Introduction
Large-scale efforts to detect invasive wood-inhabiting insects occur in North America and elsewhere (Brockerhoff et al. Reference Brockerhoff, Jones, Kimberley, Suckling and Donaldson2006; Rabaglia et al. Reference Rabaglia, Duerr, Acciavatti and Ragenovich2008). Scolytinae (Coleoptera: Curculionidae), Cerambycidae (Coleoptera), and Siricidae (Hymenoptera) are common targets of these surveys as several have successfully invaded new environments causing considerable economic and ecological impacts (Ciesla Reference Ciesla2003; Mayfield Reference Mayfield2007; Haack et al. Reference Haack, Hérard, Sun and Turgeon2010). These survey efforts generally involve deploying semiochemical-baited traps with the hopes of detecting invasive insects early in the establishment phase of invasion where eradication efforts have the best chance of success. Maximising the chances of traps intercepting invading insects relies on selecting high-risk sites for survey and various other factors that influence traps. Trapping factors, such as semiochemical blends, trap placement in an environment, and selection of trapping habitats all can influence trapping success (Miller and Rabaglia Reference Miller and Rabaglia2009; Dodds Reference Dodds2011; Graham et al. Reference Graham, Poland, McCullough and Millar2012).
For most surveys, traps are deployed in the understorey with the base of traps ∼0.5–1.0 m off the ground. Little empirical data exist to support trapping only in the understorey, but lower trap heights are logistically easier to set and maintain. Many wood-inhabiting species preferentially colonise different portions of trees but dispersal behaviour for most common species is unknown. It is possible that insects colonising upper portions of trees may not disperse below their habitat height and may be missed in sampling efforts concentrated in the understorey. Several studies have demonstrated the importance of sampling at various heights (Su and Woods Reference Su and Woods2001; Vance et al. Reference Vance, Kirby, Malcolm and Smith2003; Ulyshen and Hanula Reference Ulyshen and Hanula2007; Wermelinger et al. Reference Wermelinger, Fluckiger, Obrist and Duelli2007; Bouget et al. Reference Bouget, Brustel, Brin and Noblecourt2008), but it is unknown what influence trap height has when used in conjunction with common detection lures such as α-pinene, ethanol, ipsdienol, and ipsenol. The objective of this study was to determine the influence traps placed at two heights had on a subset of arboreal (scolytine, cerambycid, and siricid) insects in northeastern pine forests.
Materials and methods
Site
Traps were deployed in two pine stands on the Massebesic Experimental Forest near Alfred, Maine, United States of America. These stands were ∼0.5 km from one another and separated by an unthinned mature eastern white pine, Pinus strobus Linnaeus (Pinaceae) stand. Sample stands were similar in structure and both had undergone thinning treatments during the fall/winter 2008. One stand was entirely P. strobus, while the second stand contained a mix of P. strobus and red pine, Pinus resinosa Aiton. Average tree diameter was 32 cm and basal area ranged from 11 to 18 m2/ha in the stands. The thinning treatments resulted in open stand conditions with a noncontiguous canopy throughout the majority of the area.
Experimental design
Two treatments (understorey, canopy level) were selected to test the effect of trap height on arboreal insect catches. Canopy-level traps (hereafter referred to as canopy traps) were hung between two codominant trees separated by at least 15 m. Height of traps ranged from 10 to 12 m, which generally corresponded to the height of the lower third of the live crown. A Big Shot® (SherrillTree, Greensboro, North Carolina, United States of America) was used to shoot a 283.5 g (10 ounce) weighted bag with a throwline attached through the lower canopy of one tree. This throwline was then used to feed a stronger nylon rope through the lower portion of the live-crown branches. This procedure was repeated for the second tree and each end of the nylon rope was fastened to a 5 cm diameter metal ring. A smaller nylon rope was fed through the metal ring with one end tied to the multiple-funnel trap. Both ends of the horizontal line were then raised and positioned so that the metal ring guiding the vertical line was in the middle of the two trees. The opposite ends of the horizontal nylon line were then fastened to eyehooks in adjacent cut stumps. The vertical line fed through the metal ring was used to raise and lower canopy traps for insect collection. Understorey traps were hung from metal conduit poles placed in between the two co-dominant pine trees that were used to rig the canopy trap. These traps were hung so that the collection cup was ∼0.5 m from the ground. Ten replicates (five in each stand) of the two treatments were set 2 May 2011 and collections were made biweekly until 4 November 2011.
