Introduction
Wireworms, the larvae of click beetles (Coleoptera: Elateridae), are significant economic soil-dwelling pests in temperate and subtropical areas of the world (Thomas Reference Thomas1940; Jackson et al. Reference Jackson, Alves and Pereira2000; Marske and Ivie Reference Marske and Ivie2003; Vernon et al. Reference Vernon, van, Herk, Tolman, Saavedra, Clodius and Gage2008; Traugott et al. Reference Traugott, Benefer, Blackshaw, van Herk and Vernon2015). Wireworm larvae can persist in the soil for several years and are often present in agricultural fields at planting (Thomas Reference Thomas1940; Jackson et al. Reference Jackson, Alves and Pereira2000). Wireworms are generalists and feed on a wide variety of field and vegetable crops e.g., cereal, potato, sugarbeet, carrot, sugar cane, and soft roots. They inflict damage to seeds, root, stems, tubers, or other plant parts by feeding, chewing, or drilling into below-ground plant tissues and structures, thereby enhancing plant diseases, stopping plant growth, or killing plants completely (Thomas Reference Thomas1940; Keiser et al. Reference Keiser, Häberli and Stamp2012). Wireworms also cause damage to the stems later in the growing season, which stimulates excessive tillering and inhibits wheat head formation. Wireworm injury can cause wilting, stunting, crop thinning, delay in plant maturation, and seedling death, which leads to yield reduction and affects crop value (Parker and Howard Reference Parker and Howard2001; Barsics et al. Reference Barsics, Haubruge and Verheggen2013; Ritter and Richter Reference Ritter and Richter2013; Vernon and van Herk Reference Vernon and van Herk2013; van Herk and Vernon Reference van Herk and Vernon2014). When wireworm populations are extremely high, entire fields may be lost (Popov et al. Reference Popov, Barbulescu, Trotus, Vasilescu and Bucurean2001; Willis et al. Reference Willis, Abney, Holmes, Schultheis and Kennedy2010). In many fields, wireworm infestation results in an uneven plant stand, which allows weeds to outcompete the crop using up available moisture and preventing or lessening the normal tillering of adjacent uninjured plants (Thomas Reference Thomas1940).
The soil-dwelling nature of wireworms usually makes it difficult to estimate their numbers, which in turn hinders accurate forecasting of likely plant damage or crop loss. Because of this, wireworms are often managed preventively with insecticides applied at planting (Potter et al. Reference Potter, Powell, Spicer and Williams1996; Wilde et al. Reference Wilde, Roozeboom, Claassen, Janssen and Witt2004). Historically, wireworms have been controlled with inexpensive broad-spectrum insecticides (e.g., organochlorines, organophosphates, and carbamates). However, most of these pesticides have either been cancelled or restricted worldwide due to environmental and health concerns (Vernon et al. Reference Vernon, van, Herk, Tolman, Saavedra, Clodius and Gage2008; Reddy and Tangtrakulwanich Reference Reddy and Tangtrakulwanich2014), and, consequently, wireworm damage is currently increasing in the United States of America, Canada, and other parts of the world. Current chemical control relies on the use of neonicotinoids (principally imidacloprid), used as seed treatments, to provide seed and foliar protection for several weeks after planting. Neonicotinoids are widely used for control for many crop pests due to the low rates required and long residual activity of the compound (Nault et al. Reference Nault, Taylor, Urwiler, Rabaey and Hutchison2004, Reference Nault, Straub and Taylor2006; Koch et al. Reference Koch, Burkness, Hutchison and Rabaey2005; Parker Reference Parker2005; Elliott et al. Reference Elliott, Birmingham, Wilson, McAdie, Trudeau and Mineau2008; Kuhar and Alvarez Reference Kuhar and Alvarez2008). These compounds, however, repel wireworms not kill them, eventually leaving populations essentially unaffected. These pesticides have further adverse effects on the environment, especially non-target organisms (Desneux et al. Reference Desneux, Decourtye and Delpuech2007; Wilde et al. Reference Wilde, Roozeboom, Ahmad, Claassen, Gordon and Heer2007).
For this reason, there is a need to develop other alternative control options, such as environmentally safe biopesticides that might be used alone, combined with other biopesticides, or used in conjunction with a conventional pesticide. The biopesticides include the use of naturally derived compounds from microbes or plants (e.g., spinosyns, azadirachtin, and pyrethrins), living organisms (e.g., insect pathogenic fungi, Beauveria bassiana (Balsamo-Crivelli) Vuillemin (Fungi: Clavicipitaceae) and Metarhizium anisopliae (Metchnikoff) Sorokin sensu lato (Fungi: Clavicipitaceae), or combined formulations of these agents (Chandler et al. Reference Chandler, Bailey, Tatchell, Davidson, Greaves and Grant2011; Reddy and Antwi Reference Reddy and Antwi2016; Reddy et al. Reference Reddy, Antwi, Shrestha and Kuriwada2016). Such products are usually considered low-risk agents with low mammalian toxicity as well as minimal impact on non-target organisms.
