Introduction
Insecticides are generally considered critically important tools for pest management in most cropping systems, but their applications can be problematic, with potential harmful effects on non-target organisms (Tuck et al. Reference Tuck, Winqvist, Mota, Ahnström, Turnbull and Bengtsson2014), prolonged environmental persistence (Arias-Estévez et al. Reference Arias-Estévez, López-Periago, Martínez-Carballo, Simal-Gándara, Mejuto and García-Río2008), and eventually reduced effectiveness through development of insecticide resistance (Brattsten et al. Reference Brattsten, Holyoke, Leeper and Raffa1986). An important emerging alternative to conventional products are biopesticides, natural compounds derived from plants, animals, or microorganisms that have bioactivity against pests (Chandler et al. Reference Chandler, Bailey, Tatchell, Davidson, Greaves and Grant2011).
Plant-derived essential oils are biopesticides that contain complex mixtures of secondary metabolites including phenols, monoterpenes, and sesquiterpenes that help deter attack from herbivores or pathogens (Bennett and Wallsgrove Reference Bennett and Wallsgrove1994). Both commercially available formulations and rudimentary essential oil products have shown promise in controlling crop pests. For instance, EcoTrol (a rosemary oil-based pesticide) has shown to be highly effective in controlling twospotted spider mite Tetranychus urticae Koch (Acari: Tetranychidae) under greenhouse conditions (Miresmailli and Isman Reference Miresmailli and Isman2006). Likewise, in less developed economies where preharvest crop losses are high (Oerke Reference Oerke2006) and access to synthetic products is limited – unrefined products, formed of suspensions of dried and crushed plant material have been successfully used to control herbivorous pests in agricultural small-holdings, reducing crop losses (Mkindi et al. Reference Mkindi, Mpumi, Tembo, Stevenson, Ndakidemi and Mtei2017; Stevenson et al. Reference Stevenson, Isman and Belmain2017).
Despite numerous studies demonstrating insecticidal activity of essential oils, they are not as widely used or available as conventional synthetic insecticides. This is often due to reduced efficacy and persistence relative to synthetic compounds, and variable efficacy of the oils, resulting from the inherent variability in secondary metabolite profiles occurring amongst different phenotypes, parts of the plant, and environmental conditions (Isman Reference Isman2000; Angioni et al. Reference Angioni, Barra, Coroneo, Dessi and Cabras2006).
Instead of using whole essential oils for pest management, it may be adequate to use key constituent components of essential oils that have insecticidal properties (Miresmailli et al. Reference Miresmailli, Bradbury and Isman2006). In some cases, the most dominant secondary metabolites exhibit toxicity equal to that of the parent essential oils (Faraone et al. Reference Faraone, Hillier and Cutler2015; Tak et al. Reference Tak, Jovel and Isman2016). Many secondary metabolites have relatively simple organic structures (Bennett and Wallsgrove Reference Bennett and Wallsgrove1994), and can be inexpensively synthesised (Thomas Reference Thomas2007). In addition, secondary metabolites of essential oils may also act as synergists (Barile Reference Barile2013); a phenomenon where the combined toxicity of two compounds is larger than its predicted sum.
In this study, we examined the insecticidal activity of the monoterpenes linalool and thymol, the major constituents of essential oils of thyme, Thymus vulgaris Linnaeus (Lamiaceae), and lavender Lavandula angustifolia Linnaeus (Lamiaceae), respectively, against diamondback moth (Plutella xylostella (Linnaeus), Lepidoptera: Plutellidae). Diamondback moth is one of the most destructive pests of cruciferous crops worldwide (Furlong and Wright Reference Furlong and Wright2013), with annual crop damage and pest management costs estimated to be US$4–5 billion (Zalucki et al. Reference Zalucki, Shabbir, Silva, Adamson, Shu-Sheng and Furlong2012). Diamondback moth is notorious for its ability to develop resistance to multiple classes of insecticide (Furlong and Wright Reference Furlong and Wright2013). Identifying natural compounds with insecticidal or synergistic properties could be important in reducing reliance on conventional insecticides for control, while also addressing concerns of insecticide resistance and environmental pollution. We conducted experiments that examined the toxicity of linalool and thymol to diamondback moth through contact and oral exposure, and the ability of low concentrations of each monoterpene to synergise the biopesticide spinosad.