Twelve-unit multiple-funnel traps (Lindgren Reference Lindgren1983) with wet collection cups were used along with semiochemical baits to collect insects. Propylene glycol was used as the collection and preservation liquid. Traps were baited with α-pinene (releasing ∼2 g/day), ethanol (releasing ∼0.4 g/day), racemic ipsdienol, and racemic ipsenol lures. Ipsdienol and ipsenol were released from plastic bubblecap lures at rates of 0.2 and 0.4 mg/day, respectively. All release rates were provided by the manufacturer (Synergy Semiochemicals, Burnaby, British Columbia, Canada). The α-pinene and ethanol lures were changed every four weeks, whereas ipsdienol and ipsenol were changed every six weeks. Insects were collected every two weeks by straining the propylene glycol and captured insects through a paper/nylon paint filter and placing this filter in a labelled plastic sample bag. The rigging of one canopy trap was problematic throughout the latter portion of sampling and compromised sample collection on several occasions. In these instances, the understorey trap collections from the same replicate were not used in the analyses. Samples were later processed in the laboratory where scolytines, cerambycids, and siricids were separated from debris and bycatch and identified to species. No attempt was made to identify individual Pityophthorus Eichhoff (Coleoptera: Curculionidae) species because of the taxonomical challenges this genus presents. Voucher specimens were deposited in the United States Forest Service, Durham Field Office, Forest Insect Collection (Durham, New Hampshire, United States of America).
Statistical analyses
Trap catches were pooled over the entirety of the trapping period for all analyses except average species richness estimates. Only species that accounted for more than 1% of total trap catches of a family were analysed separately. The number of species each trap accumulated over the trapping period were summed and used for comparisons of average species richness. Trap catches were analysed using a generalised linear mixed model (Proc GLIMMIX) via maximum likelihood estimation with replicates as blocks. Replicates were considered a random factor and trap height was a fixed factor in the model. Data were modelled with the negative binomial function with log link (SAS version 9.3, SAS Institute, Cary, North Carolina, United States of America). Collections of Dryocoetes autographus Ratzeburg were not modelled because this species was not captured in canopy traps compared with 900 captured in understorey traps.
For all species richness and diversity measures, trap collections were pooled by treatment for the entire sampling period. Simpson's index (1-D) was calculated using the software PAST (Hammer et al. Reference Hammer, Harper and Ryan2001). Adjusted Jaccard and Sørensen abundance estimates of community similarity (Chao et al. Reference Chao, Chazdon, Colwell and Shen2006) were calculated between the trap treatments using SPADE (Chao and Shen Reference Chao and Shen2010). SPADE was also used to determine Chao1 estimates of species richness for each trap position. Individual-based rarefaction curves were calculated for understorey and canopy traps by pooling all catches by each treatment in PAST.
Results
A total of 8463 scolytine, cerambycid, and siricid specimens from 65 species were captured in understorey and canopy traps. Scolytines represented 73.9% of trap catches with specimens from 28 species and one genus (i.e., Pityophthorus), while cerambycids accounted for 25.8% of trap catches from 33 species. Hylastes opacus Erichson and Xyleborinus alni (Niisima) were the only exotic scolytines captured. No exotic cerambycids were captured. Siricids only represented 0.3% of the catch and included specimens from four native species. Significantly more total cerambycids, scolytines, and siricids were captured in understorey (567.7 ± 36.0) compared with canopy (231.3 ± 15.1) traps (F 1,9 = 97.2, P < 0.0001). Higher average species richness was captured in understorey (33.7 ± 1.8) compared with canopy (21.1 ± 1.5) traps (F 1,9 = 28.5, P = 0.0005; Fig. 1A). Understorey traps captured higher proportions of total species captured for both scolytines and cerambycids (Fig. 2). Forty-two percent of species were captured five or fewer times, and 24% of the species were captured only once (i.e., singlets).
