Introduction
The eye-spotted bud moth, Spilonota ocellana (Denis and Schiffermüller) (Lepidoptera: Tortricidae), is a polyphagous pest of European origin that occurs in many apple-growing areas across the northern hemisphere (Weires and Riedl Reference Weires and Riedl1991; Alford Reference Alford2007). Spilonota ocellana has recently become a significant economic pest of commercial apples, Malus domestica Borkhausen (Rosaceae), in the Similkameen Valley, in British Columbia, Canada (Swain Reference Swain2016). In this montane growing region at least 50% of the commercial apple orchards are organic (Mullinix Reference Mullinix2005). Organic apple producers in Canada currently have few effective options to manage S. ocellana (Edwards Reference Edwards1998) but a subset of British Columbia apple producers uses multispecies mating disruption to manage a suite of other leaf-feeding tortricid pests (Judd and Gardiner Reference Judd and Gardiner2004, Reference Judd and Gardiner2008). Commercial mating-disruption products targeting S. ocellana are not yet available in Canada, but preliminary research trials suggest it has potential (McBrien et al. Reference McBrien, Judd and Borden1998). Recent research in Sweden found that management of S. ocellana with a multispecies mating-disruption product was undermined by an influx of mated females (Porcel et al. Reference Porcel, Sjöberg, Swiergiel, Dinwiddie, Rämert and Tasin2014). The authors concluded that because apple orchards in Sweden are small and often interspersed with other crops, wooded hedgerows, or native vegetation – an agricultural landscape similar to British Columbia – successful management of S. ocellana with mating disruption requires additional adult monitoring to assess external pest pressure and avoid unexpected outbreaks (Porcel et al. Reference Porcel, Sjöberg, Swiergiel, Dinwiddie, Rämert and Tasin2014).
Management of insects with sex pheromone technologies is often vulnerable to immigration of mated females (Cardé and Minks Reference Cardé and Minks1995) and our ability to easily measure or monitor these threats is limited. Pioneering work on pear ester, the host-plant kairomone that attracts female codling moth (Light et al. Reference Light, Knight, Henrick, Rajapaska, Lingren and Dickens2001), provides the best example of how trapping female moths with chemical attractants might lead to more robust mating disruption and pest management (Knight and Light Reference Knight and Light2005a, Reference Knight and Light2005b, Reference Knight and Light2005c). Combining acetic acid with pear ester – thought to be a feeding attractant of microbial origin – was an innovation that led to a strong female lure (Landolt et al. Reference Landolt, Suckling and Judd2007). Acetic acid-pear ester lures are now commonly implemented into various codling moth pest management programmes (Knight Reference Knight2010; Hári et al. Reference Hári, Pénzes, Jósvai, Holb, Szarukán and Szólláth2011; Judd Reference Judd2016).
Besides acetic acid-pear ester lures for codling moth and more recently, Hedya nubiferana (Haworth) (Lepidoptera: Tortricidae) (Jósvai et al. Reference Jósvai, Koczor and Tóth2016), chemical attractants for female tortricid moths are rare (El-Sayed Reference El-Sayed2016). Nonetheless, during a search for tortricid attractants, Knight et al. (Reference Knight, Hilton, Basoalto and Stelinski2014) found that several leaf-feeding species, including S. ocellana, were weakly attracted by acetic acid. Collaboration with El-Sayed et al. (Reference El-Sayed, Knight, Byers, Judd and Suckling2016) led to a study in Canada showing traps baited with a combination of acetic acid and benzyl nitrile (also known as phenylacetonitrile) caught male and female S. ocellana in numbers similar to catches with sex pheromone, although El-Sayed et al. (Reference El-Sayed, Knight, Byers, Judd and Suckling2016) recommended further experiments to confirm this result. Benzyl nitrile had been identified by gas chromatography as one of several volatile aromatic benzenoid compounds released by apple leaves in response to feeding by S. ocellana and other tortricid larvae (El-Sayed et al. Reference El-Sayed, Knight, Byers, Judd and Suckling2016). If these caterpillar-induced kairomones are as attractive as sex pheromones then they could prove as useful for trapping female tortricids as sex pheromones have for male tortricids (Witzgall et al. Reference Witzgall, Kirsch and Cork2010). Furthermore, kairomones that remain attractive to adult S. ocellana in orchards treated with sex pheromones may prove more valuable for monitoring than sex pheromone lures that are often rendered ineffective by pheromone-disruption treatments (McBrien et al. Reference McBrien, Judd and Borden1998; Judd and Gardiner Reference Judd and Gardiner2004, Reference Judd and Gardiner2008).
