Introduction
Sperm competition, the process by which spermatozoa of two or more males compete to fertilise the egg(s) of a lone female, occurs when females mate with multiple males in a single breeding bout (Parker Reference Parker1970; Simmons Reference Simmons2001). Evolutionary responses that help males avoid or cope with sperm competition include morphological, physiological, and behavioural adaptations (Knowlton and Greenwell Reference Knowlton and Greenwell1984; Simmons Reference Simmons2001).
In species with internal fertilisation, a female may store sperm from competing males in her reproductive tract or sperm storage organ (e.g., spermatheca) for some time before fertilisation, thus setting the stage for intense and potentially prolonged sperm competition. In response, males may achieve first-male sperm precedence if they reduce competition from subsequent rival sperm by (i) depositing copulatory plugs to prevent female re-mating (Simmons Reference Simmons2001), (ii) reducing a female’s attractiveness to other males through substances in the seminal fluid (Simmons Reference Simmons2001), (iii) prolonging the duration, or increasing the frequency, of copulations (Thornhill Reference Thornhill1984), and (iv) by engaging in postcopulatory interactions such as grasping or guarding the mated female (Gwynne Reference Gwynne1984; Alcock Reference Alcock1994). Precopulatory mate guarding also favours first-male sperm precedence; e.g., in species where females trigger male competition as a mechanism of mate choice and females choose to mate with the strongest or fastest male (Brown et al. Reference Brown, Crespi and Choe1997), but postcopulatory rituals may represent an adaptation to sperm competition if mated females would otherwise remain receptive (Alcock Reference Alcock1994).
Prolonged interactions, whereby a male will stay near, or remain in contact with, a receptive female following insemination, may evolve in response to direct competition from rival males attempting to mate with that female (Alcock Reference Alcock1994). Under this scenario, postcopulatory interactions are expected to take longer when receptive females are limited and difficult to secure (Parker Reference Parker1974; Alcock Reference Alcock1994; Simmons Reference Simmons2001), and to intensify when the second or last male to mate (henceforth M2 male) is likely to deposit sperm that will be used to fertilise a greater proportion of eggs than sperm from the first male (henceforth M1 male) due to last-male sperm advantage (Boorman and Parker Reference Boorman and Parker1976; Simmons Reference Simmons2001).
Postcopulatory interactions may affect fertilisation in species with cryptic mate choice, whereby females manipulate sperm storage and select sperm from particular partners for egg fertilisation (Gromko et al. Reference Gromko, Gilbert and Richmond1984; Eberhard Reference Eberhard1996). Such interactions may also help advertise a male’s quality before egg fertilisation (Simmons Reference Simmons1990), or help ensure that mated females are less receptive to other males (Eberhard Reference Eberhard2009).
A specific form of postcopulatory interaction and postcopulatory rituals occur in some species of parasitoid wasps (Mackauer Reference Mackauer1969). Such rituals generally resemble courtship interactions, involving more or less stereotypical behaviour directed towards females by males, but they occur only after mating, and their adaptive significance has remained largely enigmatic (Viggiani and Battaglia Reference Viggiani and Battaglia1983; van den Assem Reference van den Assem1986; King and Fischer Reference King and Fischer2005).
Several hypotheses have been proposed to help explain the evolution of postcopulatory rituals. In some parasitoid wasps, postcopulatory rituals may have evolved in response to selection pressure from rival males (van den Assem et al. Reference van den Assem, Gijswijt and Nubel1980) who attempt to mate with a female while she is still in a receptive state from her interaction with the first-mating male. These rituals then function as a form of mate guarding by reducing the efficacy of mating attempts by rival males and/or by leading to reduced female receptivity (Allen et al. Reference Allen, Kazmer and Luck1994; King and Fischer Reference King and Fischer2005). For example, following copulation, males of the parasitoid Pteromalus puparum Linnaeus (Hymenoptera: Pteromalidae) continuously move the female’s abdomen, apparently to better detect a rival’s attempt at copulating with her (Thornhill and Alcock Reference Thornhill and Alcock1983), and males of the parasitoid Cotesia rubecula Marshall (Hymenoptera: Braconidae) implement female mimicry to distract their rivals (Field and Keller Reference Field and Keller1993).