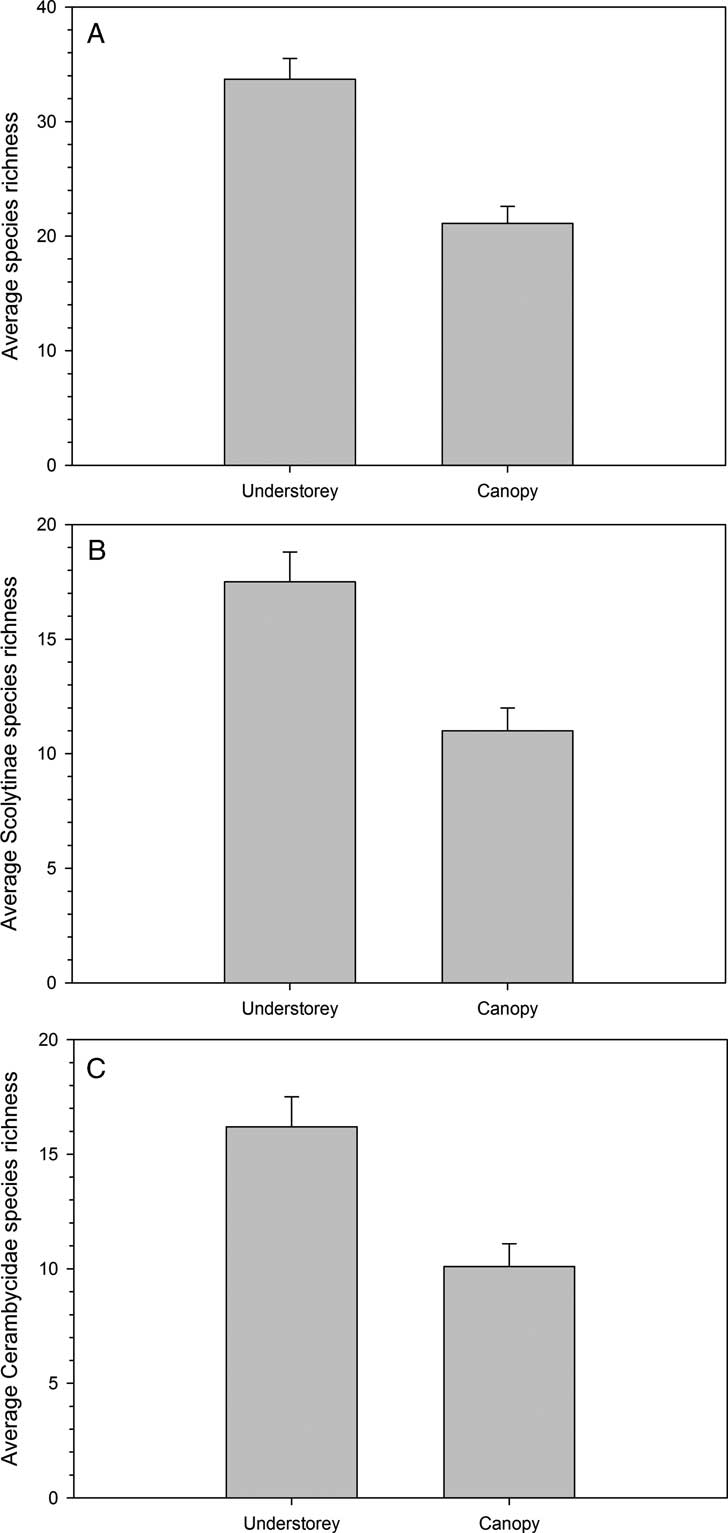
Fig. 1 Average total (A), Scolytinae (B), and Cerambycidae (C) species richness captured in understorey and canopy traps baited with α-pinene, ethanol, ipsdienol, and ipsenol in southern Maine, United States of America.
