McAbee does appear to be exceptional.
The recent rate of discovery is high – only three families had been recognised in the Okanagan Highlands fauna until the late 1950s and eight by the mid-1990s (Douglas and Stockey Reference Douglas and Stockey1996). This will certainly continue to increase through the near future, not only from new compression fossils, but particularly as Okanagan Highlands amber is thoroughly examined. The Okanagan Highlands Hymenoptera can now be seen as among the more diverse family assemblages older than that in Baltic amber (Tables 2–3). The family numbers of these deposits reflect differential histories of attention to some degree (e.g., insects of Baltic amber and Florissant shales have been treated in detail for over a century), and some include old records with identifications that are not to modern standards or which employ outdated family concepts, and so should be taken as generalities only. Still, informative patterns are emerging, and the formation and character of the Okanagan Highlands Hymenoptera community can be set in the context of preceding crises and opportunities in an increasingly angiosperm-dominated world punctuated by large-scale extinction, recovery, and dispersal events.
Table 2 Hymenoptera family numbers in major Cretaceous through Eocene deposits.

References: Cretaceous ambers: Rasnitsyn et al. (Reference Rasnitsyn, Bashkuev and Kopylov2016); Orapa, Botswana: Brothers and Rasnitsyn (Reference Brothers and Rasnitsyn2003); Menat, France: Piton (Reference Piton1940) and Nel (Reference Nel1992, Reference Nel2004), plus Formicidae, recognised here; Fur Formation, Denmark: Rust (Reference Rust1990, 1998); Green River Formation: summaries of Wilson (Reference Wilson1978a), Grande (Reference Grande1984), Dehon et al. (Reference Dehon, Michez, Nel, Engel and De Meulemeester2014), and A.P.R. (personal observation); Oise amber, France: Nel and Brasero (Reference Nel and Brasero2010); Fushun amber, China: Wang et al. (Reference Wang, Rust and Engel2014a); Indian Cambay amber: Rust et al. (Reference Rust, Singh and Rana2010); Sakhalin amber, Russia: Zherikhin (Reference Zherikhin1978), Simutnik (Reference Simutnik2014), and A.P.R. (personal observation); Kishenehn Formation: Huber and Greenwalt (Reference Huber and Greenwalt2011), Greenwalt and Engel (2014), LaPolla and Greenwalt (Reference LaPolla and Greenwalt2015), and D. Greenwalt (personal communication); Messel, Germany: Lutz (Reference Lutz1990), Wappler and Engel (Reference Wappler and Engel2003); Eckfelt, Germany: Wappler (Reference Wappler2003); Rovno amber: Perkovsky et al. (Reference Perkovsky, Zosimovich and Vlaskin2010); Baltic amber: see updated list Table 3 and references therein; Florissant: Meyer (Reference Meyer2003); Bembridge Marls: Antropov et al. (Reference Belokobylskij2014).
Table 3 Hymenoptera families present in Baltic amber.

Note: Modified from Weitschat and Wichard (A Reference Weitschat and Wichard1998, B Reference Weitschat and Wichard2010), with additional information from CSchedl (Reference Schedl2011), DVilhelmsen and Engel (Reference Vilhelmsen and Engel2012), EKupryjanowicz (Reference Kupryjanowicz2001), FBuhl (Reference Buhl2002), and GBurks et al. (Reference Burks, Heraty, Pinto and Grimaldi2015). Gasteruptiidae appears in Weitschat and Wichard (Reference Weitschat and Wichard2010) as Aulacidae.
Context
The establishment of angiosperm-dominated ecosystems
Perhaps the most profound biotic interactions that form and maintain modern terrestrial ecosystems are between angiosperms and Hymenoptera. Flowering plants are first confidently seen in the early Cretaceous and begin to define most terrestrial ecosystems by the mid-Cretaceous (Heimhofer et al. Reference Heimhofer, Hochuli, Burla, Dinis and Weissert2005; Doyle Reference Doyle2015), but the transition to an overwhelmingly angiosperm flora was only completed around Okanagan Highlands time (Niklas et al. Reference Niklas, Tiffney and Knoll1983; Cleal and Cascales-Miñana Reference Cleal and Cascales-Miñana2014). Today, they comprise almost 90% of land-plant species (Pennisi Reference Pennisi2009).
The Cretaceous-Paleogene extinction crisis
The end-Cretaceous extinction event appears to have had little or no effect on insects at the family level (e.g., Grimaldi and Engel Reference Grimaldi and Engel2005; Rasnitsyn et al. Reference Rasnitsyn, Bashkuev and Kopylov2016; Perkovsky and Węgierek Reference Perkovsky and Węgierek2017). At lower taxonomic levels, however, ichnofossil evidence indicates that they suffered a significant disruption and losses in North America (with few exceptions), stabilising and recovering diversity by the end of the Paleocene (Labandeira et al. Reference Labandeira, Johnson and Wilf2002; Wilf et al. Reference Wilf, Labandeira, Johnson and Ellis2006; Currano et al. Reference Currano, Wilf, Wing, Labandeira, Lovelock and Royer2008). Outside this region, however, insect–plant interactions appear to have suffered less, had a quicker recovery, or both (Wilf et al. Reference Wilf, Labandeira, Johnson, Cúneo and Dilcher2005b; Iglesias et al. Reference Iglesias, Wilf, Johnson, Zamuner, Cúneo, Matheos and Singer2007; Wappler and Denk Reference Wappler and Denk2011; Wappler et al. Reference Wappler, De Meulemeester, Aytekin, Michez and Engel2012; Donovan et al. Reference Donovan, Iglesias, Wilf, Labandeira and Cúneo2016). Plants suffered extensive impacts, of apparently differing intensities and durations across the globe (e.g., Wilf and Johnson Reference Wilf and Johnson2004; Wilf et al. Reference Wilf, Johnson, Cúneo, Smith, Singer and Gandolfo2005a; Iglesias et al. Reference Iglesias, Wilf, Johnson, Zamuner, Cúneo, Matheos and Singer2007; Barreda et al. Reference Barden and Grimaldi2012; Wappler et al. Reference Wappler, De Meulemeester, Aytekin, Michez and Engel2012). Changes in leaf morphologies suggest a shift in forest ecosystem functioning (Blonder et al. Reference Blonder, Royer, Johnson, Miller and Enquist2014). The Okanagan Highlands shows forests that had rebounded to high, modern tropical levels of plant diversity (woody dicotyledons) (Wilf et al. Reference Wilf, Cúneo, Johnson, Hicks, Wing and Obradovich2003; Archibald et al. Reference Archibald2010; Smith et al. Reference Smith, Basinger and Greenwood2012).
The Paleocene/Eocene transition
The Ypresian was a time of Holarctic intercontinental dispersal, explaining some biogeographic patterns. Land connections between North America, Europe, and East Asia facilitated large-scale dispersal of plants and mammals (Manchester Reference Manchester1999; Tiffney Reference Tiffney2000; Bowen et al. Reference Bowen, Clyde and Koch2002). Insects share numerous closely related taxa between northern continents in the Eocene (Archibald 2005, Reference Archibald2009; Archibald et al. Reference Archibald and Rasnitsyn2005, Reference Archibald, Bossert, Greenwood and Farrell2006, Reference Archibald, Greenwood, Smith, Mathewes and Basinger2011b; Archibald and Makarkin Reference Archibald, Johnson, Mathewes and Greenwood2006; Petrulevičius et al. Reference Petrulevičius, Nel, Rust, Bechly and Kohls2007; Makarkin and Archibald Reference Makarkin and Archibald2013; Dlussky et al. Reference Dlussky, Rasnitsyn and Perfilieva2015). This follows a brief hyperthermal spike in global temperatures by at least 5 °C at the Paleocene/Eocene boundary (Zachos et al. Reference Zachos, Dickens and Zeebe2008).