In general, rival males who are not detected or distracted could possibly mate with a female. In such circumstances, some males may attempt to increase the chances that their sperm is selected by strategically repositioning or removing rival sperm from entering the female’s storage organ (Gromko et al. Reference Gromko, Gilbert and Richmond1984; Thornhill Reference Thornhill1984; Simmons Reference Simmons2001), via prolonged duration in copula, multiple copulatory bouts, and/or specialised morphological structures (Thornhill and Alcock Reference Thornhill and Alcock1983). Generally, successful sperm removers and sperm repositioners require between seven seconds and 20 minutes of copulation time (Waage Reference Waage1984); most parasitoids do not devote that much time to the copulatory stage within a mating sequence (Gordh and DeBach Reference Gordh and DeBach1978; Allen et al. Reference Allen, Kazmer and Luck1994; King and Fischer Reference King and Fischer2005; Ablard et al. Reference Ablard, Fairhurst, Andersen, Schaefer and Gries2011).
Multiple matings are rare among parasitoids (Gordh and DeBach Reference Gordh and DeBach1978; Ridley Reference Ridley1993; Benelli et al. Reference Benelli, Bonsignori, Stefanini, Dario and Canale2013). Males that reposition and/or remove sperm tend to achieve last-male sperm precedence. Last-male sperm precedence is rare and the mechanism is ambiguous. For example, males of the parasitoid wasp Diachasmimorpha longicaudata Ashmead (Hymenoptera: Braconidae) achieve sperm precedence when they re-mate a female after 24 hours; the underlying mechanism could be the loss of M1 sperm from the female’s storage organ between copulations (Simmons Reference Simmons2001), with M1 male sperm not being used or retained by the female (Martinez-Martinez et al. Reference Martinez-Martinez, Leyva-Vazquez and Mojica1993). It is also unclear whether D. longicaudata males who achieve first-male sperm precedence also perform a precopulatory ritual, and/or a postcopulatory ritual that functions as a form of mate guarding, as evidenced in males of the parasitoid wasps Nasonia vitripennis Walker (Hymenoptera: Pteromalidae), Aphytis melinus DeBach (Hymenoptera: Aphelinidae), and Lariophagus distinguendus Förster (Hymenoptera: Pteromalidae) (Holmes Reference Holmes1974; van den Assem et al. Reference van den Assem, van Lersel and Los-den Hartogh1989; Allen et al. Reference Allen, Kazmer and Luck1994; Kuhbandner et al. Reference Kuhbandner, Sperling, Mori and Ruther2012; Benelli et al. Reference Benelli, Bonsignori, Stefanini, Dario and Canale2013).
Both precopulatory and postcopulatory rituals exist in the quasi-gregarious (with one offspring per aggregated host), 2-mm egg parasitoid wasp Ooencyrtus kuvanae Howard (Hymenoptera: Encyrtidae). Courtship and mating take place on egg masses of host gypsy moth, Lymantria dispar dispar Linnaeus (Lepidoptera: Erebidae). An egg mass measures 2–3 cm across and contains several hundred eggs covered in setae (Brown Reference Brown1984). Eggs in the uppermost layer are parasitised by female wasps that insert a single egg into each accessible host egg. By fertilising an egg, arrhenotokous females produce a daughter, and by not fertilising it, they produce a son. Haploid sons derive their entire genome from their mother, whereas daughters are diploid and receive genes from both parents. Between three to four weeks, wasps complete development inside host eggs and emerge en masse as sexually mature adults that can live four to six weeks. Females emerge up to one day later than males, are immediately receptive to mating, and are about twice as numerous as males. However, a local, adult male-biased sex ratio occurs frequently among nonsiblings (Somjee et al. Reference Somjee, Ablard, Crespi, Schaefer and Gries2011) because males typically remain on the host egg mass as long as there are mating opportunities, whereas mated females disperse within 24 hours, seeking new gypsy moth egg masses (Brown Reference Brown1984).
Courtship is mediated by a close-range sex pheromone that attracts males to females (Ablard et al. Reference Ablard, Gries, Khaskin, Schaefer and Gries2012). Males then implement one of two alternative mating tactics. They either pheromone-tag a female and at a later time engage her in the mating sequence, or they immediately engage her in the mating sequence (Ablard et al. Reference Ablard, Schaefer and Gries2013). The mating sequence consists of a brief (~4 seconds) precopulatory ritual, mating (4–9 seconds), and a relatively longer (15–67 seconds) postcopulatory ritual (Ablard et al. Reference Ablard, Fairhurst, Andersen, Schaefer and Gries2011).