Laboratory studies have examined the effect of different isolates and strains of Beauveria Vuillemin and Metarhizium Sorokīn on mortality in two wireworm species: Agriotes lineatus (Linnaeus) and A. obscurus (Linnaeus) (Coleoptera: Elateridae) (Kabaluk et al. Reference Kabaluk, Goettel, Erlandson, Ericsson, Duke and Vernon2005; Ericsson et al. Reference Ericsson, Kabaluk, Goettel and Myers2007; Ansari et al. Reference Ansari, Evans and Butt2009). Under laboratory conditions, the combined use of M. anisopliae and spinosyn increased mortality of these two species (A. lineatus and A. obscurus), compared with either one used alone (Ericsson et al. Reference Ericsson, Kabaluk, Goettel and Myers2007). Under field conditions in Canada, Kabaluk and Ericsson (Reference Kabaluk and Ericsson2007) found that the use of field corn (Zea mays Linnaeus; Poaceae) seed treated with M. brunneum Petch (F52) conidia significantly enhanced corn yield compared with an untreated control, while no effect of spinosyn alone or in combination with this fungus was found in corn fields infested with A. obscurus (Kabaluk and Ericsson Reference Kabaluk and Ericsson2007). In Germany and Italy, Ladurner et al. (Reference Ladurner, Quentin, Franceschini, Benuzzi and Ehlers2009) tested several biopesticide products alone or in combination with synthetic insecticides in potato (Solanum tuberosum Linnaeus; Solanaceae) fields and found another B. bassiana, strain ATCC 74040, to be a promising agent for the management of A. obscurus.
However, the potential value of biopesticides for management of other wireworms, such as Limonius californicus (Mannerheim) and Hypnoidus bicolor (Eschscholtz) (Coleoptera: Elateridae), common in Montana, is poorly understood. Except for two initial studies by Tharp et al. (Reference Tharp, Blodgett and Jaronski2005) and Reddy et al. (Reference Reddy, Tangtrakulwanich, Wu, Miller, Ophus, Prewett and Jaronski2014), who found an effect on these wireworm species with insect pathogenic fungi applied as seed treatments in potato crops and spring wheat, respectively. Insect pathogenic fungus use against wireworm management in Montana spring wheat production in one of the studies was encouraging (Reddy et al. Reference Reddy, Tangtrakulwanich, Wu, Miller, Ophus, Prewett and Jaronski2014). Their study indicated that three insect pathogenic fungi (M. brunneum F52, B. bassiana GHA, and M. robertsii Bischoff et al. DWR 346) applied as seed treatments were effective in controlling L. californicus and H. bicolor in spring wheat. All three fungi, when applied as granules in furrow or as banded soil drenches, significantly increased the plant stand and yields under moderate wireworm pressure, supporting their value in the management of this pest. However, environmental factors such as temperature and soil moisture are likely to influence the fungal efficacy rate because higher temperature and lower moisture can often reduce fungal germination rate and thereby reducing efficacy (Jaronski Reference Jaronski2010; Shrestha et al. Reference Shrestha, Enkegaard and Stenberg2015). It was therefore necessary to explore other biopesticides along with these previously used fungal agents for wireworm management in Montana. In addition, if any biopesticides were found to be compatible with the currently available imidacloprid insecticide for wireworm management, it may reduce the application rates and eventually cost less for the growers.
The aim of this two-year study was to evaluate the ability of several biopesticides used alone, mixed with other biopesticides, or in conjunction with a conventional pesticide (imidacloprid), to manage wireworms in Montana spring wheat Triticum aestivum Linnaeus (Poaceae) (variety: Duclair). The efficacy of biopesticide treatments was assessed based on plant stand protection, effects on larval wireworm populations, and grain yield.
Materials and methods
Study sites
Before starting our experiments, we sampled extensively for wireworms at each farm site using soil sampling bait trap method (Reddy et al. Reference Reddy, Tangtrakulwanich, Wu, Miller, Ophus, Prewett and Jaronski2014) to confirm the presence of adequate densities of wireworms. The experiments were carried out in two spring wheat fields, one each at Ledger (48°18'26.9244"N, 111°51'34.4376"W) and Valier (48°18'37.4148"N, 112°25'19.0956"W), in the Golden Triangle area of Montana from April to September in 2015 and 2016. Experimental plots were seeded on 16 April and 16 May in 2015 and 2016, respectively, at the Ledger location and on 28 April and 31 May in 2015 and 2016, respectively, at the Valier location. Both sites had the same cropping history (growing cereal crops mainly year after year) and the wireworm incidence was from moderate to high levels.
The hard red spring wheat variety “Duclair” (Lanning et al. Reference Lanning, Carlson, Lamb, Nash, Wichman and Kephart2011) was seeded at both locations at a rate of 22 seeds per 30 cm with a four-row plot drill spaced 0.3 m. Before seeding, the herbicide glyphosate (RT3; Monsanto Company, St. Louis, Missouri, United States of America) was applied at the rate of 2.5 L/ha for weed control, following local farming practices. Fertiliser (N, P, and K) was applied at a ratio of 224.2, 0, and 22.4 kg/ha by broadcast application during planting, and an additional fertiliser application (N, P, and K at a ratio of 12.3, 25.2, and 0 kg/ha) was applied through the seed plot drill at seeding. The experimental plots received 5 cm of water via overhead irrigation 30 days after treatment in the all sites.
Experimental design
In both years (2015 and 2016), the experimental design was a randomised complete block design (RCBD) with four replications per treatment. The number of treatments were 17 and 12 respectively in 2015 and 2016. Plots were 3.6×1.2 m, separated by 0.60 m buffer zones to avoid cross contamination of treatments. The numbers of plant stand, wireworms in the bait traps, and the seed yield in each plot were recorded to assess the effectiveness of each treatment.