The Okanagan Highlands
In the Ypresian of far-western North America, the Okanagan Highlands depositional basins were formed as tectonic uplift raised the interior of southern British Columbia and northern Washington (Ewing Reference Ewing1980), an upland of considerable elevation (estimates: Wolfe et al. Reference Wolfe, Forest and Molnar1998; Tribe Reference Tribe2005; Smith et al. Reference Smith, Basinger and Greenwood2009) spanning about 1000 km north to south (Archibald et al. Reference Archibald, Greenwood and Mathewes2011a). These localities are mostly coincident with the early Eocene Climatic Optimum, an interval of several million years with the highest sustained (i.e., excepting brief hyperthermal events) global temperatures of the Cenozoic (Zachos et al. Reference Zachos, Dickens and Zeebe2008). The montane Okanagan Highlands localities, however, mostly had upper microthermal (⩽13 °C) mean annual temperature at all sites except at Quilchena, which is estimated to have been a few degrees warmer (Mathewes et al. Reference Mathewes, Greenwood and Archibald2016). Winters were mild, with coldest-month mean temperatures ⩾8 °C indicated at most localities, implying low temperature seasonality (Archibald et al. Reference Archibald2010, Reference Archibald and Mathewes2014). Only the coeval Tadushi Formation of Pacific coastal Russia might also represent an Ypresian insect assemblage preserved in such a cooler upland (Archibald et al. Reference Archibald and Rasnitsyn2005; age of the Tadushi: Popov and Grebennikov Reference Popov and Grebennikov2001), although some others may have had a mixture of source communities possibly including such an environment (e.g., the Fur Formation: Archibald and Makarkin Reference Archibald, Johnson, Mathewes and Greenwood2006). The Tadushi, however, contains fewer and less diverse Hymenoptera than the Okanagan Highlands despite large collections, a fact that cannot easily be explained as a taphonomic artefact, and may then represent a community with a genuinely smaller component of the order. Insect-bearing localities of the Kishenehn Formation (Montana) and at Florissant (Colorado) of the United States of America also represent uplands, but these were younger, after the Ypresian and the early Eocene Climatic Optimum (Meyer Reference Meyer2003; Fan et al. Reference Fan, Constenius and Dettman2017).
The temperate, seasonally equable, and mesic sites of the Okanagan Highlands supported closed canopy forests that were in many ways like those of the modern eastern deciduous forest of North America. It was composed in large part of plant genera that today inhabit forests of this region, and some that range in low latitudes or in East Asia, as well as others that are now extinct (Greenwood et al. Reference Greenwood, Archibald, Mathewes and Moss2005; Moss et al. Reference Moss, Greenwood and Archibald2005; Archibald et al. Reference Archibald, Greenwood and Mathewes2011a). Insect alpha diversity has been measured at McAbee as similar to that of a modern lowland Neotropical rainforest, and there was high insect beta diversity across the series (Archibald et al. Reference Archibald2010, Reference Archibald and Farrell2013). Its mild winters allowed frost-intolerant plants now restricted to low latitudes to coexist with temperate-climate flora characteristic of its mean annual temperature values, e.g., palms (Arecaceae) and spruce (Pinaceae) (Archibald and Farrell Reference Archibald, Cover and Moreau2003; Archibald et al. Reference Archibald, Greenwood and Mathewes2011a, Reference Archibald and Mathewes2014). It records the first appearances and expansions of numerous genera of prominent plant families associated with temperate latitudes today (DeVore and Pigg Reference DeVore and Pigg2007, Reference DeVore and Pigg2009). Graham (Reference Graham2011) speculated that the Okanagan Highlands might represent the origin of boreal forests that subsequently spread into the lowlands by about 10 million years ago in the globally cooler Neogene.
The Hymenoptera assemblages
All 22 modern superfamilies of Hymenoptera were in existence by Okanagan Highlands times, 15 of which we find in these collections. There are no known extinct superfamilies of the order in the Cenozoic. Okanagan Highlands absences are mainly in the Parasitica: the Evanioidea, Megalyroidea, Platygastroidea, and Stephanoidea, but also a few in the Symphyta: the Xyeloidea, Xiphydrioidea, and Orussoidea. Many absent Parasitica are very small, and we suspect that some might appear as Okanagan Highlands amber is more thoroughly investigated. Other missing groups might have been regionally absent, unsuited for the temperate, mesic upland environment; e.g., the lack of Scelionidae, well represented in some Cretaceous and Priabonian assemblages, might be explained by their preference for warmer, drier habitats (Zherikhin et al. Reference Zherikhin, Sukacheva and Rasnitsyn2009). Some specimens belong to families for which few fossils are known, such as Trigonalidae, Siricidae, Peradeniidae, and Monomachidae (its single confirmed fossil, see above), and these may have been rare within the landscape.
Biogeography
As with plants, Okanagan Highlands insects constitute a mixture of those that are today associated with various climates of differing latitudes, which can also be explained by the combination of mostly microthermal mean annual temperature values with mild winters; i.e., low temperature seasonality in a temperate setting (Archibald and Farrell Reference Archibald, Cover and Moreau2003; Archibald et al. Reference Archibald2010). In plants, this is most clearly seen at lower taxonomic levels; although here we examine Hymenoptera families, these show broad trends consistent with this pattern (Benson Reference Benson1946; Smith Reference Smith1988; Brothers and Finnamore Reference Brothers and Finnamore1993; Finnamore and Michener Reference Finnamore and Michener1993; Gibson Reference Gibson1993; Goulet Reference Goulet1993; Masner Reference Masner1993a, Reference Masner1993b; Mason Reference Mason1993; Michener Reference Michener1993; Ritchie Reference Ritchie1993; Sharkey Reference Sharkey1993; Wahl Reference Wahl1993; Schiff et al. Reference Schiff, Goulet, Smith, Boudreault, Wilson and Scheffler2012):
Cosmopolitan: Cimbicidae, Megaspilidae, Trigonalidae, Ichneumonidae, Braconidae, Diapriidae, Mymarommatoidea, Proctotrupidae, Cynipidae, Figitidae, Chalcidoidea, Sphecidae (sensu stricto), Megachilidae, Apidae, Vespidae, Formicidae;
Cosmopolitan, trending to temperate: Heloridae, Chrysididae;
Temperate and predominantly temperate: Cephidae, Pamphiliidae, Siricidae, Tenthredinidae, Peradeniidae, Roproniidae;
Predominantly tropical: Monomachidae, Pompilidae, Scoliidae.
Along with the expected presence of groups with predominantly modern northern distributions, such as various Symphyta, we see taxa like the Peradeniidae and Myrmeciinae (Formicidae) that join other Okanagan Highlands insects that are today native to the Australia/Southeast Asia region, like Megymenum Guérin-Méneville (Hemiptera: Dinidoridae), and species of Nymphidae (Neuroptera) and Mastotermitidae (Isoptera). Monomachidae are today restricted to the Southern Hemisphere north into tropical Mexico (Johnson and Musetti Reference Johnson and Musetti2012). Some groups show deeper biogeographic patterns, e.g., the myrmeciine ants include species found in the Green River Formation of mid-continental United States of America, the Lutetian of Patagonia, and Baltic amber, and the extinct genus Ypresiomyrma is also known from the Ypresian Fur Formation of Denmark and the Priabonian Bol’shaya Svetlovodnaya (Biamo) of Pacific-coastal Russia, showing wide-spread Eocene dispersal before the later restriction of Myrmeciinae to the Australian region (Archibald et al. Reference Archibald, Bossert, Greenwood and Farrell2006 and references therein; Dlussky et al. Reference Dlussky, Rasnitsyn and Perfilieva2015). The sole living genus of Peradeniidae, Peradenia Naumann and Masner, today restricted to Australia, is also known from Baltic amber (Johnson et al. Reference Johnson, Musetti and Janzen2001) (the McAbee specimen is not yet determined to genus).