The overall objective of the present study was to gather information that may facilitate development of kairomone-based trapping technologies for female S. ocellana. Specific objectives of the current study are: (1) to confirm the relative attraction of S. ocellana by acetic acid, benzyl nitrile, 2-phenylethanol, and various combinations; (2) to evaluate several lure and trap parameters that might improve the use of these attractants for trapping female S. ocellana; and (3) to measure the attraction of these kairomones relative to a sex pheromone when used in apple orchards under management with pheromone disruption.
Materials and methods
Test locations
All trapping experiments were conducted in commercial organic apple orchards near Cawston (49°10.89'N, 119°46.29'W, elevation 400 m) in the Similkameen Valley, British Columbia, Canada. Orchards were composed of several plantings (=blocks) of dwarfing, high-density superspindle trees of mixed varieties (Ambrosia, Gala, Granny Smith, and Spartan) with an average tree height of 3–4 m and 1200–5444 apple trees/ha. One trap height experiment was conducted in a 5-m-tall Spartan block with a 3.6×3.5 m tree-row spacing and 600 tree/ha.
Chemicals
Glacial acetic acid (AA), benzyl nitrile (BN), 2-phenylethanol (PET), and dichloromethane solvent were purchased in 99% purity from Sigma-Aldrich (St. Louis, Missouri, United States of America). Two S. ocellana pheromone components (McBrien et al. Reference McBrien, Gries, Gries, Borden, Judd, King and Slessor1991), Z8-tetradecenyl acetate (Z-8-14:OAc), and Z8-tetradecenyl alcohol (Z-8-14:OH) were purchased in 99% isomeric purity from Pherobank (Wageningen, The Netherlands). Acetic acid was stored at ambient laboratory temperature (20 °C) while all other compounds were stored at −20 °C until used.
Release devices
In most experiments acetic acid was dispensed from an 8-mL polypropylene vial (Nalg-Nunc International, Rochester, New York, United States of America). Each vial contained 3 mL of glacial acetic acid applied to two cotton balls. Volatilised acetic acid was emitted through a 3-mm diameter hole drilled in the lid of each vial. This standard acetic acid release device is hereafter referred to as an acetic-acid co-lure. Red natural rubber septa (VWR International, Mississauga, Ontario, Canada), grey halobutyl rubber septa (West Pharmaceutical Services, Lionville, Pennsylvania, United States of America) and white synthetic rubber septa (VWR International) were used to release benzyl nitrile, 2-phenylethanol, and pheromone alone and in combination in various experiments. All rubber septa were extracted with dichloromethane for 24 hours and air dried in a fume hood overnight before use. To construct composite binary or ternary lures combining AA+BN, AA+PET, or AA+BN plus sex pheromone we drilled a second aperture (5 mm diameter) in the lid of our acetic-acid co-lure into which we inserted the narrow end of each rubber septum. Benzyl nitrile, 2-phenylethanol, and sex pheromone were dissolved in dichloromethane and loaded into and volatilised from the large wells of these rubber septa.
Proprietary membrane-based polymeric cup dispensers (Trécé Incorporated, Adair, Oklahoma, United States of America) having a 1.8-cm diameter release surface were used in one experiment to release benzyl nitrile (TRE-1381), 2-phenylethanol (TRE-1256), and BN-PET (TRE-1379) alone, or in a mixture with acetic acid as AA-BN (TRE-1378), AA-PET (TRE-1377), or AA-BN-PET (TRE-1380). All loadings of membrane dispensers are considered proprietary.
Traps
Pherocon® VI style, white plastic delta traps with polybutene sticky liners (Trécé Incorporated) were used in most experiments. Multipher®-I white plastic bucket traps with green lids and inner funnels (Solida Incorporated, Saint-Ferréol-les-Neiges, Quebec, Canada) and all-transparent plastic bucket traps (UnitrapsTM; International Pheromone Systems, Wirral, United Kingdom) were used in two experiments. Food-grade propylene glycol (Sigma-Aldrich) and Vapona (VaportapeTM II; Hercon Environmental, Emigsville, Pennsylvania, United States of America) were used as killing agents in bucket traps.