During the precopulatory ritual, the females are placed in a “trance-like” (unmoving, unresponsive) state (henceforth “trance”) that persists for some time after copulation (Ablard et al. Reference Ablard, Fairhurst, Andersen, Schaefer and Gries2011). The behavioural mechanisms underlying the postcopulatory ritual resemble those of the precopulatory ritual; the male interlocks the female’s antennae with his and then proceeds to strike her antennae with his legs. In contrast to the precopulatory ritual, he uses his forelegs in a random rather than repetitive or synchronous pattern of strikes. The female then strikes back at the male with her forelegs. If she is interrupted and becomes motionless, the male resumes his strikes. Thus, the postcopulatory ritual may function as a form of mate guarding to accelerate the “awakening” of the entranced mated female, who then rejects all mating attempts by other males over the course of her lifetime, ensuring paternity of the M1 male (Ablard et al. Reference Ablard, Fairhurst, Andersen, Schaefer and Gries2011).
Following our reports (Ablard et al. Reference Ablard, Fairhurst, Andersen, Schaefer and Gries2011, Reference Ablard, Gries, Khaskin, Schaefer and Gries2012, Reference Ablard, Schaefer and Gries2013) that O. kuvanae females mate only once and then become unattractive to males, we most recently noticed “sneaker” (M2) males in the O. kuvanae mating system (K.M.A., personal observation). In highly competitive settings, these M2 males do not directly compete for mating opportunities. Instead, M2 males copulate with an in-trance female, when she is either in copula with a M1 male, or postmating when she is coming out of the trance and engaged in the postcopulatory ritual with the M1 male, thus possibly siring some or all of the female’s daughters.
Interpretation of the adaptive significance of male and female mating behaviour in O. kuvanae, and other species with postcopulatory rituals, depends critically on patterns of sperm precedence and use. In this study, we investigated the presence of sperm competition in O. kuvanae by testing paternity of M1 and M2 males using DNA microsatellite analysis. We predicted that (1) there is first-male sperm precedence, as reported in parasitoids where, like in O. kuvanae, males perform a precopulatory and a postcopulatory ritual, and mated females remain receptive only briefly; (2) males will not have multiple or lengthy copulatory bouts that are often associated with long periods of receptivity in females and last-male sperm precedence; (3) the postcopulatory ritual represents a male tactic to reduce sperm competition; and (4) that males do not possess morphological adaptations for removal of rival male sperm because such adaptations are associated with lengthy, rather than brief, copulatory bouts.
Materials and methods
Experimental insects
A new colony of O. kuvanae was started with specimens field collected from Quercus Linnaeus (Fagaceae) hardwood forests in the town of North East, Maryland, United States of America (39°36′N, 75°55′W). All insects were reared under a 16:8 hour light:dark photoperiod at 22–25 °C and 50–70% relative humidity (Hofstetter and Raffa Reference Hofstetter and Raffa1997) in the Global Forest Quarantine Facility of Simon Fraser University, Burnaby, British Columbia, Canada. They were contained in Plexiglass cages (40×40×30 cm) and provided with cotton wicks (1×10 cm; Richmond Dental, Charlotte, North Carolina, United States of America) soaked in a 30% honey water solution (w:v) every two days. Ten gypsy moth egg masses, supplied by the United States Forest Service (Hamden, Connecticut, United States of America), were introduced to be parasitised by female wasps. Fourteen days later, parasitised egg masses were removed and 1000 eggs were placed singly into translucent plastic cups (103.5 mL) and secured with a lid. Emergent insects were separated by sex and size under a microscope and used in the experiment within one day of emergence to avoid adverse effects associated with ageing (van den Assem Reference van den Assem1996).
Attaining twice-mated females
To produce twice-mated females, four males without prior contact with a female were confined with one virgin female (n=10) in a Petri dish (30 mm diameter). This competitive setting increased the likelihood that the female would be mated by a sneaker (M2) male while she was still in the trance and receptive state following the precopulatory ritual and copulation, and before the completion of the postcopulatory ritual with the M1 male. Following the completion of the postcopulatory ritual by the M1 and/or M2 male, the two males and the twice-mated female were removed from the arena; the two males that did not mate were discarded. Two observers using a digital voice recorder equipped with a time tracker continuously tracked and documented the mating order of the two males that mated, their number of copulatory bouts, time spent in copula, and the number of postcopulatory ritual bouts and time spent engaged in the postcopulatory ritual. Due to the small size of the insects, morphological markers were not used. Mated females were placed singly in glass jars provisioned with food and 40 gypsy moth eggs, which according to pre-experiments provide sufficient oviposition opportunities to a twice-mated female (K.M.A., personal observation). After 21 days, the females were removed and the 40 eggs were placed singly into plastic cups to prevent mating between offspring. Emergent daughters and sons were counted. Parents and daughters were stored singly in 2.0 mL Qiagen® sterile microcentrifuge tubes (Company, Toronto, Ontario, Canada) at −80°C until DNA extraction.