Materials and methods
Plant and insect material
Larvae used in experiments were from a laboratory colony of diamondback moth maintained on the Agricultural Campus, Dalhousie University (Truro, Nova Scotia, Canada). Insects were reared on cabbage plants, Brassica oleracea Linnaeus (Brassicaceae), in BugDorm (Bioquip Products, Compton, California, United States of America) rearing cages (47.5×47.5×93 cm) inside a walk-in environmental chamber (22±2 °C, 16:8 light:dark hours, 65±5% relative humidity). Second instars were used in experiments. Cabbage plants (Copenhagen market variety) were grown from seed in Pro-Mix (Premier Tech Home & Garden, Mississauga, Ontario, Canada) potting medium in an insecticide-free greenhouse and were watered as needed. Four to six-week-old plants were used for the experiments and for sustaining the diamondback moth colony. Foliage was rinsed with distilled water before use in experiments.
Treatment solutions
Serial dilutions of spinosad were prepared from formulated Entrust 80 WP insecticide (spinosad 800 g/L; Dow AgroSciences, Calgary, Alberta, Canada). Linalool (97%) and thymol (98%) were purchased from Alfa Aesar (Ward Hill, Massachusetts, United States of America). Test solutions were prepared using distilled water as a solvent and Polysorbate 80 (Tween 80; Sigma-Aldrich, Saint Louis, Missouri, United States of America) as a surfactant (0.5% v/v). As thymol was partially insoluble in water, all tests involving thymol included acetone (1.0% v/v) to allow solubilisation. When testing the synergistic effect of thymol, spinosad solutions additionally included 1.0% v/v acetone to serve as a positive control. When testing the toxicity of secondary metabolites, we used concentrations ranging from 10 000 to 100 000 ppm and 3000 to 100 000 ppm for linalool and thymol, respectively. When testing for synergism, spinosad concentrations ranged from 1 to 10 000 ppm. Concentrations of linalool and thymol were included as synergists at maximum no observable effect concentrations of 5000 and 1000 ppm, respectively. For further detail on test concentrations see Supplementary Material Table 1.
Bioassays
For direct contact exposure with individual compounds, cohorts of five second instars were placed in 90 mm diameter Petri dishes. A 2-mL application of test solution was administered to each cohort using a Potter tower (Burkard Scientific, Uxbridge, United Kingdom) at 78 kPa. Immediately following treatment, larvae were transferred to a 15 mm diameter untreated cabbage leaf disc held in a 55 mm diameter Petri dish lined with filter paper. For oral exposure, treatments were applied to leaf discs using the Potter tower, as described above. Leaf discs were left to dry for 10–15 min and then transferred to clean plastic Petri dishes as above. Cohorts of five untreated larvae were then randomly selected and placed on each disc using a fine paint brush. For both direct contact and oral toxicity bioassays, larval mortality was assessed with a dissecting microscope at 10× magnification, 24 hours after exposure. Larvae that did not respond to gentle prodding with a blunt probe were considered dead.
In synergism experiments, the concentration of synergist used should be a maximum dose that causes no observable toxicity (Scott Reference Scott1990). A range of spinosad concentrations that caused ~10–90% mortality was used. Insects or leaf discs were treated with spinosad+linalool/thymol mixtures using a Potter tower, and mortality was assessed after 24 hours as described above.
Each experiment was conducted as a complete randomised block design. Each block was comprised of four concentrations of a compound plus a control, replicated in triplicate. Fresh treatment solutions were prepared for each bioassay replicate. Experiments were repeated in time, beginning on three separate days, so that every concentration of compound was replicated n=9 times. This resulted in a total of 225 larvae per compound used for determining acute toxicity.
Data analysis
We used generalised linear models with a binomial error structure and a probit link function to model the relationship between larval survival and the log10 concentration of each compound. When model residuals indicated overdispersion, a quasibinomial error structure was used instead (Crawley Reference Crawley2012). The slopes and intercepts of these models were then used to estimate the median lethal concentration (LC50) and its 95% confidence limits. We determined whether linalool or thymol enhanced the toxicity of spinosad using a ratio test (Wheeler et al. Reference Wheeler, Park and Bailer2006). This test calculates the ratio between the toxicity of a compound with and without a synergist. If the confidence limits of the ratio are above, and do not include a value of 1, the synergism is statistically significant (Wheeler et al. Reference Wheeler, Park and Bailer2006). All analyses were conducted using R 3.3.2 (R Core Team 2012). The LC50 for each compound was estimated using the package “MASS” (Venables and Ripley Reference Venables and Ripley2002), and the ratio tests were conducted with the package “drc” (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015).