Last occurrences/relictual taxa
Some taxa that were previously only known from the Cretaceous or were prominent then are last seen or are unexpectedly well represented in the Okanagan Highlands. Cuspilongus cachcreekensis Archibald and Rasnitsyn (Symphyta: Cephidae) from McAbee bears a distinctive, exceptionally long ovipositor (Fig. 2B), a trait shared within the family only by its sole congener and only other member of the Cuspilonginae, C. ghilarovi Rasnitsyn from the Aptian of Bon-Tsagaan, and so represents the single occurrence of this ancient lineage persisting into the Ypresian (Archibald and Rasnitsyn Reference Archibald, Morse, Greenwood and Mathewes2015; Kopylov and Rasnitsyn Reference Kopylov and Rasnitsyn2016). We find three specimens (Republic and Allenby Formation) belonging to the Archaeoscoliinae (Scoliidae), in contrast to only one reported from the very large, extensively collected Florissant assemblage. These are the only known occurrences of archaeoscoliines in the Cenozoic, a group that was diverse through the Cretaceous, found in Kazakhstan, Brazil, China, Spain, Siberia, and Mongolia (summarised: Rasnitsyn and Martínez-Delclòs Reference Rasnitsyn and Martínez-Delclòs1999; Zhang Reference Zhang2004). Several Okanagan Highlands diaprioids from Driftwood Canyon, Horsefly River, and McAbee appear to belong to basal Diapriidae sensu lato not previously known after the early Cretaceous. There is an unexpectedly large representation of angarosphecid Spheciformes, which were prominent in the early Cretaceous (see family treatment above) and were previously unknown in the Cenozoic outside of a single species in the Okanagan Highlands. Further, it is surprising that so large a number of other Okanagan Highlands Hymenoptera cannot be easily attributed to any family, which is unusual for the Cenozoic, more typical of the Mesozoic.
Earliest occurrences/taxon expansions
These fossils include the oldest confirmed occurrences of Monomachidae (but see a possible early Cretaceous occurrence, above), Trigonalidae, Peradeniidae, Pompilidae, and Sphecidae (sensu stricto), and perhaps others such as the Halictidae, although these await confirmation. Importantly, they also show the earliest evidence of notable diversifications within other families that were previously minor community elements. We discuss these below by trophic guilds, grouping fungivores with herbivores and separate from pollinivorous bees, treating parasitoids apart from predators, and considering ants separately by their wide range of roles.
Herbivores, fungivores
Fossils of siricomorph Symphyta are rare in the Okanagan Highlands – two of Siricidae, one of Pamphiliidae, and three of Cephidae – and are generally rare in the fossil record, and so new specimens are important (Archibald and Rasnitsyn Reference Archibald, Morse, Greenwood and Mathewes2015 and references therein). Okanagan Highlands forests contained many of the plant genera that they are associated with today, including some within the angiosperm families Rosaceae, Betulaceae, Cornaceae, Sapindaceae, Fagaceae, Juglandaceae, Oleaceae, Salicaceae, Ulmaceae, and Grossulariaceae, and the conifer families Pinaceae and Cupressaceae; many are characteristic of modern northern temperate latitudes, and some are early or earliest-known occurrences (Pigg et al. Reference Pigg, Manchester and Wehr2003; DeVore and Pigg Reference DeVore and Pigg2007, Reference DeVore and Pigg2009). These, together with a climatic regime of mean annual temperature values, provide the first appearance of the combination of conditions in the Okanagan Highlands that these Symphyta overwhelmingly prefer today.
The major diversity of modern herbivorous Hymenoptera is found in the Tenthredinoidea, which today has 7169 described species in six extant families (Aguiar et al. Reference Aguiar, Deans and Engel2013). Tenthredinoid families associated with angiosperms are its most species-rich: the Argidae, Cimbicidae, Pergidae, and Tenthredinidae (except Selandriinae). The Tenthredinidae overwhelmingly dominate the superfamily with 5500 species (Aguiar et al. Reference Aguiar, Deans and Engel2013; Isaka and Sato Reference Isaka and Sato2015). Molecular analysis indicates that the basal host plants of tenthredinoids were angiosperms, with a few groups subsequently switching to gymnosperms (Diprionidae) or pteridophytes (Blasticotomidae and Selandriinae) (Isaka and Sato Reference Isaka and Sato2015); however, while the fossil record of Blasticotomidae is not older than Florissant, they are morphologically closest to the predominantly Jurassic (and therefore pre-angiosperm) Xyelotomidae (Gao et al. Reference Gao, Ren and Shih2009) implying the likelihood of a long ghost lineage. It further suggests that higher-level Tenthredinoidea would have been largely in place well before Okanagan Highlands time, although there are no fossils reported for Diprionidae older than Baltic amber (Schedl Reference Schedl2008) and for Argidae and Blasticotomidae older than Florissant (Zhelochovtzev and Rasnitsyn Reference Zhelochovtzev and Rasnitsyn1972, but a tentative argid from the Paleocene of Menat, France was reported by Nel Reference Nel2004), and there is only equivocal fossil evidence of Pergidae (Krogmann et al. Reference Krogmann, Engel, Bechly and Nel2013).
The Mesozoic record of Tenthredinidae is small and little known. Palaeathalia laiyangensis Zhang from the early Cretaceous of Laiyang, China (Barremian to early Aptian) (Zhang Reference Zhang1985) was placed in the family, and leaf damage attributed to tenthredinids was described from the late Cretaceous (Turonian) Ora Formation of Israel (Krassilov and Rasnitsyn Reference Krassilov and Rasnitsyn2008). Grimaldi and Engel (Reference Grimaldi and Engel2005) and Vilhelmsen and Engel (Reference Vilhelmsen and Engel2012) subsequently considered all Mesozoic specimens assigned to the superfamily to be most conservatively treated as putative stem-group Tenthredinoidea. We believe P. laiyangensis to be a confident tenthredinid, however, which is supported by further undescribed fossils from the Cretaceous of Mongolia (Rasnitsyn Reference Rasnitsyn1980: Bon Tsagaan: Aptian; 17 specimens, possibly six others), Transbaikalia (Baissa: Valanginian; one to three specimens), and the Ola Formation (Obeshchayushchiy, Magadan Region, southeastern Siberia: Santonian to mid-Campanian; six to seven specimens) (A.P.R., personal observation). From this preliminary examination, it appears that these all either belong to Palaeathalia Zhang or differ slightly and are surely closely related, with little variation within each site assemblage, consistent with low Mesozoic diversity within the family.
In the Paleocene, there is one described species of Tenthredinidae from Menat, France (Piton Reference Piton1940; see review of Vilhelmsen and Engel Reference Vilhelmsen and Engel2012). We know of no undescribed Paleogene specimens older than the Okanagan Highlands, when the explosive species radiation within the family is now seen, a diversification that was previously thought to take place in the Priabonian (e.g., see Florissant: Meyer Reference Meyer2003). In the Okanagan Highlands, two workers collecting an unbiased insect sample for about three weeks in a very limited portion of the McAbee recovered 26 tenthredinids (13% of Hymenoptera), which included 20 undescribed species assigned to the Tenthredininae, Allantinae, Blennocampinae, and Nematinae (Archibald et al. Reference Archibald2010). Rice (Reference Rice1968) reported further species of Allantinae at Horsefly River and in the Allenby Formation. The family has also been found elsewhere throughout the Okanagan Highlands, at Driftwood Canyon, Quilchena, and Republic (numerous specimens, exemplars listed above). In collections from the coeval Tadushi Formation (eastern Sikhote-Alin, Russian Far East: Ypresian) there are 26 tenthredinoid fossils (about 30% of Hymenoptera), with 12 wings that can be assigned to the Tenthredinidae in at least several unidentified subfamilies; although determination of Tadushi tenthredinid morphospecies diversity is difficult, as they are of varied preservation quality and all are incomplete, they preliminarily appear modern and diverse compared with the Cretaceous fossils discussed above (A.P.R., personal observation).