General experimental design
All trapping experiments were run as randomised complete block designs with 5–12 replicates (Zar Reference Zar1984). Unless stated otherwise, all traps were hung from wires within apple trees at ~1.7 m above ground with 20 m between the treatment traps within each replicate (=block) and these statistical blocks were separated by at least 30 m. All traps were also at least 30 m from the borders of any orchard. Within each experiment all sticky-trap liners were replaced at least weekly and returned to the laboratory where moths were counted and sexed.
Lure development experiments
Experiment 1 was conducted to determine the relative attraction of acetic acid, benzyl nitrile, and AA+BN lures using rubber septa to dispense benzyl nitrile rather than plastic sachets (El-Sayed et al. Reference El-Sayed, Knight, Byers, Judd and Suckling2016). Rubber septa are commonly used to produce commercial sex pheromone lures. Demonstrated effectiveness of septa for release of benzenoid compounds facilitates production of experimental lures for trapping development work on S. ocellana and other tortricid moths. Use of septa also allows us to compare and combine kairomones and pheromones using devices with known loadings and release properties not always available with proprietary products. Small red septa (250 µL well load volume) were used in experiment 1.
Four treatments were included in experiment 1: (1) a blank control, consisting of an empty 8-mL vial+blank septum; (2) an acetic-acid treatment, consisting of an acetic-acid co-lure+blank septum; (3) a benzyl-nitrile treatment, consisting of an empty 8-mL vial+septum loaded with 10 mg of benzyl nitrile; and (4) an AA+BN binary treatment, consisting of an acetic-acid co-lure+septum loaded with 10 mg of benzyl nitrile. These four treatments excluded any possibility that traps and lures differed in any way except their chemical constituents. All of these composite lures were attached at the centre and on the side, inner surface, of a delta trap using a 2.5-cm length of Velcro® industrial sticky-back tape (Canadian Tire, Penticton, British Columbia, Canada). Experiment 1 had five replicates and was conducted from 6–20 June 2016.
Experiment 2 was conducted with prototype commercial membrane dispensers (Trécé Incorporated) to measure the relative attraction of AA+BN, AA+PET, and AA+BN-PET when the acetic acid was mixed in the same dispenser as the aromatic compounds or presented as a separate acetic-acid co-lure. There were seven treatments in this experiment: (1) acetic-acid co-lure+benzyl nitrile [TRE-1381], (2) acetic-acid co-lure+PET [TRE-1256], (3) acetic-acid co-lure+BN-PET [TRE-1379], (4) AA-BN mixed [TRE-1378], (5) AA-PET mixed [TRE-1377]), (6) AA-BN-PET mixed [TRE-1380], and (7) an acetic-acid co-lure. All acetic-acid co-lures were attached to delta traps with Velcro as described before. All other lures were pinned inside each delta trap at the centre apex. Experiment 2 was conducted with five replicates from 1–15 June 2016.
Experiments 3A and 3B were conducted to determine if the type of rubber septum used to release benzyl nitrile had any influence on moth catch. In experiment 3A we compared catches of S. ocellana in delta traps baited with an acetic-acid co-lure in combination with red, grey, or white rubber septa loaded with 10 mg of benzyl nitrile. The composite AA+BN lures were assembled and attached to traps as before. This experiment was conducted with six replicates from 6–20 June 2016.
Experiment 3B was conducted to determine if the size, surface area or internal well volume of a rubber septum used to release benzyl nitrile affected moth catch. We compared catches of S. ocellana in delta traps baited with an acetic-acid co-lure in combination with either large (1000 µL well volume) or small (250 µL well volume) red rubber septa loaded with 100 mg of benzyl nitrile. The assumption is that a larger septum absorbs more material upon initial loading and has a larger surface area for release than a smaller septum. Composite AA+BN lures with small septa were assembled and attached as before. For the AA+BN lures with large septa the acetic-acid co-lure was attached as before but the septum was pinned beside it rather than inserted into its lid. This experiment was conducted with five replicates from 6–20 June 2016.
Experiments 4A and 4B, respectively, were conducted to determine the relationship between catches of S. ocellana and the amount of benzyl nitrile, or 2-phenylethanol, loaded into rubber septa when combined with an acetic-acid co-lure. Red septa were loaded with increasing doses (1, 5, 10, 50, or 100 mg) of either benzyl nitrile or 2-phenylethanol, to conduct two separate dose-response experiments, 4A and 4B, respectively. Delta traps were baited as before with composite binary AA+BN or AA+PET lures each consisting of an acetic-acid co-lure and red septum with varying loads of benzyl nitrile or 2-phenylethanol. Experiments 4A and 4B had 10 and five replicates, respectively, and were conducted from 6 June–20 July 2016.