DNA library construction, screening, and enrichment
Methods for DNA library construction, enrichment, and screening are published elsewhere (Jones et al. Reference Jones, Levine and Banks2002) and were applied by Genetic Identification Services (GIS, Chatsworth, California, United States of America). Genomic DNA was partially restricted with a cocktail of seven blunt-end cutting enzymes. Fragments that ranged between 300 and 700 base pairs in length were adapted and subjected to magnetic bead capture (CPG, Lincoln Park, New Jersey, United States of America), using biotinylated capture molecules. Libraries were prepared in parallel, using Biotin-CA(15), -AAG(12), -AAT(12), and -ATG(12) as capture molecules in a protocol provided by CPG (Lincoln Park, New Jersey, United States of America). Captured molecules were amplified and restricted with HindIII to remove the adapters. The resulting fragments were ligated into the HindIII site of pUC19. Recombinant molecules were electroporated into Escherichia coli (Enterobacteriales: Enterobacteriaceae) DH5α. Recombinant clones were selected at random for sequencing, and enrichment levels were expressed as the fraction of sequences that contained a microsatellite. Sequences were obtained on an ABI 377 or an ABI 3730, using ABI Prism Taq dye terminator cycle sequencing methodology. Microsatellite-containing sequences were identified by inspection, polymerase chain reaction (PCR) primers were designed using DesignerPCR version 1.03 (Research Genetics Inc., Huntsville, Alabama, United States of America), and they were purchased from Integrated DNA Technologies (Coralville, Iowa, United States of America). The optimal amplification reaction mix for all primer pairs consisted of 1× Biolase© Buffer from a 10× stock solution supplied by Bioline (Taunton, Massachusetts, United States of America), 2 mM MgCl2, 0.2 mM of each dNTPs, 6 µM of each primer, 0.025 U/µL Biolase DNA Polymerase (Bioline), and 0.2 ng/µL template DNA in a 50-µL final reaction volume. Samples were amplified in a Perkin-Elmer-Cetus thermal cycler by an initial denaturation at 94 °C (180 seconds), followed by 35 cycles of 94 °C (40 seconds), 55 °C (40 seconds), and 72 °C (30 seconds), with a final extension of 72 °C (240 seconds). DNA was extracted using the DNeasy Blood and Tissue® kit (Qiagen, Germantown, Maryland, United States of America) according to the manufacturer’s protocol. Microsatellite loci were amplified in 10-µL reactions in the following reaction mix: MgCl2, 2 mM; dNTPs (premixed), 0.2 mM each; primers, 0.3 µM each; Biolase DNA Polymerase® (Bioline), 0.025 U/µL; template DNA, 0.2 ng/µL. PCR was conducted in a RoboCycler Gradient 96® thermocycler (Stratagene, La Jolla, California, United States of America), using the same protocol as above. PCR products were separated on 3.5% agarose gels, and stained with ethidium bromide to identify polymorphic loci; six loci were polymorphic (A1, A3, A106a, A107, B105, and D106) and limited to this study.
DNA extraction
Frozen-stored specimens were transferred to a bed of ice before being crushed with a sterile plastic micropipette tip. Immediately postcrushing, DNA was extracted using the microLysis®-Plus kit (Gel, San Francisco, California, United States of America) following the manufacturer’s protocol except that 40 instead of 20 µL of microLysis®-Plus were used.