The oldest described species of Cimbicidae is from the Paleocene (Nel Reference Nel2004), and no undescribed specimens pre-date this to our knowledge. This is followed by a species from the Green River Formation (Cockerell Reference Cockerell1925) and those reported here from the coeval Okanagan Highlands, which include specimens from McAbee, the Allenby Formation, and Republic (above). McAbee specimens are assigned to the subfamilies Cimbicinae and Coryninae or Pachylostictinae (Archibald Reference Archibald2007). The unbiased McAbee sample has six specimens (3% of Hymenoptera) (Archibald et al. Reference Archibald2010); including these, we found a total of 14 in all collections examined.
Pollinators: bees
Bees are well known for their essential role today as the most important angiosperm pollinators (e.g., Regal Reference Regal1977; Michener Reference Michener2007). Confident bee fossils first appear with three species with one specimen each from the Paleocene of Menat: Paleoepeolus micheneri Dehon et al. (Apidae), Probombus hirsutus Piton (Megachilidae), and Paleohabropoda oudardi Michez and Rasmont (Apidae) (Piton Reference Piton1940; Nel and Petrulevičius Reference Nel and Petrulevičius2003; Michez et al. Reference Michez, De Meulemeester, Rasmont, Nel and Patiny2009; Dehon et al. Reference Dehon, Perrard, Engel, Nel and Michez2017). Molecular analyses support an older, cryptic Cretaceous record, but these were calibrated with fossils that include the advanced bee Cretotrigona prisca (Michener and Grimaldi) as Cretaceous, which we consider most likely younger (Cenozoic) and some using a Lutetian age for the rich record of Priabonian Baltic amber bees (e.g., Rehan et al. Reference Rehan, Chapman, Craigie, Richards, Cooper and Schwarz2010; Cardinal and Danforth Reference Cardinal and Danforth2013; Martins et al. Reference Martins, Melo and Renner2014), or including the Cenomanian Melittosphex burmensis Poinar and Danforth, now considered to be in an unresolved position near crabronids and bees (Branstetter et al. Reference Branstetter, Danforth and Pitts2017).
Proxy evidence supporting the existence of bees deep into the Cretaceous is provided by Turonian flowers with morphology today associated with bee pollination (Crepet and Nixon Reference Crepet and Nixon1998; Crepet Reference Crepet2008). There was a variety of lineages of Jurassic and Cretaceous insect pollinators of various orders, some not associated with pollination today, some pre-dating angiosperms and associated with other plant groups, and others with angiosperms or stem-group proangiosperms (e.g., Ren Reference Ren1998; Krassilov and Rasnitsyn Reference Krassilov and Rasnitsyn1999; Krassilov et al. Reference Krassilov, Tekleva, Meyer-Melikyan and Rasnitsyn2003; Ren et al. Reference Ren, Labandeira and Santiago-Blay2009; Labandeira Reference Labandeira2010; Peñalver et al. Reference Peñalver, Arillo, Pérez-de la Fuente, Riccio, Delclòs, Barrón and Grimaldi2015; Labandeira et al. Reference Labandeira, Yang and Santiago-Blay2016; Lu et al. Reference Lu, Zhang and Liu2016; Makarkin Reference Makarkin2016). It is thought that the onset of angiosperms was associated not only with extinctions of some of these insect groups, but also with lateral transfer to angiosperm pollination in others, and origins of new angiosperm pollinators, some of which subsequently went extinct. This raises the possibility that one or more of these extinct insect groups might have been originally associated with these floral structures, which could have then been later exploited by bees as floral exaptations and so are today exclusively associated with them.
After the Paleocene, we see a rapid expansion of bee fossils beginning in Okanagan Highlands time, i.e., the latter half of the Ypresian. Okanagan Highlands bees include seven body fossils: the previously reported Halictus? saveneyi (Engel and Archibald Reference Engel and Archibald2003) from Quilchena (a possible halictid), and six new body-fossil specimens reported here from Republic (one possible halictid, one of undetermined family), McAbee (one apid, one possible halictid, one of undetermined family) and Driftwood Canyon (one of undetermined family). None appears to be conspecific. Ichnofossil evidence further indicates the presence of Megachilidae at Republic, McAbee, and Horsefly River (see above, Fig. 14H–I). Coeval specimens in Green River Formation shale (leaf damage, Megachilidae: Labandeira Reference Labandeira2002; one body specimen, Apidae: Dehon et al. Reference Dehon, Michez, Nel, Engel and De Meulemeester2014), French Oise amber (one specimen, Melittidae: Michez et al. Reference Michez, Nel, Menier and Rasmont2007), Indian Cambay amber (four specimens, three species in Apidae belonging to Electrapini and Melikertini: Engel et al. Reference Engel, Ortega-Blanco, Nascimbene and Singh2013a), and Chinese Fushun amber (one specimen, Apidae: Engel and Michener Reference Engel and Michener2013), broaden the Ypresian record.
The Paleocene P. micheneri from Menat has morphology characteristic of cleptoparasitism (Dehon et al. Reference Dehon, Perrard, Engel, Nel and Michez2017), and the Ypresian Oise melittid and some Lutetian German bees possess structures indicating specialised relationships with flowers (Michez et al. Reference Michez, Nel, Menier and Rasmont2007; Wappler et al. Reference Wappler, Labandeira, Engel, Zetter and Grímsson2015). These either support the notion of a long, cryptic, Cretaceous presence of bees, or alternatively that they had a later origin, burst of diversification, and rapid evolution of specialised behaviour and sophisticated pollination syndromes in concert with the onset of early Paleogene angiosperm modernisation.
Predators
Okanagan Highlands spheciform Apoidea include three specimens that are the earliest records of the Sphecidae (sensu stricto), previously known in Priabonian and younger deposits (see above). The superfamily is dominated by the Angarosphecidae, perhaps a paraphyletic grade of stem-apoids. These were plentiful and diverse in the later early Cretaceous and were thought to be long extinct by Okanagan Highlands time until Eosphecium was reported at Quilchena (Pulawski et al. Reference Pulawski, Rasnitsyn, Brothers and Archibald2000). The extent of the representation of these primitive wasps in the Okanagan Highlands reported here is unexpected.
In the Vespoidea, the Scoliidae and Pompilidae are present, but rare. The McAbee pompilid is the oldest confident record of its family. The Vespidae is well represented, with 14 specimens distributed among most major Okanagan Highlands localities: at Driftwood Canyon (two), McAbee (four), Quilchena (two), the Allenby Formation (one), and at Republic (five). It is a surprise that they are so well represented in the Okanagan Highlands, and a greater one yet that we can refer 12 to eusocial subfamilies. Because of their conservative wing morphology, a precise estimate of their species diversity is premature here; however, their relative sizes, shapes, and venations indicate that a number of species is represented, and that they are not repeated specimens of the same or a few species. Depending on the finalised number, this could be a larger representation of eusocial Vespidae than at any other site or site group (e.g., Baltic amber) in the fossil record. The plentiful and diverse Formicidae are treated separately, below.