Biopesticide product application
Biopesticide or synthetic pesticide product rates were based on the respective manufacturers’ recommendations (see Tables 1–2 for 2015 and 2016, respectively). Some of the treatments and formulations that failed to reduce wireworm numbers or to protect plant stands in 2015 were not included in 2016 (Table 2). In 2015 and 2016, imidacloprid (as Gaucho 600, Bayer Crop Science, Raleigh, North Carolina, United States of America) was applied as a seed treatment. No fungicide was added to the seeds treated with imidacloprid. Imidacloprid+B. bassiana GHA (Mycotrol ESO, LAM International, Butte, Montana, United States of America) and imidacloprid+M. brunneum F52 (Met52 EC, Novozymes Biologicals, Salem, Virginia, United States of America) treatments were applied to the rows by spraying Mycotrol and Met52 as a soil drench to the base of plants grown from seed treated with imidacloprid. The granular formulation of the entomopathogen B. bassiana ANT-03 (BioCeres G; Anatis Bioprotection, St.-Jacques-le-Mineur, Québec, Canada) was applied by placing 21.6 g of product to each row by hand. In 2016, Gaucho and a heat-killed formulation of the bacterium Burkholderia Yabuuchi et al. (Burkholderiaceae) strain A396 (Venerate XC) were applied as seed treatments. A formulation of the bacterium Chromobacterium subtsugae Martin et al. (Bacteria: Neisseriaceae) (Grandevo SC, Marrone BioInnovations, Davis, California, United States of America), F52 formulated as microsclerotia or on a corn grit granule, and the organophosphate insecticide phorate (Thimet 20-G) were all applied in furrow.
Table 1 Materials and rates of application in each treatment, 2015.

* Gaucho 600, seed treatment application rate unit (mL/45.35 kg seed).
† Entrust WP, application rate unit (g/L).
‡ Gaucho 600, seed treatment application rate unit (35.49 mL/45.35 kg seed).
§ BioCeres GR, application rate unit (20 g/m2).
Table 2 Material, rate, and method of application in each treatment, 2016.

All other treatment combinations used in 2015 and 2016 (see Tables 1–2) were tank mixed and applied to rows as a soil drench. A 92-mL spray suspension was applied per row in each application. Spray treatments were applied to plots with a SOLO four-gallon backpack sprayer #425 (SOLO; Newport News, Virginia, United States of America) with a flat spray nozzle, 144.8-kPa valve (21.0 psi), and calibrated at 816.89 L/ha. Spray applications were made 14 days after seeding.
Plant stand counts
The number of emerged wheat seedlings was determined along a 1-m strip in the middle of the centremost two rows of each plot as a measure of initial plant stand protection. The starting and ending points of the sample areas were marked with plastic labels so that the same seedlings could be recounted before and after treatments. In both 2015 and 2016, wheat seedlings were counted again 28 days after treatment application.
Larval wireworm sampling
“Stocking bait” traps, described in (Reddy et al. Reference Reddy, Tangtrakulwanich, Wu, Miller, Ophus, Prewett and Jaronski2014), were used to detect wireworms and to estimate their relative abundance. The stocking bait traps were placed evenly along the centre of each plot, spaced 1 m apart. To make the baits, about 90 g of wheat seed was placed in a nylon stocking, which was then tied shut with a string, leaving a tail end of about 30 cm. These traps were immersed in water for 24 hours to stimulate the grain to start germinating before being placed in holes 7–15 cm deep and positioned so as to maximise the contact of the grain mixture with the soil as much as possible. The strings were left above the soil surface to help locate the traps later. The traps were then covered with about 3–5 cm of soil. A 12×12 cm piece of black poly ethylene was placed over the covered holes and four metal pegs were used to secure these pieces of polythene to the soil. This system is required to avoid entry by ground squirrels (G.V.P. Reddy, personal observation).
In 2015, three stocking traps, spaced 1 m apart, were placed in the middle row of each plot. These traps were deployed one week before the spray applications. Just before treatments were applied, one trap from each plot was removed to estimate the pre-treatment wireworm density in plots (one bait per plot, with four replicates of each treatment). The second and third traps were sequentially removed 14 and 28 days after treatment. Larvae found in traps were counted in the laboratory. A similar procedure was used for 2016 wireworm samplings, except that just two stocking bait traps per plot were used, with one sampled before treatments and the other 28 days after treatments. Wireworm species were identified in 2016 using morphological keys developed by Etzler et al. (Reference Etzler, Wanner, Morales-Rodriguez and Ivie2014).
Crop yield
A Hege 140 plot combine was used to sample the plots for yield assessment. Wheat seeds were cleaned with a seed processor (Almaco, Nevada, Iowa, United States of America) and weighed on a scale to determine yield per plot at the Western Triangle Agricultural Research Center (WTARC) seed laboratory in Conrad, Montana.