Results
Both linalool and thymol were toxic to diamondback moth larvae, with similar toxicity for each compound regardless of exposure route (Table 1). The toxicity of spinosad alone was three orders of magnitude greater than either linalool or thymol (Table 1). Through the ratio test, we found that linalool significantly synergised the toxicity of spinosad (Table 1) through contact exposure (Fig. 1: A, 95% confidence limits=1.02–1.98) and oral exposure (Fig. 1: B, 95% confidence limits=1.03–2.04). The contact toxicity of spinosad was enhanced 2.0-fold by the addition of linalool, and the oral toxicity of spinosad was enhanced 2.3-fold by the addition of linalool (Table 1). Through the ratio test, we found thymol did not synergise the toxicity of spinosad through contact exposure (Fig. 1: C, 95% confidence limits=0.81–1.36) or oral exposure (Fig. 1: D, 95% confidence limits=0.87–1.41). The highest control mortality (11.1%) occurred in the combined spinosad+0.5% linalool topical bioassay. In all other bioassays control mortality was below 9.0%.
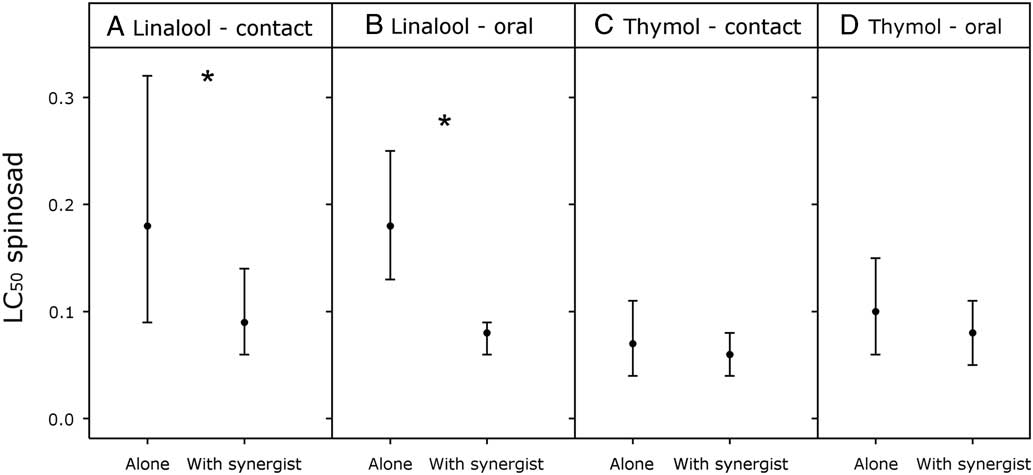
Fig. 1 Toxicity of spinosad to second instar diamondback moth with or without a secondary metabolite synergist. Values shown are the LC50s for spinosad with 95% confidence intervals. Asterisks identify assays where the monoterpene synergised spinosad, as determined using the ratio test.
Table 1 Median lethal concentration (LC50), and 95% confidence limits (95% CL) of two monoterpenes and spinosad, with and without synergists to second instar Plutella xylostella.

Note: Larvae were treated either by contact or oral exposure.
Discussion
We found that both linalool and thymol were toxic to diamondback moth larvae. A study using Musca domestica Linnaeus (Diptera: Muscidae) showed that linalool inhibits the neurotransmitter acetyl cholinesterase (López and Pascual-Villalobos Reference López and Pascual-Villalobos2010). A second study using Drosophila melanogaster Meigen (Diptera: Drosophilidae), showed that thymol obtains its toxicity by interfering with post-synaptic gamma-aminobutyric acid receptors (Priestley et al. Reference Priestley, Williamson, Wafford and Sattelle2003). Using contact exposure assays with diamondback moth larvae, Kumrungsee et al. (Reference Kumrungsee, Pluempanupat, Koul and Bullangpoti2014) reported an LD50 of 0.22 µg for thymol, but found that linalool did not on its own induce mortality rates of 50%, even when topical application exceeded 15 µg per larva. Although we found linalool and thymol to be toxic to diamondback moth in our experiments, their toxicity was more than three orders of magnitude lower than that of spinosad, and other widely used synthetic insecticides used to control diamondback moth (Hill and Foster Reference Hill and Foster2000). Moreover, given their low levels of persistence (Ngamo et al. Reference Ngamo, Ngatanko, Ngassoum, Mapongmestsem and Hance2007; Hu and Coats Reference Hu and Coats2008), it is unlikely that either of these compounds will serve as stand-alone insecticides in the field.