Parasitoids
About 75% of insect parasitoids belong to the Hymenoptera, most in the paraphyletic Parasitica, which constitutes the largest number of described species of the order (LaSalle and Gauld Reference LaSalle and Gauld1992; LaSalle Reference LaSalle1993). Perhaps 77–99% remain undescribed, however, and they may represent some 80–85% of Hymenoptera and 20% of all insects (LaSalle and Gauld Reference LaSalle and Gauld1992). It is thought that food chains consisting of green plants, insect herbivores and the Parasitica contain over half of all metazoan species (May Reference May1988). A typical English garden was found to have over 500 species of Ichneumonidae alone (Owen et al. Reference Owen, Townes and Townes1981). They include many keystone species essential in regulating phytophagous insect populations and so maintaining plant diversity and ecosystem stability (Pimm and Lawton Reference Pimm and Lawton1978; Hawkins and Lawton Reference Hawkins and Lawton1987; LaSalle and Gauld Reference LaSalle and Gauld1992; Hawkins Reference Hawkins1993; LaSalle Reference LaSalle1993).
The fossil record shows prominent parasitoid taxa undergoing major diversifications in the Paleogene, notably within the Chalcidoidea, Diaprioidea, and Ichneumonoidea (general overviews by Rasnitsyn Reference Rasnitsyn2002; Zherikhin Reference Zherikhin2002; of individual groups by Grimaldi and Engel Reference Grimaldi and Engel2005), and with major changes within the Platygastroidea.
Platygastroidea
Platygastroids are tiny (mostly 1–2 mm in length) endoparasitoids of insect eggs (Masner Reference Masner1993c). The Platygastridae (sensu stricto) underwent a burst of diversification in the Cenozoic, in contrast to the Scelionidae, which flourished in the Cretaceous (Rasnitsyn Reference Rasnitsyn1975; Ortega-Blanco et al. Reference Ortega-Blanco, McKellar and Engel2014). Neraudeau et al. (Reference Neraudeau, Perrichot and Colin2008) reported four undescribed specimens of Platygastridae from uppermost Albian-lowermost Cenomanian French amber, but they meant Platygastridae (sensu lato), and the specimens referred to belong to Scelionidae (V. Perrichot, personal communication). Therefore, the earliest confident record of Platygastridae (sensu stricto) is in the Lutetian Kishenehn Formation (Talamas and Buffington Reference Talamas and Buffington2015). They subsequently appear in Priabonian Baltic (four genera) and Scandanavian (three genera) ambers, and then in Miocene Dominican amber (25 genera) (Scandanavian: Buhl Reference Buhl2002; Baltic and Dominican: Talamas and Buffington Reference Talamas and Buffington2015). Talamas and Buffington (Reference Talamas and Buffington2015) suggest that many of the species-level records of these are in need of revision. Platygastroids are currently unknown in the Okanagan Highlands.
Chalcidoidea
The chalcidoids are very small (3–5 mm or less in length), species-rich but poorly known wasps that are found in a wide variety of habitats worldwide (Gibson Reference Gibson1993). Although their fossil record extends to the earliest Cretaceous, they remained a relatively small group until they are found in greater abundance in the Priabonian, diversifying towards their modern ecological importance, perhaps in concert with their known primary hosts, Lepidoptera and brachyceran flies (Zherikhin Reference Zherikhin2002; Rasnitsyn et al. Reference Rasnitsyn, Basibuyuk and Quicke2004; Zherikhin et al. Reference Zherikhin, Sukacheva and Rasnitsyn2009). Consistent with their minute size, their fossil record is almost entirely in amber, rarely in shale (Grimaldi and Engel Reference Grimaldi and Engel2005). In the Okanagan Highlands, they are known not only from the single specimen in Hat Creek amber, but also in shale, with one from McAbee and three from Driftwood Canyon, where small to minute insects are commonly preserved.
Diaprioidea
Today the superfamily is among the more speciose of the Parasitica (although far smaller than the Ichneumonoidea), and is dominated by the Diapriidae, which has 2048 of its 2109 species (Aguiar et al. Reference Aguiar, Deans and Engel2013). They were scarcely known before the Priabonian (Rasnitsyn Reference Rasnitsyn2002; Zherikhin Reference Zherikhin2002), until large numbers of Belytinae were reported in the Lutetian Kishenehn Formation of Montana (Greenwalt and Labandeira Reference Greenwalt and Labandeira2013). However, it is currently undetermined if these constitute many or few species. In the Okanagan Highlands assemblage, many appear modern: three belong to the diapriid subfamily Belytinae, and a further one may also be a belytine, but might also belong to the Ismaridae, and five wing specimens belong to the Diapriidae sensu lato (Diapriidae+Ismaridae: Sharkey et al. Reference Sharkey, Carpenter and Vilhelmsen2012). Seven surprisingly primitive specimens are apparently allied to early Cretaceous basal Diapriidae sensu lato (see above). From our preliminary observations, we estimate conservatively that there is a minimum of 10 species of the family in these collections.
Ichneumonoidea
The Ichneumonoidea are widely considered to be the most important group of entomophagous insects today (LaSalle and Gauld Reference LaSalle and Gauld1992). They comprise the great majority of described modern Parasitica, and dominate the Hymenoptera in species numbers: Ichneumonidae have some 24 025 described species and Braconidae some 19 205 (Aguiar et al. Reference Aguiar, Deans and Engel2013), which is thought to be a small portion of a much greater total diversity of both – the Ichneumonidae alone may have more than 100 000 species (Rasnitsyn Reference Rasnitsyn1978).
The fossil record of the superfamily begins in the early Cretaceous, with all families present: the extant Ichneumonidae and Braconidae, and its extinct groups Praeichneumonidae (five described species), possibly paraphyletic with regard to extant Ichneumonoids (Kopylov Reference Kopylov2012); and the Tanychorinae (22 species), described as a subfamily of the Ichneumonidae, but probably basal to Ichneumonidae+Braconidae (Kopylov Reference Kopylov2010). The Ichneumonidae (excluding the Tanychorinae) have 37 described Cretaceous species, 12 in the early Cretaceous and 25 in the late Cretaceous (recently summarised by Menier et al. Reference Menier, Nel, Waller and De Ploëg2004; Li et al. Reference Li, Kopylov, Shih and Ren2017). These are represented by numerous specimens in shale deposits; all 12 early Cretaceous species, and 15 of the late Cretaceous species (Li et al. Reference Li, Kopylov, Shih and Ren2017). At Baissa, they are 1.1% of Hymenoptera; at Khasurtyi, 0%; at Bon-Tsagan, 10.1%; at Obeshchayushchii, 29%; and at Orapa, 4.6% (here, Tanychorinae are excluded, altering slightly the data provided by Kopylov Reference Kopylov2010 and Brothers and Rasnitsyn Reference Brothers and Rasnitsyn2003). In the Paleocene, a single species is known from Menat (Piton Reference Piton1940), and in a collection of 2000 insects from the earliest Ypresian Fur Formation of Denmark there were 23 ichneumonids, all belonging to a single named species (Rust Reference Rust1990), with a few other unnamed ones found in subsequently examined collections (Rust Reference Rust1999). They were previously known to be diverse first in the Priabonian (Bembridge Marls: Antropov et al. Reference Belokobylskij2014; Florissant: Meyer Reference Meyer2003; further, see checklist of Menier et al. Reference Menier, Nel, Waller and De Ploëg2004), but here we see them both plentiful and diverse in the Okanagan Highlands. In samples taken from unbiased collecting (Archibald) at Driftwood Canyon, Horsefly River and McAbee, and from the SFU Quilchena collections (Mathewes) and the Stonerose Interpretive Center collection of Republic insects, there were 117 ichneumonid specimens with 94 morphospecies, almost three times the known species from the Cretaceous (Archibald et al. Reference Archibald2010, Reference Archibald and Farrell2013).