Statistical analyses
The data were analysed using SAS 9.4 (SAS Institute 2015). Analysis of covariance was used to determine the impact of treatments on wireworm larval population and yield levels. Treatment differences were assessed using Fisher’s least significant (LSD) test. Paired t-test was used to assess the effect of treatment on plant stand counts before and after the treatment application within each group. To compare among the treatments for plant stand counts, the plant survival percentage was calculated as (number of plant counts after treatment application/total number of plant counts before treatment application)×100, prior to data subjected to analysis. One-way analysis of variance was used to determine the treatment effects on plant survival percentage followed by post hoc Tukey test for means comparisons.
Results
Plant stand count
In the 2015 Ledger study site, the paired t-test result showed that there was a significant reduction in plant stand counts after the treatment applications on nearly all treated groups, except for Gaucho 600 (G), Met52 EC+Entrust WP (Met+E), and BioCeres GR (Bi)-treated plots (Table 3). In contrast, regardless of treatments, there was a significant reduction in plant stand counts after treatment application at the Valier study site (Table 3).
Table 3 Paired t-test comparisons of plant stand count recorded before and 28 days after biopesticides or synthetic pesticide application at the two study locations of Montana, 2015. SE, standard error.

W, water; G, Gaucho 600; E, Entrust WP; M-1, M-1 (25 g/L); M-2, M-2 (50 g/L); Met, Met52 EC; My, Mycotrol ESO; My+Met, Mycotrol ESO+Met52 EC; My+Az, Mycotrol ESO+Aza-Direct; My+E, Mycotrol ESO+Entrust WP; My+G, Mycotrol ESO+Gaucho 600; Met+Az, Met52 EC+Aza-Direct; Met+E, Met52 EC+Entrust WP; Met+G, Met52 EC+Gaucho 600; XPE, Xpectro OD; Bi, BioCeres; XPU, Xpulse OD.
When comparing the treatment groups, the 2015 study showed significant effects on plants survival percentage after treatment applications at the Ledger (F=3.50; df=16, 119; P=0.001) and Valier (F=2.00; df=16, 119; P=0.01) study sites. Among treatments, significantly higher mean plant survival percentage (±standard error) were observed at the Ledger location for Mycotrol ESO (My) (78.91±6.99), Met52 EC+Entrust WP (Met+E) (76.57±7.01), Gaucho 600 (G) (73.93±7.03), Met52 EC+Gaucho 600 (Met +G) (71.24±2.57), and BioCeres GR (Bi) (70.61±7.41) treatments than in water control (W) (40.05±2.12) (Fig. 1). Other treatments were not significantly different from water control (Fig. 1). At the Valier location, Met52 EC (Met) treatment had significantly higher mean percentage plant survival (±standard error) (65.86±5.02) as compared with water control (W) (41.18±1.96) (Fig. 1).
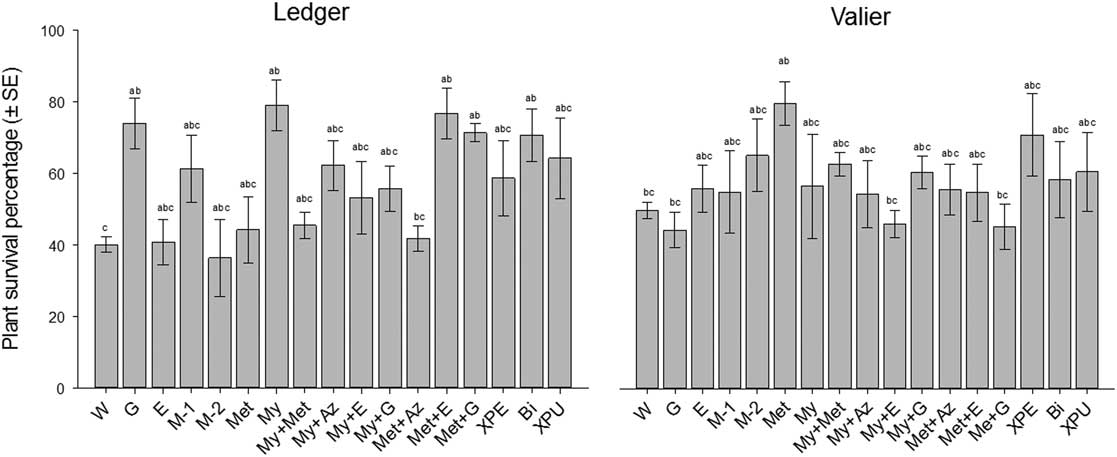
Fig. 1 Mean percentage survival of wheat plants (±standard error (SE)) 28 days after biopesticide or synthetic insecticide application at the Ledger and Valier of Montana, 2015. The number of replicates per treatment was four and each replicate has two repetitions. Bars bearing the same letters are not significantly different (Tukey test, P>0.05). W, water; G, Gaucho 600; E, Entrust WP; M-1, M-1 (25 g/L); M-2, M-2 (50 g/L); Met, Met52 EC; My, Mycotrol ESO; My+Met, Mycotrol ESO+Met52 EC; My+Az, Mycotrol ESO+Aza-Direct; My+E, Mycotrol ESO+Entrust WP; My+G, Mycotrol ESO+Gaucho 600; Met+Az, Met52 EC+Aza-Direct; Met+E, Met52 EC+Entrust WP; Met+G, Met52 EC+Gaucho 600; XPE, Xpectro OD; Bi, BioCeres GR; XPU, Xpulse OD.