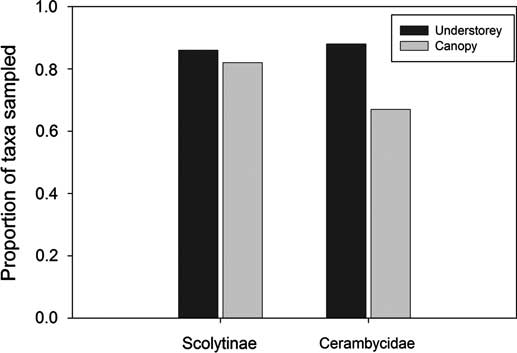
Fig. 2 Proportion of total Scolytinae and Cerambycidae taxa sampled with understorey and canopy traps in southern Maine, United States of America.
Scolytinae
Dendroctonus valens LeConte and Ips grandicollis (Eichhoff) were the most abundant scolytines, representing 24% and 21% of total scolytine captures, respectively. Other abundant species included D. autographus (14%), Orthotomicus caelatus (Eichhoff) (13%), Ips pini (Say) (8%), and Pityogenes hopkinsi Swaine (7%). Singlets accounted for 18% of the scolytine species captured. Pityophthorus was treated as a genus, but at least four morphospecies were present in the collections.
Average species richness of scolytines was higher in understorey compared with canopy traps (F 1,9 = 14.6, P = 0.004; Fig. 1B). More total scolytines were also captured in understorey than canopy traps (F 1,9 = 80.9, P < 0.0001; Table 1). Dendroctonus valens (F 1,9 = 200.2, P < 0.0001), D. autographus, H. opacus (F 1,9 = 76.6, P < 0.0001), O. caelatus (F 1,9 = 182.6, P < 0.0001), and Gnathotrichus materiarius (Fitch) (F 1,9 = 49.4, P < 0.0001) were all more abundant in understorey compared with canopy traps. Only I. pini (F 1,9 = 8.6, P = 0.02) and P. hopkinsi (F 1,9 = 10.3, P = 0.01) were more abundant in canopy traps. Species that were captured in similar numbers at the two trap heights included I. grandicollis (F 1,9 = 2.4, P = 0.2), Pityokteines sparsus (LeConte) (F 1,9 = 0.09, P = 0.8), and Pityophthorus Eichhoff species (F 1,9 = 0.9, P = 0.4).
Table 1 Mean ± SE of scolytines and cerambycids captured in understorey and canopy traps baited with α-pinene, ethanol, ipsdienol, and ipsenol in southern Maine, United States of America.
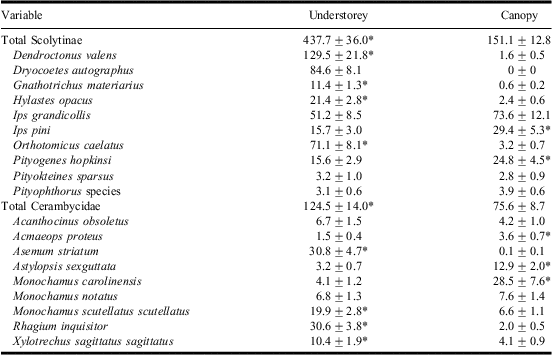
*P < 0.05.
Cerambycidae
Monochamus carolinensis (Olivier) and Rhagium inquisitor (Linnaeus) were the most abundant cerambycids, representing 18% and 17% of total cerambycid captures, respectively. Other abundant species included Asemum striatum (Linnaeus) (15%), Monochamus scutellatus scutellatus (Say) (14%), Astylopsis sexguttata (Say), Monochamus notatus (Drury) (8%), and Xylotrechus sagitattus sagittatus (Germar) (7%). Singlets accounted for 27% of the cerambycid species captured.