The Braconidae (including the Eoichneumonidae) have ~36 species in the Cretaceous (Perrichot et al. Reference Perrichot, Nel and Quicke2009; Ortega-Blanco et al. Reference Ortega-Blanco, Delclòs and Engel2011a; Belokobylskij Reference Barreda, Cúneo, Wilf, Currano, Scasso and Brinkhuis2012), but this is difficult to enumerate with precision because of undescribed specimens in current collections (A.P.R., personal observation) and many questionable published identifications in need of revision (Belokobylskij Reference Barreda, Cúneo, Wilf, Currano, Scasso and Brinkhuis2012). However, they reasonably appear to have a similar species richness in the Cretaceous as the Ichneumonidae. They are also seen as diverse first in the Priabonian (Baltic amber, Bembridge Marls, and Florissant: reviewed by Belokobylskij Reference Belokobylskij2014). While they are present in the Okanagan Highlands, Braconidae are much less well represented than the Ichneumonidae in both species and number of specimens. The unbiased McAbee sample contains only six specimens, two of which were complete enough to assign to two species. The upland Tadushi Formation also has a small number of braconid specimens relative to ichneumonids (A.P.R., personal observation), but the reverse is true for the hot, lowland Green River Formation (A.P.R., personal observation: 21 Braconidae, 11 Ichneumonidae, unbiased collection in the Paleontological Institute of the Russian Academy of Sciences, Moscow, Russia; 105 versus 83 respectively, general collection in the National Museum of Natural History, Smithsonian Institution, Washington, District of Columbia, United States of America, A.P.R. determined in 1988). Ichneumonidae dominate in the Lutetian Kishenehn Formation of Montana (125 versus 67 Braconidae: D. Greenwalt, personal communication). It is not yet determined whether these braconid fossils contain a few or many species. The differing patterns of relative abundance of Braconidae and Ichneumonidae among these localities may be due to a regionally varying factor such as climate, e.g., the cooler Okanagan Highlands versus the warmer Green River Formation. Today, Ichneumonidae far outnumber Braconidae in cool, wet northern Siberia (S. Belokobylskij and D. Kasparyan, personal communication), and in samples from Harvard Forest in Massachusetts there were about three times the ichneumonid specimens as braconids, but in the tropical lowland forest at La Selva, Costa Rica, braconids were about double the number of ichneumonids (Archibald et al. Reference Archibald2010). Li et al. (Reference Li, Kopylov, Shih and Ren2017) found this same pattern present in the Cretaceous, with Braconidae distributed widely across latitudes and Ichneumonidae prevalent outside of the tropics.
Ants: predators, herbivores, fungivores
The diversification of ant species and their increase in abundance in communities would have marked an important event in the development of modern terrestrial ecosystems. Today, they are ubiquitous, prospering in a wide variety of ecological niches worldwide, excluding the polar regions and isolated Pacific islands, comprising about 15–20% of global animal biomass, and about 25% in the tropics; in the Amazon terra firme rainforest, each hectare is thought to support over eight million individual ants; 43 species in 28 genera were recovered from a single tree in the Peruvian Amazon (Fittkau and Klinge Reference Fittkau and Klinge1973; Wilson Reference Wilson1987; Hölldobler and Wilson Reference Hölldobler and Wilson1990; Schultz Reference Schultz2000). Ants are extremely efficient foragers, with large numbers of workers constantly monitoring large areas and rapidly mass-recruiting nest mates to exploit discovered food items. They are among the leading invertebrate predators in most terrestrial habitats, and in the tropics dead insects are scavenged within minutes (Carroll and Janzen Reference Carroll and Janzen1973; Leston Reference Leston1973; Hölldobler and Wilson Reference Hölldobler and Wilson1990 and references therein). Ant predation protects plants from their herbivores: a colony of Formica rufa Linnaeus fed upon 21 700 caterpillars (Lepidoptera and Symphyta) in a day, and one of F. polyctena Förster took about six million prey items per year from a 0.33 ha plot (Hölldobler and Wilson Reference Hölldobler and Wilson1990 and references therein). Leaf-cutter ants are among the major herbivores of the Neotropics, as shown by recently measured consumption rates on Barro Colorado Island in Panama (Herz et al. Reference Herz, Beyschlag and Hölldobler2007). They transport large amounts of nutrients in the form of leaf cuttings up to 6 m underground, modifying soil composition and facilitating deep root growth, and act as soil turners in many regions, in some surpassing termites and earthworms (reviewed by Hölldobler and Wilson Reference Hölldobler and Wilson1990; LaSalle and Gauld 1993). In the humid tropics, keystone ant mosaics control rates of insect herbivory, defending against it in return for rewards (e.g., domatia, extrafloral nectaries), or promoting it by tending herbivores (e.g., aphids, caterpillars) (Leston Reference Leston1978; Gilbert Reference Gilbert1980; Pierce Reference Pierce1985). These relationships are highlighted by the evolution of ant domatia in over 90 genera of 36 plant families, and of extrafloral nectaries in at least 68 angiosperm families (Elias Reference Elias1983; Hölldobler and Wilson Reference Hölldobler and Wilson1990). In some regions, they are the principle granivores, dispersing seeds of many plants, reducing their competition, buffering the effects of local disturbance, and promoting growth in the nutrient-rich soils of their nests (Hölldobler and Wilson Reference Hölldobler and Wilson1990; LaSalle and Gauld 1993). In New York State, United States of America, they disperse 37–48% of aboveground herbaceous biomass, half of all stems (Handel et al. Reference Handel, Fisch and Schatz1981). Major insect pollinators (bees, some Diptera) may have flourished in this role at least in part through resistance to predation by ants (Leston Reference Leston1973), and flowers may have evolved characters to exclude them (Faegri and van der Pijl Reference Faegri and van der Pijl1979). In these ways, ants strongly influence differential floral and invertebrate patchiness, taxon compositions and diversities across the landscape, and so are a major factor controlling overall biotic composition, diversity, and distributions (Leston Reference Leston1978; Gilbert Reference Gilbert1980; Majer Reference Majer1983).
While molecular analyses indicate that many higher-level ant taxa were established in the Cretaceous, only a few are confirmed by fossils (Engel and Grimaldi Reference Engel and Grimaldi2005; Moreau et al. Reference Moreau, Bell, Vila, Archibald and Pierce2006; LaPolla et al. Reference LaPolla, Dlussky and Perrichot2013). Most recent hypotheses on the diversification of ants use “diversification” to mean divergence of their higher-level sub-taxa, although a few concern increase of richness at lower levels, which we mean here (e.g., see differing meanings by Leston Reference Leston1973; Wilson and Hölldobler Reference Wilson and Hölldobler2005; Moreau et al. Reference Moreau, Bell, Vila, Archibald and Pierce2006; Dunn et al. Reference Dunn, Gove, Barraclough, Givnish and Majer2007; Dlussky and Wedmann Reference Dlussky and Wedmann2012; Lucky et al. Reference Lucky, Trautwein, Guenard, Weiser and Dunn2013; Ward Reference Ward2014). The Cretaceous fossil record suggests a low community presence and impact of small numbers of individuals and species (Zherikhin Reference Zherikhin2002; Barden and Grimaldi Reference Aria, Perrichot and Nel2016). Cretaceous ants are discussed further in the family treatment above. Paleocene ants are little known, apart from Ancistrocerus berlandi Piton at Menat, described as a member of the Eumenidae (see Piton Reference Piton1940: fig. 91) but which is surely an ant, and possible fragmentary evidence from the Paskapoo Formation of Alberta, Canada (Mitchell and Wighton Reference Mitchell and Wighton1979).