In contrast to the 2015 study, there was no significant decline in nearly all treated groups when plant stand counts compared between before and after the treatment application within each group in the 2016 study (Table 4). Except water (W), Gaucho 600 (G), Grandevo SC (GR), and Met52 microsclerotial granules (MM) treated groups at the Ledger, and water (W) and Thimet 20-G (T2) (5.61 kg/ha) at the Valier were significantly different (Table 4). Similar pattern was also observed when mean plant survival percentage were compared among treatment groups. No significant differences recorded on survival percentage at Ledger (F=1.00; df=11, 84; P=0.44) and Valier (F=1.81; df=11, 84; P=0.06) locations (Fig. 2). Consequently, the 2016 study indicated non-significant effect of biopesticide treatments on plant stand count at both field locations.
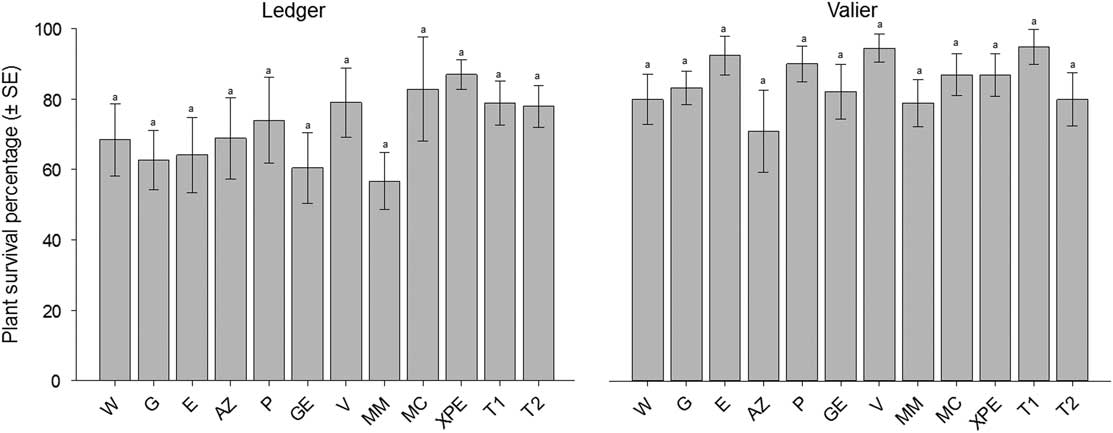
Fig. 2 Mean percentage survival of wheat plants (±standard error (SE)) 28 days after biopesticide or synthetic insecticide application at the Ledger and Valier of Montana, 2016. The number of replicates per treatment was four and each replicate has two repetitions. Bars bearing the same letters are not significantly different (Tukey test, P>0.05). W, water; G, Gaucho 600; E, Entrust WP; AZ, Aza-Direct; P, PyGanic 1.4 EC; GR, Grandevo SC; V, Venerate XC; MM, Met52 microsclerotial granules; MC, Met52 corn grit granules; XPE, Xpectro OD; T1, Thimet 20-G (2.80 kg/ha; T2, Thimet 20-G (5.61 kg/ha)).
Table 4 Paired t-test comparisons of plant stand count recorded before and 28 days after biopesticides or synthetic pesticide application at the two study locations of Montana, 2016. SE, standard error.

W, water; G, Gaucho 600; E, Entrust WP; AZ, Aza-Direct; P, PyGanic 1.4 EC; GR, Grandevo SC; V, Venerate XC; MM, Met52 microsclerotial granules; MC, Met52 corn grit granules; XPE, Xpectro OD; T1, Thimet 20-G (2.80 kg/ha); T2, Thimet 20-G (5.61 kg/ha).
Wireworm populations
Wireworms were successfully captured in baited stocking traps in all treatments regardless of location, except in the Mycotrol ESO+Entrust WP (My+E) treatment in 2015 pre-treatment sampling at Ledger. The mean number of wireworms per baited trap ranged from 0.00 to 3.00 and 1.75 to 5.25, respectively, at the Ledger and Valier (Table 5). In 2016 pre-treatment sampling, similar mean numbers of wireworms (0.25–4.25) were captured in baited stocking traps at the Ledger location (except that no wireworms were found in plots designated for Thimet 20-G (T1; 2.79 kg/ha, while very few wireworms (<0.80) were found at Valier (Table 6).
Table 5 Wireworm catch per baited trap in wheat seedling plots treated with biopesticides or synthetic insecticides at the two study locations of Montana, 2015.

Notes: The number of replicates per treatment was four. Means within a column followed by the same letter are not significantly different at P<0.05.
* PT, pre foliar and granular application (21 days after planting).
† 14 DPT, days after foliar and granular application (35 days after planting).
‡ 28 DPT, days after foliar and granular application (49 days after planting).
W, water; G, Gaucho 600; E, Entrust WP; M-1, M-1 (25 g/L); M-2, M-2 (50 g/L); Met, Met52 EC; My, Mycotrol ESO; My+Met, Mycotrol ESO+Met52 EC; My+Az, Mycotrol ESO+Aza-Direct; My+E, Mycotrol ESO+Entrust WP; My+G, Mycotrol ESO+Gaucho 600; Met+Az, Met52 EC+Aza-Direct; Met+E, Met52 EC+Entrust WP; Met+G, Met52 EC+Gaucho 600; XPE, Xpectro OD; Bi, BioCeres GR; XPU, Xpulse OD.