PCR
DNA paternity analyses were based on four microsatellite loci. Genomic DNA was amplified with PCR blends that contained 5.15 µL ddH2O, 1.0 µL of 10× enzyme buffer, 1.0 µL of 25 mM of MgCl2, 0.8 µL of 2.5 mM of dNTPs, 0.3 µL mix of 10 mM forward-labelled primer [700 series] (Integrated DNA Technologies), and unlabelled forward primer, 0.3 µL of 10 mM reverse primer, 0.05 µL of Taq DNA polymerase (GenScript, Piscataway, New Jersey, United States of America), and 1 µL of 2 ng/µL template DNA. The sequences of the designed primers were as follows: A1-F: 5′-CCC GTA TTA TAG ACG TTC GTA C-3′; A1-R: 5′-GCA AAA TTG CAC ATA TAC ACA G-3′; A106a-F: 5′-AGA GCA TAA GCC GTC GTC-3′; A106a-R: 5′-GCG AAG CAC ACA CAA CTG-3′; A107-F: 5′-TTG GTC TCT CTT TCT CTC CTG-3′; A107-R: 5′-GCA GTG CTG TTG CTG TTA C-3′; B105-F: 5′-TCG CTC TCT CGC TTG TTC-3′; B105-R: 5′-AGT TGG TCA GGA GGG TGA G-3′. PCR reactions were denatured at 94 °C (180 seconds), followed by 30 cycles of 94 °C (40 seconds), 58 °C (40 seconds), 72 °C (30 seconds), and a final extension step of 72 °C (320 seconds). We added 2 µL of formamide and bromophenol blue loading dye to PCR mixtures that were electrophoresed through a 10% polyacrylamide gel with a 1× TBE buffer at 1500 V and 45 °C for 1.5–2.0 hours on a LI-COR 4300 genetic analyser (Lincoln, Nebraska, United States of America). Products were visualised for paternity analysis on LI-COR gel images. Parents and offspring were run with a positive control generated from the initial testing of the primers.
A total of 157 wasps were genotyped. This large sample size is extraordinary given the requisites (i) to develop a novel and effective experimental protocol for testing paternity in an egg parasitoid wasp using DNA microsatellite markers, (ii) to visually track and attain twice-mated females (see above) and their morphologically identical mates within highly competitive settings (one female: four males), (iii) to establish and maintain large controlled broods for daily experimental replicates, and (iv) to extract DNA from extremely small and delicate specimens. Replicates (n=10) consisted of 30 parents (10 females and 20 males) and 127 daughters, totaling 157 wasps, which were genotyped. This large dataset proved appropriate for the application of robust statistical analyses.
Microstructure: the male aedeagus
The microstructure of the malesʼ aedeagus was examined by means of photomicrographic imaging and environmental scanning electron microscopy (ESEM). Aedeagi of one-day-old virgins (n=4) protruded without force when males were placed on dry ice. Photomicrographic images were obtained with a Nikon Microphot-FX EPI microscope (Japan) and SPOT software v. 4.6 (SPOT Imaging Solutions, Sterling Heights, Michigan, United States of America). ESEM images were obtained by mounting insects onto a peg (12.7 mm diameter) covered with a conductive carbon adhesive tab, and by imaging with the ESEM FEI Quanta FEG 4000 (FP Innovations, Hillsboro, Oregon, United States of America) at magnifications of 1500×, 2500×, and ⩾5000× within a chamber kept at ambient temperature, using 1.5 T pressure, an accelerating voltage of 15 kV, and a Gaseous Secondary Electron Detector with a 1-mm aperture.
Statistical analyses
A paired t-test was used to compare the mean number of copulations, the mean duration in copula, and the mean duration of the postcopulatory ritual recorded from M1 and M2 males. The data were tested for normal distribution using a Kolmogorov–Smirnov test. A Pearson’s correlation was used to test for a linear relationship between (i) the duration of copulation and the number of daughters sired by M1 and M2 males, and (ii) the duration of the postcopulatory ritual and the number of daughters sired by M1 and M2 males.
Fragment sizes (base pair) were scored from LI-COR gel images and assigned paternity probabilities with the computer program COLONY v 2.0 (Jones and Wang Reference Jones and Wang2010), which assigns paternity based on maximum-likelihood. To accurately assign paternity, COLONY requires additional information regarding the mating and genetic system of the species. For these analyses, females were considered polygamous because they could mate with more than one male, while males were considered monogamous because they were constrained to mate with only one female in our experiment. This programme also allowed us to specify the genetic background of the species, which is haplodiploid. Other parameters were constrained to reflect the facts that the female in the experiment was the only possible mother and that each male had a 50% chance of being the sire of the offspring. In addition, we specified a low genotyping error rate (0.00001) and indicated that inbreeding may occur in this species.
A male was assigned paternity for each daughter within a brood if the COLONY-issued probability was 1.000, except for replicates 3, 8, and 10 where paternity was assigned to a total of 14 males whose overall probability of paternity did not equal 1.000, but averaged 0.70. Replicate 3 resulted in an average probability of 0.50 for four daughters; replicate 8 resulted in an average probability of 0.80 for eight daughters; and replicate 10 resulted in an average probability of 0.70 for two daughters. All probabilities were individually tested for each replicate using a binomial distribution. For each replicate, exclusion probabilities were calculated in Microsoft Excel (Microsoft Canada, Mississauga, Ontario, Canada) on alleles of an individual locus; a mean was then calculated for all loci. Descriptive statistics were used to calculate P 2 values, and a paired t-test was used to compare the mean number of offspring sired by M1 and M2 males. Nonpaternity assignment analyses were run with PASW v. 18.0 software. The confidence interval for all tests was set at 95%.