Ants first appear common and diverse in the Ypresian. A single ant species has been reported from the earliest Ypresian Fur Formation of Denmark (Rust and Andersen Reference Rust and Andersen1999) and 40 species in French Oise amber (approximately coeval with McAbee) (Aria et al. Reference Archibald, Rasnitsyn and Akhmetiev2011; LaPolla et al. Reference LaPolla, Dlussky and Perrichot2013); 27 species in Chinese Fushun amber (Hong Reference Hong2002); 16 species from the Green River Formation (Dlussky and Rasnitsyn Reference Dlussky and Rasnitsyn2003); and a diverse collection of ants in Cambay amber, but with no estimation of species number (Rust et al. Reference Rust, Singh and Rana2010). In the Okanagan Highlands, ants have been previously reported from a variety of sites (see family treatment above). Here, we conservatively find a preliminary estimate of about 50 Okanagan Highlands ant species in the collections examined and the above references, excluding numerous wing specimens and others that may well be recognised as distinct species in the future. We expect this number to grow, particularly as Okanagan Highlands amber is thoroughly examined.
Conclusions
The Okanagan Highlands show Hymenoptera in transition, with the last or among the last occurrences of some taxa that were diverse in, or were previously only known in the Mesozoic (e.g., Angarosphecidae, Archaeoscoliinae, Cuspilonginae, some Diaprioidea), and the first appearances of some modern taxa (e.g., Sphecidae sensu stricto, Pompilidae, Cephinae).
Biogeography
The mixture of taxa that today inhabit both temperate and low-latitude tropical climates is consistent with mostly upper microthermal mean annual temperatures combined with mild, likely frost-free winters, and is also seen in plants. The community is also consistent with Holarctic dispersals through intercontinental connections as seen in plants and mammals. There is also a component that is today restricted to the Australian region.
Diversifications within ecologically key carnivore and herbivore groups
Phytophagous Hymenoptera species numbers increased towards modern prominence at a time of completion of the transition to angiosperm-dominated ecosystems, and the first evidence of temperate northern forests that would later spread through northern latitudes and the first appearances of many plant genera that characterise those forests today. This combination of climate and flora that Symphyta are mostly associated with today is not seen before the Okanagan Highlands. There is a great increase of species within the Tenthredinoidea, most importantly in the Tenthredinidae (at least 20 species in four subfamilies at McAbee alone), but also in the less diverse Cimbicidae. The beginning of the expansion of bees is seen in the latter half of the Ypresian, from three in the Paleocene to 14: seven in the Okanagan Highlands plus numerous specimens of Megachilidae leaf damage, one in the Green River Formation plus Megachilidae damage, one in Oise amber, four in Indian Cambay amber, and one in Fushun amber, indicating a shift towards modern pollination relationships.
Carnivorous Hymenoptera show notable diversifications within the Parasitica, in the Diapriodea and significantly in the highly ecologically important Ichneumonidae, with almost three times the number of species in the Okanagan Highlands as is known from the Cretaceous. Predators show diversifications within Vespidae (14 specimens, perhaps a few if any conspecific, 12 placed in eusocial subfamilies; only two specimens in two Paleocene species of eusocial Vespidae predate this) and ants (a minimum 50 species in the Okanagan Highlands, 30 in Oise amber; 27 in Fushin amber, 16 in the Green River Formation, and an unknown number in Indian Cambay amber; all earlier records are low diversity).
Diversifications within eusocial Hymenoptera
The establishment of the dominance of social insects is a major turning point in terrestrial ecology. May (Reference May1988) noted that, to a first order of approximation, insects comprise all animal life on Earth, and Hölldobler and Wilson (Reference Hölldobler and Wilson1990), taking a similarly broad point of view, went further to say that essentially all insects today are social insects. Engel et al. (Reference Engel, Grimaldi and Krishna2009, Reference Engel, Barden, Riccio and Grimaldi2016) showed six termite species with various castes in Cenomanian Burmese amber, although they speculated that termites may not have begun their ascent to ecological prominence until the Priabonian, not achieving it until the Miocene. Rasnitsyn and Ross (Reference Rasnitsyn and Ross2000), however, found a higher presence of termites in Burmese amber (about 91 of 1198 insects), indicating that they may have been prominent as far back as the mid-Cretaceous.
The record of eusocial Hymenoptera in the Cretaceous, however, is limited. Ants are found deep into the Cretaceous, but with a small presence, and eusocial wasps are indicated only by a single late Cretaceous fossil paper-wasp nest (Carpenter and Rasnitsyn Reference Carpenter and Rasnitsyn1990). It is not yet known if any Okanagan Highlands bees belong to social taxa. The oldest evidence of eusocial bees is the Ypresian Meliponini from Fushun amber and Electrapini and Melikertini from Cambay amber (Engel and Michener Reference Engel and Michener2013; Engel et al. Reference Engel, Ortega-Blanco, Nascimbene and Singh2013a).
Might these diversifications of ecologically key Hymenoptera groups be older, masked by preservational bias?
The insect-rich Danish Fur Formation, immediately following the Paleocene/Eocene boundary in the earliest Ypresian, has no reported bees or social vespids, but bears plentiful specimens of ants and ichneumonids. Of the 2000 insects examined by Rust and Andersen (Reference Rust and Andersen1999) in Fur Formation collections, however, all of the 101 ant specimens belong to the myrmeciine species Ypresiomyrma rebekkae (Rust and Andersen), and the ichneumons were also all placed in a single species by Rust (Reference Rust1990), who later estimated a few further, undescribed species in new collections (Rust Reference Rust1999). The Fur, however, is a marine formation, and may have been highly selective for the subset of its source insect communities that flew near the shore at elevations and perhaps times of year with favourable winds to transport them to offshore depositional sites coinciding with the diatom blooms within which they are preserved. We know little about Paleocene insects apart from ichnofossil evidence, as they are barely represented in insect body-fossil localities. The richest Paleocene insect fossil record at Menat suggests that while bees, social Vespidae, ants, Ichneumonidae, Tenthredinidae, and Cimbicidae were present, they were not important community elements. The Menat record is, however, poor for Hymenoptera in general (Piton Reference Piton1940; Nel and Auvray Reference Nel and Auvray2006) and appears unexpectedly unbalanced, perhaps for unknown taphonomic reasons. The only Hymenoptera reported from the Paleocene insect assemblage of the Paskapoo Formation in Alberta, Canada are possible ant fragments (Mitchell and Wighton Reference Mitchell and Wighton1979).
If the lower-level diversifications of these key groups were earlier than the second half of the Ypresian, perhaps masked in the Fur and Paskapoo Formations and at Menat by taphonomic factors specific to those sites, they might also have been obscured in late Cretaceous deposits by yet other taphonomic processes. The Maastrichtian lacks insect localities except for North Dakota (United States of America) amber, which is little known (Rasnitsyn et al. Reference Rasnitsyn, Bashkuev and Kopylov2016). The next oldest is Campanian Canadian amber, which is strongly biased towards smaller species (McKellar and Engel Reference McKellar and Engel2012). Late Cretaceous Hymenoptera are primarily known in amber. Even in larger-sized ambers, however, there appears to be a tendency to capture smaller insects relative to shale deposits (A.P.R., personal observation), potentially under-representing such groups as Symphyta, non-chrysidoid Aculeata and Ichneumonidae.
There are, however, some indirect reasons to suspect that at least some of the diversifications of these ecologically key hymenopteran taxa commenced in or soon before Okanagan Highlands times. Low-diversity Paleocene forests, at least in North America, diversifying to high, modern tropical levels of species diversity seen in various regions such as the Okanagan Highlands, coupled with the appearance of new forest types and diversifications of temperate plant families preferred by Symphyta, and the bottleneck and re-establishment of plant-insect interactions indicated by the North American leaf-damage record, would be consistent with diversification of phytophagous Hymenoptera and then others at higher trophic levels in the Ypresian. The aftermath of the Paleocene-Eocene Thermal Maximum shows diversifications in other organisms (e.g., mammals: Gingerich Reference Gingerich1987; Rea et al. Reference Rea, Zachos, Owen and Gingerich1990), associated not only with its dramatic and brief spike in global temperature, but also with Holarctic intercontinental dispersals.