Experiments 5A and 5B were conducted to measure the attraction of an AA+BN binary lure relative to a sex pheromone lure, and to test the hypothesis (El-Sayed et al. Reference El-Sayed, Knight, Byers, Judd and Suckling2016) that combining this kairomone with a sex pheromone might increase catches of male S. ocellana. Small red septa were used to make all benzyl nitrile and sex pheromone lures in these experiments. The sex pheromone lure contained a 99:1 blend of Z-8-14:OAc and Z-8-14:OH (McBrien et al. Reference McBrien, Gries, Gries, Borden, Judd, King and Slessor1991). In experiment 5A we evaluated three treatments: (1) a 1-mg, sex pheromone septum lure, (2) an acetic-acid co-lure+10 mg benzyl-nitrile septum lure, and (3) an acetic-acid co-lure+a single-septum lure containing 10 mg of benzyl nitrile and 1 mg of sex pheromone. The latter treatment tested the idea that combining different semiochemicals in the same septum might lead to a more attractive male lure, and secondarily, it tested whether the addition of sex pheromone had any effect on female response to the binary kairomone lure. Experiment 5A had nine replicates and was conducted from 20 June to 5 July 2016.
In experiment 5B we reduced the load of our pheromone lures to a female equivalent amount (McBrien et al. Reference McBrien, Gries, Gries, Borden, Judd, King and Slessor1991) and retested three treatments: (1) a 0.01-mg, sex pheromone septum, (2) an acetic-acid co-lure+10 mg benzyl-nitrile septum, and (3) an acetic-acid co-lure+10 mg benzyl-nitrile septum+0.01 mg sex pheromone septum. The latter treatment retested the hypothesis that combining these different semiochemicals could lead to a more attractive lure for males, but in this case we presented benzyl nitrile and sex pheromone on separate septa, thus avoiding any chemical interaction on the release substrate. The sex pheromone septum was pinned to the benzyl-nitrile septum. Experiment 5B had 12 replicates and was conducted from 27 June to 11 July 2016 in a separate planting located ~100 m from experiment 5A.
Trapping development experiments
Three experiments were conducted to compare trap types. In experiment 6 we tested whether non-saturating bucket traps would be useful for trapping S. ocellana with kairomones. We compared catches in delta traps, Multipher-I traps and Unitraps when each was baited with our standard AA+BN composite lure. As before, composite AA+BN lures were attached in the upright position at the centre and inside delta traps. For each of the non-saturating traps we rotated the AA+BN lure through 90° and attached it horizontally to the underside of each bucket trap lid using Velcro tape. Vapona was placed in the bottom of each bucket trap to kill and retain all insects caught. All delta trap sticky liners were replaced each week to prevent saturation but all non-saturating traps were emptied only once at the end of the experiment. All moths were returned to the laboratory where they were identified and sexed. This experiment was conducted with 10 replicates from 6–20 July 2016.
Experiment 7 was conducted to measure the effect of different killing agents on catches of S. ocellana in Multipher-I traps baited with the AA+BN binary lures described and attached as in experiment 6. Vapona was used as the standard conventional killing agent and it was compared to propylene glycol (250 mL). Multipher-I traps with no killing agent served as a negative control. Experiment 7 had five replicates and was conducted from 26 July to 9 August 2016.
Experiment 8 was conducted to determine if trap height in the tree canopy had any impact on female moth catch as little is known about the behaviour of females. Delta traps were baited as before with standard composite binary AA+BN lures. Ten sets (statistical blocks) of two traps were deployed with one member in each set hung 1.5 m above ground and the second member of each set hung 3.5 m above ground and directly above a 1.5-m trap. Each of the 10 traps hung at the 3.5 m height was attached by wire to one end of a 2-m long bamboo pole and hooked over a branch near the top of the tree canopy. Each set of traps was separated by 30 m. Experiment 8 had 10 replicates and was conducted from 8–22 July 2016.