Results
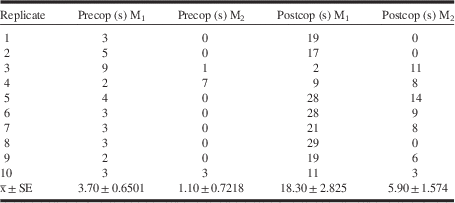
Postcopulatory ritual behaviour of M1 and M2 males
Seventy percent of M2 males engaged the female in a postcopulatory ritual, either by performing the ritual concurrently (n=3) with M1 males, or after they physically prevented the ritual (n=4) of M1 males. The time M1 and M2 spent engaged in the postcopulatory ritual differed (M1:
![]() ${\rm \bar{X}}$
=18.30± 2.825 seconds, M2:
${\rm \bar{X}}$
=18.30± 2.825 seconds, M2:
![]() ${\rm \bar{X}}$
=5.90±1.574 seconds; t
9=3.730, P=0.005) (Table 1).
${\rm \bar{X}}$
=5.90±1.574 seconds; t
9=3.730, P=0.005) (Table 1).
Table 1 Precopulatory ritual (precop) and postcopulatory ritual (postcop) duration (seconds) of the first male (M1) and the second male (M2) to mate.
Paternity assignment
Nearly all (98%) daughters were assigned to sires. The exclusion probability (probability that potential sires were excluded on genetic incompatibility alone) averaged 84% over all replicates (Table 2). M1 males sired more daughters than M2 males (M1:
![]() ${\rm \bar{x}}$
=11.60±1.899, M2:
${\rm \bar{x}}$
=11.60±1.899, M2:
![]() ${\rm \bar{x}}$
=1.10±0.823; t
9=4.426, P=0.002). Mixed paternity was inferred for only two of 10 broods, resulting in an overall low P
2 value (Table 3).
${\rm \bar{x}}$
=1.10±0.823; t
9=4.426, P=0.002). Mixed paternity was inferred for only two of 10 broods, resulting in an overall low P
2 value (Table 3).
Table 2 Loci, alleles of parents, daughter genotypes and their proportions.

Note: P-value representing probability that the first male to mate (M1 male) sired each daughter (n), and mean exclusion probability (EP) proportion for each replicate.
Table 3 Number of daughters sired by the first male (M1) and the second male (M2) to mate.

Notes: P 2 values (proportions of offspring sired by M2 male), 95% confidence interval (CI) limits, and list of key courtship and mating behaviour (pcr=postcopulatory ritual).
1 88% of M1 males were first to perform the precopulatory ritual; M2 males performed the precopulatory and postcopulatory rituals in replicates 3, 4, and 10, and had shared paternity in replicates 3 and 10.
Copulation behaviour of M1 and M2 males
Eight out of 10 M2 males copulated with the female while the M1 male was engaging her in the postcopulatory ritual. Two M2 males copulated with the female shortly after the M1 male had begun copulating. The number of copulations M1 (n=14) and M2 (n=13) males attained did not differ (M1:
![]() ${\rm \bar{x}}$
=1.40±0.221, M2:
${\rm \bar{x}}$
=1.40±0.221, M2:
![]() ${\rm \bar{x}}$
=1.30± 0.153; t
9=1.000, P=0.343), and copulation durations of M1 and M2 males did not differ (M1:
${\rm \bar{x}}$
=1.30± 0.153; t
9=1.000, P=0.343), and copulation durations of M1 and M2 males did not differ (M1:
![]() ${\rm \bar{x}}$
=9.40±1.869 seconds, M2:
${\rm \bar{x}}$
=9.40±1.869 seconds, M2:
![]() ${\rm \bar{x}}$
=10.30± 1.309 seconds; t
9=−0.462, P=0.655). There was no correlation between (i) the mean copulation duration and the mean number of offspring sired by M1 males (r=−0.199, P=0.582), and (ii) the mean duration of copulation and the mean number of offspring sired by M2 males (r=0.018, P=0.962).