The Ichneumonidae feed on a wide variety of immature holometabolous insects and various Chelicerata, but most commonly upon larvae of the Symphyta and Lepidoptera. We know that the most diverse symphytans, the Tenthredinidae, were at least somewhat numerous as individuals in the Cretaceous (above), and their high species richness is first seen in the Okanagan Highlands; however, they are scarcely known in amber – even in Priabonian Baltic amber after they are seen to be diverse in shale deposits (Vilhelmsen and Engel Reference Vilhelmsen and Engel2012) – and so their species diversity is unknown between their presence in the Santonian to mid-Campanian Ola Formation shale (see above) and the Okanagan Highlands. The fossil record shows Lepidoptera since the later early Jurassic, and a few rare specimens of diverse higher taxa of moths since the middle Jurassic (Zhang et al. Reference Zhang, Shih and Labandeira2013); molecular analyses indicate that its higher-level clades were in place during the Cretaceous, followed by a burst of diversification of modern lineages below that level in the Paleogene (Lepidoptera in general: Wahlberg et al. Reference Wahlberg, Wheat and Peña2013; butterflies: Wahlberg et al. Reference Wahlberg, Leneveu, Kodandaramaiah, Peña, Nylin, Freitas and Brower2009; Heikkilä et al. Reference Heikkilä, Kaila, Mutanen, Peña and Wahlberg2012).
The argument for a post-Campanian diversification of ant lower taxa appears stronger, as Canadian amber should show this, but does not. The Fur and Menat records are suggestive, but inconclusive that high ant species diversity was not in place before the mid-Ypresian. An earlier history and cryptic diversity of bees is possible, as many are small, which, like ants, could have been preserved in Cretaceous ambers even of smaller size. Ants and bees are common and diverse in Priabonian Northern European and Miocene Dominican and Mexican ambers. Although the Maastrichtian and Paleocene insect body fossil record is depauperate, there is a good leaf record, and the characteristic leaf damage of megachilids first appears in the Ypresian of the Okanagan Highlands and the Green River Formation, soon after the oldest megachilid body fossil from Menat. Modernisation of angiosperm communities in the early Paleogene could be consistent with a concomitant diversification of bees. The tiny Diapriidae should be well represented, but have a small fossil record in Cretaceous ambers (see family treatment above).
For these reasons, while a mid to later Ypresian diversification of bees, ants, social vespids, Tenthredinoidea, Diaprioidea, and Ichneumonidae is possible, it remains somewhat speculative, perhaps clouded by differential taphonomic factors influencing fossil assemblage compositions between it and the late Cretaceous, and resting on the assumptions discussed above. Confirmation awaits future discoveries of Paleocene and more comparable late Cretaceous insect localities. What is clear now, however, is that these events were all underway at least as early as the mid-late Ypresian.
The Okanagan Highlands
If this diversification was a phenomenon of the latter half of the Ypresian, did the Okanagan Highlands play a particular role? Ants are also plentiful and diverse in some coeval deposits discussed above; however, there is scant evidence of higher species diversities of other key hymenopteran groups discussed until later. If the Okanagan Highlands were different beyond possessing a favourable taphonomic setting promoting unusually high fidelity of fossil assemblage to community representation, i.e., if it was in fact composed of communities where at least some of these changes were first occurring, then why? The unique combination of an Ypresian temperate upland in a warm early Eocene Climatic Optimum world with a diversifying modern temperate flora and high-diversity insect and plant community at a time of large-scale intercontinental dispersal immediately presents itself for consideration. Detailed comparison of Okanagan Highlands Hymenoptera with other taphonomically equivalent assemblages of the later half of the Ypresian might suggest answers, e.g., of Okanagan Highlands ambers with Oise, Cambay, and Fushun ambers, bearing in mind that we know none of these to have represented temperate, montane communities, nor do we know any such in the Paleocene or late Cretaceous.
The lower-level diversifications in these ecologically key Hymenoptera groups represent the onset of a major phase in insect evolution, not by the appearance of new body plans or higher-level taxa, but of the great lower-level expansion and impact of key functional groups within communities, ones that profoundly affect modern terrestrial ecosystems. While this change or parts of it may have commenced undetected in the later Cretaceous or the Paleocene, we now see the broad sweep of it certainly in place by the latter half of the Ypresian, most clearly in the diverse, temperate uplands of the Okanagan Highlands.
Acknowledgements
The authors thank the curators, collection managers, and museum volunteers who loaned and expedited loans of specimens: Katherine Meade, Travis Wellman, Michael Sternberg, Karl Volkman, and Catherine Brown of the Stonerose Interpretive Center; Marji Johns and Richard Hebda of the Royal British Columbia Museum; Nancy Van Wagoner of Thompson Rivers University; Caroline Strömberg and Regan Dunn of the Burke Museum; Robin Irwin and Kathy Simpkins of the Princeton and District Museum and Archives; and Pat Trask of the Courtenay and District Museum. They thank the collectors who donated fossils to these institutions that were part of this study – in particular the authors thank late John Leahy and David Langevin, who donated a number of specimens from McAbee; the following who contributed specimens to the Stonerose Interpretive Center in Republic: Karon and Bob Ambrogi, John Arnold, Cathy Bailey, Shane and Mathew Balcom, Lisa Barksdale, Patty Capps, Karen Gutierrez, Carma Henry, Melanie DeVore and Kathleen Pigg, Peg Johnson, Rick Johnson, Laura Jones, Rose Jenkins, Bob Kelton, Rob Krause, Mary Ellen Martinsen, Nils Larsen, Tracy Larson, Shannon Miller, Liam Milliken, Christian McGovern, Harrison McMillan, Vickie Norman, Gloria Perchynski, Susan and Roger Rosbach, Ron and Arlene Sakit, Elizabeth Seccomb, Debbie Smith, Michael Sternberg, Karl Volkman, Steve Wang, Travis Wellman, Joanne West, Pat White, Gregg Wilson, and Judy Wold; Muriel Morris, and Ed Staples and Nienke Klaver, who contributed Allenby Formation fossils to the RBCM and the Princeton Museum and Archives respectively; and Christopher West, who made the Driftwood Canyon ant available to them. The authors thank many people who facilitated and helped in fieldwork, including John Howard and others at British Columbia Parks in Smithers British Columbia, and Terry McCullough of British Columbia Hydro for work at Hat Creek. They thank S.A. Belokobylskij, Melanie DeVore, Dale Greenwalt, Dmitriy Kasparyan, Dmitry Kopylov, Conrad Labandeira, Vincent Perrichot, Kathleen Pigg, and Junfeng Zhang for providing information. They also thank Marlow Pellatt of Parks Canada for access to digital microphotography equipment and Ryan McKellar (Royal Saskatchewan Museum, Regina, Saskatchewan) for assistance with amber preparation and photography. Archibald’s fieldwork was supported by a Putnam Expeditionary grant (Harvard University), and by Natural Sciences and Engineering Research Council of Canada Discovery grants to Mathewes (grant 3835) and David Greenwood (Brandon University, Brandon, Manitoba; grant 311934), which also provided infrastructure support for research; for Rasnitsyn’s work was supported by the A.A. Borissiak Paleontological Institute, Russian Academy of Sciences, and in part by the Program of the Presidium of the Russian Academy of Sciences number 22 “The evolution of the organic world and planetary processes”; Brothers had financial support from the University of KwaZulu-Natal Research Office.



