Seasonal monitoring
One half of each of five organic apple blocks ranging in size from 4–8 ha was treated with an experimental pheromone disruption formulation (Isomate®-LR/ESBM [LRESX22], Shin-etsu Fine Chemicals Division, Tokyo, Japan) while the other half of each of these apple blocks was left untreated. Each pheromone-treated plot received 750 Isomate-LR/ESBM dispensers/ha and each twin-tube dispenser contained 560 mg of active ingredient consisting of the following tortricid pheromone components: Z-11-tetradecenyl acetate (42.8%), Z-11-tetradecenol (1.1%), Z-11-tetradecenal (1.1%), Z-9-tetradecenyl acetate (5.0%), Z-8-14:OAc (45.7%), and Z-8-14:OH (4.3%). This pheromone formulation has activity against several sympatric leafroller species including S. ocellana. Each apple block was monitored from 1 May until 15 September 2016 with two kairomone-baited delta traps and two pheromone-baited delta traps that were deployed on the diagonals of a 30×30 m2 in the centre of each block. Each kairomone-baited trap contained the standard AA+BN lure. Each pheromone trap was baited with 1 mg of sex pheromone (McBrien et al. Reference McBrien, Gries, Gries, Borden, Judd, King and Slessor1991) loaded on a small red septum that was pinned inside each delta trap at the centre apex. All lures were replaced every three weeks. All delta trap sticky liners were replaced weekly and returned to the laboratory where moths were identified, counted, and sexed.
Statistical analyses
All insect count data were tested for normality (Kolmogorov–Smirnov test) and equality of variances (Levine’s median test) to ensure they met the assumptions of an analysis of variance (ANOVA). Any non-normal data sets were normalised and variances stabilised using a √(x+0.5) transformation (Zar Reference Zar1984). All trapping experiments were analysed by randomised block or factorial ANOVA and mean catches were compared using Tukey’s honest significant difference multiple-comparison test following significant ANOVAs. Paired trap lure means from the seasonal monitoring experiment (experiment 9) were compared using two-tailed, paired t-tests. All statistical analyses were performed with experimental error rates set at α=0.05 using SigmaPlot® 12.5 (SYSTAT Software, San Jose, California, United States of America).
Results
In experiment 1 traps baited with the AA+BN binary lure (acetic-acid co-lure+benzyl-nitrile septum) caught significantly more total S. ocellana than traps baited with either of the individual components (Fig. 1). Acetic-acid-baited traps caught significantly more total S. ocellana than benzyl-nitrile-baited traps, but traps baited with benzyl nitrile alone caught no more moths than a blank trap (Fig. 1). Moth catches in this experiment were predominately female and likely reflect the population sex ratio in the test orchard at the time this experiment was conducted.

Fig. 1 Mean (+standard error (SE)) total number of Spilonota ocellana moths caught during experiment 1 (6–20 June 2016) in sticky white delta traps baited with red rubber septa loaded with 10 mg of benzyl nitrile, an acetic-acid (AA) co-lure containing 3 mL of acetic acid in 3-mm open 8-mL polypropylene vial, or their combination, relative to blank traps. Bars followed by a common letter are not significantly different (Tukey’s honest significant difference test, α=0.05) following significant (P<0.05) analysis of variance.
Experiment 2 was conducted with proprietary membrane dispensers to compare different sources of acetic acid in combination with different aromatic blends (Table 1). Catches in traps baited with acetic-acid co-lures on their own were not included in any statistical analyses but were used as a positive control and are shown for relative comparison (Table 1). Two-way factorial ANOVA (three aromatic blends versus two acetic-acid sources) showed that the aromatic compounds had a significant effect on male (F(2,24)=17.887, P<0.001), female (F(2,24)=4.544, P=0.021), and total moth catches (F(2,24)=11.064, P<0.001) (Table 1). Significantly more male (F(1,24)=4.477, P=0.045) and total moths (F(1,24)=4.357, P=0.048) were caught when acetic acid was presented as a co-lure than when mixed in the same dispenser, but female catches were unaffected by the acetic-acid source (F(1,24)=2.287, P=0.144). Given there was a significant acetic-acid source×aromatic blend interaction for male moth catch (F(2,24)=4.219, P<0.027) we chose to compare blends within each acetic-acid source separately as shown in (Table 1).
Table 1 Catches of Spilonota ocellana moths during experiment 2 (1–15 June 2016) in sticky white delta traps baited with various membrane dispensers loaded with the aromatic compounds benzyl nitrile (BN) or 2-phenylethanol (PET) alone or in combination, and released with acetic acid in the form of a co-lure or mixed in the same dispenser.