${\rm \bar{x}}$
=10.30± 1.309 seconds; t
9=−0.462, P=0.655). There was no correlation between (i) the mean copulation duration and the mean number of offspring sired by M1 males (r=−0.199, P=0.582), and (ii) the mean duration of copulation and the mean number of offspring sired by M2 males (r=0.018, P=0.962).
Microstructure of the males’ aedeagus
The aedeagus (~7 µm in length; SE=0.04) of males has no morphological characteristics indicative of a function in sperm removal or displacement. The pointed, rather than arched, tip lacks hooks and spines (Fig. 1A). The grappling hooks (Fig. 1B) are likely clasping organs that help grasp the female during copulation.

Fig. 1 Environmental scanning electron micrograph (A) and photomicrographic image (B) of the proximal tip of a male Ooencyrtus kuvanae aedeagus. Note the absence of spines, which could serve in sperm removal.
Discussion
First-male sperm precedence
In the O. kuvanae mating system, the high P 1 value (0.91) for the first-mating (M1) male and the corresponding low P 2 value (0.09) of sneaker (M2) males are suggestive of strong first-male sperm precedence, assuming that M2 males did transfer sperm (Martel et al. Reference Martel, Damiens and Boivin2008). In other insect species, low P 2 values usually stem from female preference for a M1 male, low numbers of copulations, short durations in copula, adaptations to first-male sperm usage, unsuccessful copulations due to poor performance, or effective postcopulatory guarding by M1 males (Simmons Reference Simmons2001; Shuster and Wade Reference Shuster and Wade2003). In our study, the high fertilisation success of M1 males was not associated with the number of copulations or time spent in copula, however M1 males of O. kuvanae engage the mated female in a postcopulatory ritual as a form of postcopulatory mate guarding which is associated with a state of nonreceptivity in the female (Ablard et al. Reference Ablard, Fairhurst, Andersen, Schaefer and Gries2011).
Although M1 males sired all of the offspring in many matings, our observations and microsatellite data show that M2 males can mate successfully, but sire very few offspring (low P 2 value). This outcome may be associated with the effective postcopulatory guarding by M1 males that decreases the receptivity of the female. Alternatively, morphological attributes of the female reproductive tract and/or cryptic sperm choice by females may have influenced the fertilisation success of M1 and M2 males.
Copulation behaviour between M1 and M2 males
In our study, M1 and M2 males of O. kuvanae copulated equally often, suggesting that the number of copulations does not function as a male-driven mechanism to increase their fertilisation success. For example, when both the M1 and the M2 male copulated twice with the same female in each of replicates 3 and 9, and for circa the same duration each time, the M2 male in replicate 3 shared paternity and sired more daughters than the M1 male, whereas the M2 male in replicate 9 sired no daughters (Table 3). By contrast, competing males of the fly Dryomyza anilis Fallén (Diptera: Dryomyzidae), and of the scorpionfly Panorpa germanica Linnaeus (Mecoptera: Panorpidae), increase their fertilisation success with the number of copulatory bouts (Otronen Reference Otronen1994; Kock and Sauer Reference Kock and Sauer2007).
The duration of a male’s copulatory bout can increase the number of offspring he sires (Simmons Reference Simmons2001) but this does not apply to male O. kuvanae; M1 males sired 10 times more offspring than M2 males, yet M2 males remained in copula on average for 50% longer than M1 males. These results firmly corroborate our prediction that the number of copulations and the time spent in copula are not male-driven adaptations to sperm competition.
Prolonged duration of a copulatory bout may also provide the time needed for sneaker males to remove M1 male sperm and deliver their own (Simmons Reference Simmons2001). However, similar to Trichogramma euproctidis (Girault) (Hymenoptera: Trichogrammatidae) (Damiens and Boivin Reference Damiens and Boivin2006), this does not apply to the O. kuvanae mating system; the aedeagus of males simply lacks any attributes that could facilitate removal or displacement of M1 male sperm by M2 males. In contrast, in mating systems with last-male sperm precedence, the males’ aedeagus of some species assumes a unique shape or is fitted with spines or hooks capable of displacing a competitor’s sperm (Thornhill and Alcock Reference Thornhill and Alcock1983). For example, in the dragonfly Sympetrum rubicundulum Say (Odonata: Libellulidae) two long and coiled structures of the males’ aedeagus fit into a paired spermatheca and push M2 sperm deeper into the spermatheca while flushing out M1 male sperm, resulting in last-male sperm precedence (Thornhill and Alcock Reference Thornhill and Alcock1983).