Table 6 Wireworm catch per baited trap on wheat seedling plots treated with biopesticides or synthetic insecticides at the two study locations of Montana, 2016.

Notes: The number of replicates per treatment was four. Means within a column followed by the same letter are not significantly different at P<0.05.
* PT, pre foliar and granular application (21 days after planting).
† 28 DPT, days after foliar and granular application (49 days after planting).
W, water; G, Gaucho 600; E, Entrust WP; AZ, Aza-Direct; P, PyGanic 1.4 EC; GR, Grandevo SC; V, Venerate XC; MM, Met52 microsclerotial granules; MC, Met52 corn grit granules; XPE, Xpectro OD; T1, Thimet 20-G (2.80 kg/ha); T2, Thimet 20-G (5.61 kg/ha).
In 2015 significant differences in overall wireworm populations were observed at Ledger (F=1.56; df=17, 118; P<0.05), but not at Valier (F=0.59; df=17, 118; P>0.05) (Table 5). Unexpectedly, the mean wireworm populations (±standard error) at Ledger were significantly higher in some of the biopesticide treatment plots compared with the water control at 14 days (F=1.01; df=16, 51; P<0.05) and 28 days (F=1.70; df=16, 51; P<0.05). The Met52 EC+Entrust WP (Met+E) (5.50±2.18), M-1 (25 g/L) (5.00±1.68), and Entrust WP (E) (4.00±0.71) treatments had significantly higher wireworm populations than did the water control treatment (1.63±0.73) 14 days after treatment (Table 5). At 28 days, only the Mycotrol ESO (My) treatment had a significantly higher wireworm population (4.00±2.83) than did the water control (1.00±0.57) (Table 5).
In 2016, treatments had no significant effect on wireworm populations 28 days after treatment: Valier (F=1.12; df=11, 35; P>0.05), Ledger (F=1.20; df=11, 35; P>0.05) (Table 6). Across the treatments, the mean number of wireworms per bait trap varied from 0.00 to 0.75 and 0.00 to 1.25, respectively, at Ledger and Valier (Table 6).
Wireworm species composition
In the 2016 study, wireworm species collected from Valier and Ledger were L. californicus, H. bicolor, and Aeolus mellilus Say irrespective of study locations. In both locations, H. bicolor was the most predominant species followed by L. californicus and A. mellilus at both before and 28 days after treatment. The total number of H. bicolor, L. californicus, and A. mellilus individuals recorded at Ledger were 57, 8, and 2, respectively, and the comparing value for Valier were 24, 12, and 4, respectively.
Crop yield
Average wheat grain yield for 2015 ranged from 3436 to 4743 kg/ha and 2448 to 3541 kg/ha respectively, at the Ledger and Valier locations (Fig. 3). The corresponding values for 2016 were 1017–1867 kg/ha and 514–762 kg/ha, respectively (Fig. 4). Treatments showed significant impact on grain yield in 2015 at both Ledger (F=1.04; df=16, 51; P<0.05) and Valier (F=0.81; df=16, 51; P<0.05), while in 2016 a significant effect of treatments was observed at Ledger (F=1.26; df=11, 36; P<0.05) but not Valier (F=0.51; df=11, 36; P>0.05) (Fig. 4).
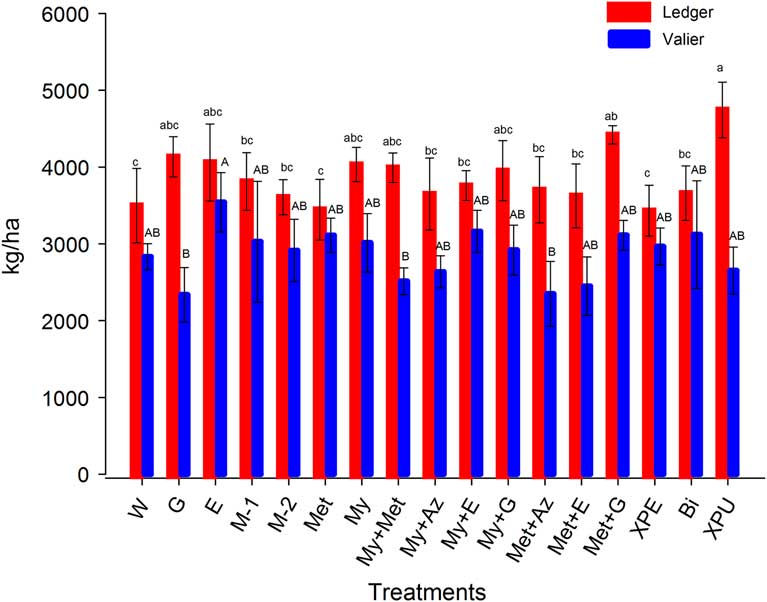
Fig. 3 Wheat yield produced in different treatments at the two study locations of Montana, (mean±standard error (SE)), 2015. The number of replicates per treatment was four. Bars bearing the same letters are not significantly different (Fisher’s least significant test, P>0.05). W, water; G, Gaucho 600; E, Entrust WP; M-1, M-1 (25 g/L); M-2, M-2 (50 g/L); Met, Met52 EC; My, Mycotrol ESO; My+Met, Mycotrol ESO+Met52 EC; My+Az, Mycotrol ESO+Aza-Direct; My+E, Mycotrol ESO+Entrust WP; My+G, Mycotrol ESO+Gaucho 600; Met+Az, Met52 EC+Aza-Direct; Met+E, Met52 EC+Entrust WP; Met+G, Met52 EC+Gaucho 600; XPE, Xpectro OD; Bi, BioCeres GR; XPU, Xpulse OD.