Average species richness of cerambycids was higher in understorey compared with canopy traps (F 1,9 = 13.9, P = 0.005; Fig. 1C). More total cerambycids were also captured in understorey (124.5 ± 14.0) than canopy (75.6 ± 8.7) traps (F 1,9 = 21.8, P = 0.001; Table 1). Of the cerambycids statistically tested, M. scutellatus (F 1,9 = 34.9, P = 0.0002), R. inquisitor (F 1, 9 = 116.3, P < 0.0001), A. striatum (F 1,9 = 31.5, P = 0.0003), and X. sagittatus (F 1,9 = 12.44, P = 0.007) were found more often in understorey traps. Cerambycids caught at significantly higher numbers in canopy traps included M. carolinensis (F 1,9 = 49.2, P < 0.0001), Acmaeops proteus (Kirby) (F 1,9 = 7.1, P = 0.03), and A. sexguttata (Say) (F 1,9 = 52.3, P < 0.0001). Trap catches of M. notatus (F 1,9 = 0.52, P = 0.5) and Acanthocinus obsoletus (Olivier) (F 1,9 = 4.7, P = 0.06) did not significantly differ between understorey and canopy traps.
Siricidae
The most abundant siricid captured was Sirex nigricornis Fabricius (83%), followed by Tremex columba (Linnaeus) (10%). Urocerus albicornis (Fabricius) and Urocerus cressoni Norton were only captured one time each. Too few siricids were captured to statistically analyse. Of the 29 specimens collected, the majority were captured in the understorey (76%) compared with canopy (24%) traps. The three specimens of T. columba were captured exclusively in canopy traps. Only female siricids were captured.
Species richness and diversity
Species richness of understorey (56) and canopy traps (47) were not significantly different (Table 2). There were 38 shared species between understorey and canopy traps with more unique species found in understorey traps (Fig. 3). Simpson's Index (1-D) estimates were statistically higher for understorey compared with canopy traps (Table 2). Adjusted Jaccard abundance (0.94 ± 0.05) and Sørensen abundance (0.97 ± 0.03) similarity indices suggested traps sampled similar communities.
Table 2 Species richness, abundance, and diversity estimates in understorey and canopy traps baited with α-pinene, ethanol, ipsdienol, and ipsenol in southern Maine, United States of America.

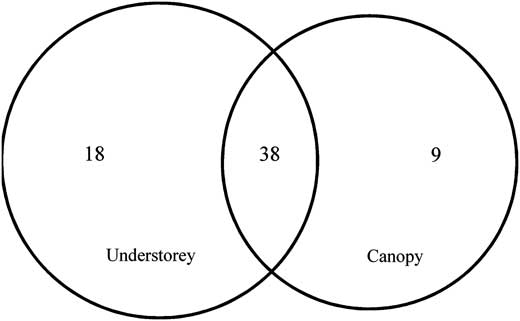
Fig. 3 Venn diagram depicting the number of species captured in understorey and canopy traps, similarity among trap catches, and unique species captured at each height in southern Maine, United States of America.
Individual-based rarefaction curves for understorey and canopy traps followed similar patterns with neither reaching an asymptote (Fig. 4). While understorey traps captured more individuals, rarefaction curves suggest canopy traps have the potential to capture higher species richness. Chao1 estimated species richness was slightly higher in understorey traps compared with canopy traps (Table 2).
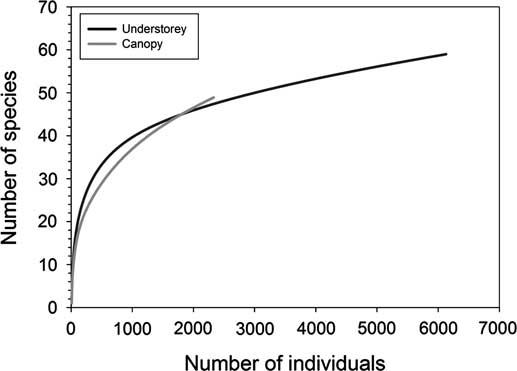
Fig. 4 Individual-based rarefaction curves for understorey and canopy traps baited with α-pinene, ethanol, ipsdienol, and ipsenol in southern Maine, United States of America.