Mate guarding
As the reproductive success of M1 and M2 O. kuvanae males was not coupled to the number and duration of copulations with the same female, or aedeagus morphology, the underlying mechanisms of first-male sperm precedence in O. kuvanae appear to include both precopulatory and postcopulatory mate guarding as adaptations to reduce sperm competition. The precopulatory ritual is associated with the female entering a “trance” and receptive state (Ablard et al. Reference Ablard, Fairhurst, Andersen, Schaefer and Gries2011). Effectively, a female exhibits mate choice by engaging in the precopulatory ritual with the first male to contact her. He proceeds to mate with her and then immediately performs the postcopulatory ritual, which results in her exit from the trance and becoming unreceptive (Ablard et al. Reference Ablard, Fairhurst, Andersen, Schaefer and Gries2011). Males that first encounter a female may be favoured in species such as O. kuvanae that engage in intense, time-limited scramble competition among males for matings. Potential sperm choice by females would favour, by default, the first male to perform the postcopulatory ritual, which typically is the same male to have contacted and engaged her in the precopulatory ritual. Whether M1 males would sire most offspring had they not performed the postcopulatory ritual first has yet to be determined.
In seven out of 10 replicates where the M1 male sired all of the daughters, he was first to engage the female in the precopulatory ritual and in the postcopulatory ritual (Table 3). Conversely, the M2 males in replicates 1, 2, 5–7, and 9 who did not sire daughters (Table 3) did not perform the precopulatory ritual; yet all attempted the post-copulatory ritual. These data suggest a linked effect of precopulatory and postcopulatory rituals on paternity. For example, of the three replicates where the M1 and the M2 males performed both the precopulatory and postcopulatory rituals, two replicates (3 and 10) (Table 3) resulted in shared paternity. In replicate 3, the M1 male performed the precopulatory ritual but failed to initiate the postcopulatory ritual before the M2 male did. In replicate 10, both the M1 and the M2 male performed the precopulatory ritual and in the same order engaged the female in the postcopulatory ritual. These results combined clearly indicate that sperm competition can occur in O. kuvanae. Observations that (i) males invariably engage a female in the postcopulatory ritual immediately after mating (Ablard et al. Reference Ablard, Fairhurst, Andersen, Schaefer and Gries2011), (ii) fiercely compete over postcopulatory ritual rights (this study), and (iii) share paternity if the M2 male performs the precopulatory and postcopulatory ritual, all suggest that first-male sperm precedence is linked to the precopulatory and postcopulatory rituals, and that the completion of the postcopulatory ritual renders the mated female unreceptive and helps prevent sperm competition.
Theoretical models of mating systems predict that males should abandon their mates immediately after mating, if there is strong first-male sperm precedence (Simmons Reference Simmons2001). Such behaviour negates postcopulatory rituals in species where male adaptations to sperm competition could rely solely on precopulatory ritual performance, or chemical substances in seminal fluid of the first male to mate, which immediately inhibit female receptivity (Simmons Reference Simmons2001). In the absence or the presence of competitors, male O. kuvanae never abandon their receptive mate immediately after copulation, and complete the postcopulatory ritual even if they then forego mating opportunities with other females. This behaviour corroborates the importance of the postcopulatory ritual as a form of mate guarding, which functions to ensure paternity in the context of sperm competition (Simmons Reference Simmons2001).
Conclusion
In summary, our study demonstrates that first-male sperm precedence is most prevalent in O. kuvanae, but that “sneaker” males are also capable of achieving paternity. The underlying mechanisms do not entail more frequent or prolonged copulatory bouts, or morphological characteristics of the males’ aedeagus. Instead, our data suggest that the precopulatory and postcopulatory rituals may function as an adaptation to sperm competition, with the postcopulatory ritual accelerating the awakening of an in-trance female, thereby effectively and quickly closing her window of receptivity.
Acknowledgements
The authors thank M. Todd, G. Sadowski, F. Fernando, and K. Jones (Genetic Identification Services) for technical assistance with the DNA library construction, screening, and enrichment; U. Somjee for technical assistance with the experiments; J. Drummond for ESEM and photomicrographic imaging; M. Christie for his exclusion probability program; J. Wolfe for formatting the tables; M. Keena (Hamden) for supplying reared gypsy moth eggs; D. Stover for helpful suggestions; and two anonymous reviewers for meticulous reviews and constructive comments. This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) – Discovery Grant and by an NSERC – Industrial Research Chair to G.G., with Contech Enterprises, and Global Forest Science (GF-18–2007–226; GF-18–2007–227) as sponsors.