Fig. 4 Wheat yield produced in different treatments at the two study locations of Montana, (mean±standard error (SE)), 2016. The number of replicates per treatment was four. Bars bearing the same letters are not significantly different (Fisher’s least significant test, P>0.05). W, water; G, Gaucho 600; E, Entrust WP; AZ, Aza-Direct; P, PyGanic 1.4 EC; GR, Grandevo SC; V, Venerate XC; MM, Met52 microsclerotial granules; MC, Met52 corn grit granules; XPE, Xpectro OD; T1, Thimet 20-G (2.80 kg/ha); T2, Thimet 20-G (5.61 kg/ha).
At Ledger in 2015, yields in the Xpulse (XPU) (4743.70±363.12 kg/ha) and Met52 EC+Gaucho 600 (Met+G) (4420±49.65 kg/ha) plots were significantly higher than in the control treatment (3498±484.40 kg/ha) (Fig. 3). In contrast, at Valier, grain yields in the Entrust WP (E), Mycotrol+Entrust WP (My+E), BioCeres GR (Bi), Met52 EC+Gaucho 600 (Met+G), and Met52 EC (Met) treatments were only numerically higher than the control (Fig. 3). At Ledger in 2016, Xpectro OD (XPE), and Met52 Microsclerotial granules (MM) treatments had comparatively higher yields than water control treatment but without significant differences (Fig. 4).
Discussion
Wireworms are resurging as pests on many crops due to the fact that most pesticides used traditionally for their management have either been withdrawn or restricted due to environmental or health concerns (Adhikari and Reddy Reference Adhikari and Reddy2017). In these two-year field studies, we examined several commercial or experimental biopesticides for their potential to manage wireworms in spring wheat in the Golden Triangle area of Montana. Assessment of efficacy was based on the plant stand protection, wireworm larval populations, and grain yield.
Protecting wheat seedling stands from wireworm feeding in Montana and western Canada is often needed to achieve maximum crop yield (Ester and Huiting Reference Ester and Huiting2007; Kabaluk and Ericsson Reference Kabaluk and Ericsson2007; Vernon et al. Reference Vernon, Clodius and Harding2009; Reddy et al. Reference Reddy, Tangtrakulwanich, Wu, Miller, Ophus, Prewett and Jaronski2014). Imidacloprid is considered the benchmark pesticide against which to measure wireworm control of other products (AgInfomatics 2014), and in our 2015 trial it provided effective stand protection (as determined by percent wheat survival) at Ledger location. At the Valier location, plant stand counts were nearly twofold higher in imidacloprid treated plot compared with water control after treatment application but without significant difference. Our observations suggest that imidacloprid provided wheat seedlings early season protection because wireworms become moribund after exposure to this seed treatment (van Herk et al. Reference van Herk, Vernon, Clodius, Harding and Tolman2007, Reference van Herk, Vernon, Tolman and Saavedra2008; Vernon et al. Reference Vernon, van, Herk, Tolman, Saavedra, Clodius and Gage2008, Reference Vernon, Clodius and Harding2009). The exposure of wireworms to neonicotinoid (i.e., imidacloprid, clothianidin) insecticides causes prolonged periods of subacute toxicity (without persistence) characterised by immobility, loss of coordination, and inability to feed after which larvae recover (van Herk et al. Reference van Herk, Vernon, Clodius, Harding and Tolman2007, Reference van Herk, Vernon, Tolman and Saavedra2008; Vernon et al. Reference Vernon, van, Herk, Tolman, Saavedra, Clodius and Gage2008).
Among several biopesticide treatments in the 2015 study, we found that entomopathogenic fungus treatments alone or in combination with imidacloprid resulted in significantly higher plant stand compared with the control. However, this effect was found only in one of four site-years. These results were comparable with that of the imidacloprid treatment. The impact of similar types of treatments on plant stand density has been examined previously (Ester and Huiting Reference Ester and Huiting2007; Kabaluk and Ericsson Reference Kabaluk and Ericsson2007; Reddy et al. Reference Reddy, Tangtrakulwanich, Wu, Miller, Ophus, Prewett and Jaronski2014). For example, Kabaluk and Ericsson (Reference Kabaluk and Ericsson2007) reported that application of M. anisopliae (F52) alone resulted in significant increase in stand density of field corn infested with wireworms (A. obscurus) but did not enhance stand density when this fungus was combined with spinosyn. On the other hand, a study by Ester and Huiting (Reference Ester and Huiting2007) found no effect from use of B. bassiana or its combination with imidacloprid on the stem density in potato fields infested with Agriotes species, even though a significant reduction in wireworm damage was observed in potato tubers with both treatments.
Limited information exists regarding the impact of entomopathogenic fungi alone or in combination with imidacloprid on wheat stand density, except for the study by Reddy et al. (Reference Reddy, Tangtrakulwanich, Wu, Miller, Ophus, Prewett and Jaronski2014). The authors found that the use of several entomopathogenic fungi including M. brunneum F52 granules and B. bassiana GHA granules resulted in significantly higher wheat seedling protection compared with the control, in spring wheat infested by two wireworm species: L. californicus and H. bicolor. This result is consistent with our study, suggesting that entomopathogenic fungi such as B. bassiana GHA (Mycotrol), B. bassiana ANT-03 (BioCeres GR), and M. brunneum F52 have the ability to protect wheat plant seedlings from wireworm damage.