The known variability of plant essential oils (Angioni et al. Reference Angioni, Barra, Coroneo, Dessi and Cabras2006), suggests that identifying and subsequently purifying or synthesising key constituents that give essential oils their insecticidal properties might offer greater promise for commercialisation. In some cases, dominant secondary metabolites have similar toxicity as their parent essential oils. For example, Faraone et al. (Reference Faraone, Hillier and Cutler2015) found that essential oils from lavender and thyme respectively consisted of 54.3% linalool and 72.7% thymol. When tested against the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae), the contact exposure toxicity of both secondary metabolites was essentially equal to that of their respective parent oils. Similarly, work by Tak et al. (Reference Tak, Jovel and Isman2016) found the toxicity of lemongrass essential oil to third instar Trichoplasia ni (Hübner) (Lepidoptera: Geometridae), was equivalent to citral, its dominant secondary metabolite. However in contrast, other studies have shown that parent oils have greater toxicity than their dominant constituent parts (Jiang et al. Reference Jiang, Akhtar, Bradbury, Zhang and Isman2009).
We found that oral and topical exposure of linalool synergised the toxicity of spinosad, a pesticide that works through targeting binding sites on nicotinic acetylcholine receptors at a unique site to other insecticides (Orr et al. Reference Orr, Shaffner, Richey and Crouse2009). We were unable to detect any synergistic properties of thymol, due to an interaction between acetone and spinosad (Table 1). The synergistic properties of plant essential oils are usually achieved by the inhibition of cytochrome P450 monooxygenases and carboxylesterases, which are enzymes responsible for metabolising insecticides (Bernard and Philogène Reference Bernard and Philogène1993). The potential of essential oils or secondary metabolites to act as synergists seems to be dependent on the compounds being studied (Tong and Bloomquist Reference Tong and Bloomquist2013; Faraone et al. Reference Faraone, Hillier and Cutler2015). For instance, Tong and Bloomquist (Reference Tong and Bloomquist2013) identified six essential oils that synergised carbaryl, but found these same six essential oils, and eight others, antagonised the toxicity of permethrin.
If dominant secondary metabolites retain properties of their parent essential oils, their potential for pest management might extend beyond their insecticidal and synergistic properties. For example, a recent study of eight essential oils for P. xylostella management showed that treating cabbage plants with black pepper, Piper nigrum Linnaeus (Piperaceae), essential oil increased larval mortality, it also deterred larval colonisation, and reduced oviposition (Sangha et al. Reference Sangha, Astatkie and Cutler2017).
Relative to conventional insecticides, linalool and thymol have low toxicity to diamondback moth larvae. Owing to the low toxicity (Kumrungsee et al. Reference Kumrungsee, Pluempanupat, Koul and Bullangpoti2014) and short persistence (Ngamo et al. Reference Ngamo, Ngatanko, Ngassoum, Mapongmestsem and Hance2007; Hu and Coats Reference Hu and Coats2008) of these secondary metabolites, it is unlikely that either could serve as standalone products for control of diamondback moth. Linalool provided only low levels of synergism in our laboratory bioassays, and it is unlikely any appreciable synergism would occur in field or greenhouse situations. Although higher levels of synergism might be achieved with whole essential oils (e.g., Faraone et al. Reference Faraone, Hillier and Cutler2015), compositional inconsistencies of these natural compounds is a significant hurdle to regulatory approval in North America and Europe (Isman Reference Isman2006). An alternative approach might be to build a synthetic blend of secondary metabolites, modelled after a particular essential oil to contain all bioactive compounds (e.g., Miresmailli et al. Reference Miresmailli, Bradbury and Isman2006). Such a mixture could be tested and formulated with different adjuvants to improve persistence and efficacy, or tested as a synergist of conventional insecticides, the latter of which might be useful in resistance management.
Acknowledgements
This work was supported by: an Atlantic Canada Opportunities Agency, Atlantic Innovation Fund (project number 197853); the Agriculture and Agri-Food Canada Organic Science Cluster II, in partnership with the Organic Federation of Canada (project number AIP-CL02); a National Science and Engineering Research Council (NSERC) Undergraduate Student Research Award to A.E.W.; and a Killam Postdoctoral Fellowship to P.M.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.4039/tce.2017.63