Discussion
Resources to conduct exotic species surveys are often limited. Placing traps to optimise trap catches (i.e., increase species richness) and sample surrounding communities as completely as possible is critical to success of these surveys. Insights gained by trapping indigenous species provide guidance for exotic species surveys as well as efforts to sample native communities. Trap type (Flechtmann et al. Reference Flechtmann, Ottati and Berisford2000; de Groot and Nott Reference de Groot and Nott2001; Dodds et al. Reference Dodds, Dubois and Hoebeke2010), surface treatments (Graham et al. Reference Graham, Mitchell, Reagel, Barbour, Millar and Hanks2010; Allison et al. Reference Allison, Johnson, Meeker, Strom and Butler2011), collection method (Morewood et al. Reference Morewood, Hein, Katinic and Borden2002; Miller and Duerr Reference Miller and Duerr2008), colour (Strom and Goyer Reference Strom and Goyer2001), and semiochemical blends (Miller and Rabaglia Reference Miller and Rabaglia2009) all can influence trap captures. Placement variables, such as habitat selection, location of traps within or near habitat patches, and height can also influence trap catches and the portion of a community sampled (Vance et al. Reference Vance, Kirby, Malcolm and Smith2003; Ulyshen and Hanula Reference Ulyshen and Hanula2007; Wermelinger et al. Reference Wermelinger, Fluckiger, Obrist and Duelli2007; Dodds et al. Reference Dodds, Dubois and Hoebeke2010; Dodds Reference Dodds2011; Graham et al. Reference Graham, Poland, McCullough and Millar2012). In the current study, traps placed in the understorey and canopy captured many of the same species, but differences in effectiveness between the treatments existed.
Species richness and abundance were higher in understorey traps compared with canopy traps for total abundance of arboreal species counted, and for scolytines and cerambycids separately. Understorey traps captured 86% of total species sampled at both heights, while canopy traps captured 72%. Proportions of scolytines and cerambycids captured were also higher in understorey than canopy traps. While both heights sampled the majority of species captured, understorey traps caught a larger proportion of the community. Average species richness and number of species were higher in understorey traps. Understorey traps also captured more unique species than canopy traps. Higher abundance of arboreal insects were also captured in understorey traps, with over 2.5 times as many insects trapped at this height compared with canopy traps. While abundance estimates are not relevant to exotic species surveys, they may be important if population monitoring is the goal of trapping efforts. Results support placement of traps in the understorey for both increasing species richness and catching larger numbers of insects.
There was variation in response of individual scolytines to the two trapping heights. Dendroctonus valens, H. opacus, O. caelatus, and D. autographus inhabit lower portions of tree boles and roots (Wood Reference Wood1982; Hoebeke Reference Hoebeke1994; Owen et al. Reference Owen, Smith and Seybold2010) and were more abundant in understorey traps. Very low numbers of each, with the exception of D. autographus, were captured in canopy traps as well, indicating that flight level or trap height does not necessarily indicate habitat preference. The ambrosia beetle G. materiarius was also more abundant in understorey traps. This species has been previously associated with lower sections of dead pine trees (Ryan et al. Reference Ryan, de Groot and Smith2011). Pityogenes hopkinsi was more abundant in canopy traps, which may relate to its habit of colonising smooth bark sections of tree boles and broken limbs and branches (Baker Reference Baker1972; Wood Reference Wood1982). Abundance of P. sparsus and Pityophthorus species was not different between the two trap heights. Because of the difficulty separating species of Pityophthorus, we did not attempt to differentiate individual species and treated the group at the genera level. Consequently, any behavioural differences of individual species may have been lost.
While no differences existed between the catches of I. grandicollis at the two heights, I. pini was twice as abundant in canopy compared with understorey traps. These results differ from previous work that documented more abundant I. pini catches in traps at 2 m compared with 9 m (Raffa Reference Raffa1991). Ips pini has been reported to colonise mid-bole of trees (Ayres et al. Reference Ayres, Ayres, Abrahamson and Teale2001) but trapping height may not relate to bole-colonisation patterns. Ips grandicollis colonised basal portions of trees (Ayres et al. Reference Ayres, Ayres, Abrahamson and Teale2001), but was captured in equal numbers at the two trapping heights used in this study.