No previous reports have examined the effect of combining entomopathogenic fungi and imidacloprid for wheat seedling protection. Our results suggest that imidacloprid and entomopathogenic fungi can work synergistically, such as the combination of imidacloprid and M. brunneum, which provided better wheat stand protection against wireworm damage than imidacloprid alone. The wheat yields support this observation, with significantly or at least numerically higher yields than the control when spring wheat was treated with combined applications of imidacloprid and M. brunneum or imidacloprid and B. bassiana GHA, respectively. Moreover, wheat plots treated with B. bassiana and azadirachtin mixture product (Xpulse) provided significantly higher grain yield compared with controls at Ledger. Our data indicate this product has some possibility for use against wireworms.
In contrast to the 2015 study, in 2016 there was a lack of any significant effect of biopesticides, including imidacloprid or other pesticide treatments, on stand counts compared with the control. The reason for this phenomenon is unclear, but abiotic factors such as temperature, rainfall and the planting date may have influenced wireworm activity beneath the soil. Campbell (Reference Campbell1937) and Milosavljevic et al. (Reference Milosavljevic, Esser and Crowder2016) reported that lower temperature and rainfall during the growing season hindered the movement of wireworm larvae towards the soil surface, thereby reducing feeding activity. The authors also reported that in general, wireworm-feeding activity begins at soil temperatures of 10 °C, with highest activity at 15–18 °C. Higher soil moisture further enhanced wireworm-feeding activity. In our study, lower average temperature and precipitation were recorded in 2016 (12 °C and 15 mm) than during 2015 (17 °C and 136 mm of rain) (Natural Resources Conservation Service 2017). These factors could have led to less wireworm activity in 2016, a possibility further supported by our results, which found a low decline rate in wheat seeding plant counts irrespective of treatment and almost no wireworms in traps after treatment applications, in contrast to 2015.
Surprisingly, we found significantly higher wireworm populations in treatments with insect pathogenic fungi, spinosyn (Entrust) alone, or insect pathogenic fungi+spinosyn compared with water control or imidacloprid treatments. These findings, especially for insect pathogenic fungi treatments, appeared to be in disagreement with the findings reported by Reddy et al. (Reference Reddy, Tangtrakulwanich, Wu, Miller, Ophus, Prewett and Jaronski2014), which found significantly lower number of wireworms in entomopathogenic fungus treatments than in the control treatment. However, this result did not occur consistently and only in one out of four site-years. This inconsistent performance of treatments may have been caused by non-uniform wireworm distribution in the plots or lack of fungal treatments due to abiotic factors such as soil temperature and moisture. Additional studies are necessary to resolve the present inconsistencies.
No other reports directly comparing insect pathogenic fungus effects on wireworms in a spring wheat crop are available. Kabaluk and Ericsson (Reference Kabaluk and Ericsson2007) reported that A. obscurus were repelled by M. anisopliae-contaminated soil in a corn trial, at a rate that increased with conidial concentration in the soil. Several studies have examined the interaction of beetle/weevil larvae with fungal pathogens in the soil or rhizosphere. Kepler and Bruck (Reference Kepler and Bruck2006) stated that black vine weevil Otiorhynchus sulcatus (Fabricius) (Coleoptera: Curculionidae) larvae were significantly more attracted to Norway spruce tree (Picea abies Linnaeus; Pinaceae) roots growing in M. anisopliae-inoculated potting media than plants grown in un-inoculated media. Similar findings were reported by Villani et al. (Reference Villani, Krueger, Schroeder, Consolie, Consolie, Preston-Wilsey and Donald1994), who observed that Japanese beetles, Popillia japonica Newman (Coleoptera: Scarabaeidae), were more attracted to oviposit in bare soil treated with M. anisopliae than non-inoculated soil, possibly in response to CO2 released during mycelial growth. Currently, no information is available on the behaviour in relation to fungus of the wireworm species (L. californicus, H. bicolor, and A. mellilus) commonly found in Montana agriculture soils. Our findings suggest that soil insect pest behaviour such as wireworms in connection with fungal exposure should be taken into consideration when developing integrated pest management strategies.
In conclusion, although variability between years and between sites was observed in the present study, the overall results indicate that insect pathogenic fungi alone or in combination with imidacloprid could be used for the management of wireworms in spring wheat in the Golden Triangle area of Montana. However, cost/benefit studies will be required if the application of biopesticide products are economical and sustainable for spring wheat growers in the Golden Triangle.
Acknowledgements
This work was supported by the Montana Wheat and Barley and United States Department of Agriculture National Institute of Food and Agriculture, Multistate Project S-1070 and, the Working Group on Improving Microbial Control of Arthropod Pests Covering Research in Montana [accession number 232056]. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the National Institute of Food and Agriculture (NIFA) or the United States Department of Agriculture (USDA). We would like to thank John H. Miller, Julie Prewett, Dawson Berg, and Kristal Judisch for their assistance with the fieldwork.