More total cerambycids were captured in understorey compared with canopy traps, however, there was variation in response to trap height by individual species. Asemum striatum, a lower bole specialist (Baker Reference Baker1972), was captured almost exclusively in understorey traps. Rhagium inquisitor, a species reported to colonise dead and dying tree boles (Baker Reference Baker1972) was also more abundant in understorey traps. Two cerambycid species, A. sexguttata and A. proteus were trapped more frequently in canopy traps. Little is known about their colonisation behaviour on host trees, but a larger percentage of A. sexguttata emerged from branch sections compared with large bolts (Reagel et al. Reference Reagel, Smith and Hanks2012). No information could be found on A. proteus colonisation behaviour.
Monochamus Dejean species abundance varied in response to trap height. The three Monochamus species captured all share a common resource (i.e., stressed and dying pines) but little is known about specific colonisation patterns. Resource partitioning of host trees is common in wood-inhabiting beetles that temporally and spatially co-occur (Nord and Knight Reference Nord and Knight1972; Paine et al. Reference Paine, Birch and Švihra1981; Ayres et al. Reference Ayres, Ayres, Abrahamson and Teale2001), but caution must be taken in drawing conclusions from trapping studies on this behaviour. While M. notatus was captured in equal numbers at the two heights, both M. carolinensis and M. scutellatus showed strong responses to different trap heights. Over six times the number of M. carolinensis were captured in canopy compared with understorey traps. The opposite was found for M. scutellatus where three times as many were found in understorey compared with canopy traps. It is unknown if this flight pattern will relate directly to colonisation behaviour on pine trees. However, in a study of Sirex noctilio (Fabricius) distribution along the length of tree boles, Ryan et al. (Reference Ryan, de Groot and Smith2011) found M. carolinensis throughout the entire tree bole with a slight increase in the upper portions. In a separate study, M. scutellatus preferred larger diameter sections of felled P. strobus in West Virginia, United States of America for oviposition (Hughes and Hughes Reference Hughes and Hughes1982). It is possible that Monochamus species are partitioning tree boles in a similar way seen in scolytines.
As in many surveys implementing semiochemical lures, low numbers of siricids were captured during the approximately six months of sampling. The majority of siricids were captured in understorey traps. However, the very low number of T. columba captured were exclusively found in canopy traps. Dodds et al. (Reference Dodds, Zylstra, Dubois and Hoebeke2012) reported relatively large numbers of T. columba in multiple-funnel traps hung at 6 m on artificially stressed pine trees.
The key component to successful surveys is maximising species richness to define a community present at a trapping site. Understorey traps captured the highest observed species richness and unique species of the two trap placements. Individual-based rarefaction curves and Chao1 estimates suggested there were species present at both heights that were not sampled during the survey. Neither rarefaction curve plateaued, but it appears that canopy traps may be capable of sampling more species over time. Chao1 species richness estimates were similar for each trap placement, with species richness higher than what was observed during the trapping period. The addition of different trap types to surveys may increase species richness by capturing insects not sampled with multiple-funnel traps (Dodds et al. Reference Dodds, Dubois and Hoebeke2010). Diversity estimates were significantly higher in understorey traps, but whether or not this was biologically meaningful is debatable. Interestingly, both Jaccard and Sorensen abundance-based similarity indices suggested very similar communities were trapped at each height.
Conclusions
Understorey traps captured more species than canopy traps and the portion of the community sampled from each height were very similar. Common practice in exotic species and other survey types is to place traps in the understorey hanging between two trees, from conduit, or on a low tree branch. Results from this study suggest traps in the understorey sample larger portions of the community present than canopy traps. However, much overlap in trap captures at understorey and canopy heights suggested there is leeway in vertical trap placement. Because unique species were captured at each height, multiple trapping heights may be beneficial in survey of high-risk forests to ensure the largest portion of the arboreal insect community present on a site is sampled.
Acknowledgements
This project would not have been possible without the invaluable field and laboratory work contributed by Garret D. Dubois and Angie Hammond, both United States Forest Service Northeastern Area employees. Jon Janelle, United States Forest Service Northern Research Station, helped with location of study sites on the Massabesic Experimental Forest. E. Richard Hoebeke, Georgia Museum of Natural History, confirmed or identified several insect specimens. Ryan P. Hanavan, United States Forest Service Northeastern Area, provided comments on a draft version of this paper.