* Acetic-acid co-lure is an 8-mL polypropylene vial containing 3 mL of acetic acid in cotton balls and sealed with a lid having a 3-mm aperture. Volume of acetic acid in mixed membrane dispensers is proprietary to Trécé.
† Means within a column and acetic-acid source followed by the same letter are not significantly different (Tukey’s honest significant difference test, α=0.05) following significant (P<0.05) analysis of variance. Acetic-acid co-lure with no aromatic compounds was not included in the statistical analyses.
Two-way randomised block ANOVA (five repetitions versus three aromatic blends) showed that when acetic acid was presented as a co-lure, traps baited with the ternary AA+BN-PET blend caught significantly more male (F(2,8)=14.256, P=0.002) and total moths (F(2,8)=17.417, P=0.001) than did traps baited with the AA+BN and AA+PET blends (Table 1). However, when using acetic-acid co-lures, traps baited with AA+BN-PET and AA+BN caught similar but significantly (F(2,8)=6.883, P=0.018) greater numbers of female moths than did traps baited with AA+PET (Table 1).
When acetic acid was mixed in the same dispenser as the aromatic compounds, traps baited with the AA-BN-PET and AA-BN blends caught similar, but significantly greater numbers of male (F(2,8)=6.306, P=0.023), female (F(2,8)=4.546, P=0.049), and total moths (F(2,8)=7.641, P=0.014), than did traps baited with the AA-PET blend, respectively (Table 1). Total moth catch provided the clearest statistical separation among treatment lures with the AA+BN-PET treatment (acetic-acid co-lure+TRE-1379) catching significantly more total moths than any other treatment lure (Table 1).
In experiment 3A catches of S. ocellana in traps baited with AA+BN binary lures made with grey rubber septa caught significantly more male (F(2,10)=4.728, P=0.036) and total moths (F(2,10)=7.686, P=0.011) than did traps baited with red or white septa (Table 2). The numbers of female S. ocellana caught were not significantly different (F(2,10)=2.710, P=0.115) in traps baited with binary lures made with grey, red, or white septa (Table 2). In experiment 3B there was no significant effect of septum size on male (F(1,9)=0.194, P=0.892), female (F(1,9)=0.012, P=0.922), or total moth catches (F(1,9)=4.37, P=0.525) when loaded with 100 mg of benzyl nitrile (Table 2).
Table 2 Influence of different rubber septa used to release benzyl nitrile on catches of Spilonota ocellana moths in sticky white delta traps baited with binary lures containing acetic acid (3 mL/3-mm open 8-mL polypropylene vial) and benzyl nitrile on rubber septa.

* Septa loaded with 10 mg of benzyl nitrile in experiment 3A and 100 mg in experiment 3B.
† Means within a column of experiment 3A followed by the same letter are not significantly different (Tukey’s honest significant difference test, α=0.05) following significant (P<0.05) analysis of variance. Means within a column for experiment 3B are not significantly different (P>0.05) by analysis of variance.
In experiment 4A, total catches of S. ocellana showed a significant (F(4,36)=6.525, P<0.001) increase with increasing loads of benzyl nitrile (Fig. 2), but there were no significant differences among mean catches with septa loaded with 10 mg or more benzyl nitrile. In experiment 4B, the loading of 2-phenylethanol on red septa had no significant effect (F(4,16)=1.565, P=0.23) on total catches of S. ocellana.

Fig. 2 Relationship between mean (±standard error) total number of Spilonota ocellana moths caught during experiment 4A (6–20 July 2016) in sticky white delta traps and the amount of benzyl nitrile loaded on red rubber septa presented in combination with an acetic-acid (AA) co-lure containing 3 mL of acetic acid in 3-mm open 8-mL polypropylene vial. Line represents best fitting nonlinear regression: y=3.556+1.329 ln(x). Means associated with a common letter are not significantly different (Tukey’s honest significant difference test, α=0.05) following significant (P<0.05) analysis of variance.
In experiment 5A, catches of male S. ocellana in traps baited with 1 mg sex pheromone lures were 6.2 times greater (F(2,16)=15.694, P<0.001) than male catches in traps baited with the standard AA+BN lure (Table 3). Adding benzyl nitrile to a septum loaded with sex pheromone, and in combination with an acetic-acid co-lure, reduced catches of male S. ocellana by 38%, relative to catches with sex pheromone alone (Table 3). In experiment 5A, total moth catch in traps baited with the ternary AA+BN-pheromone blend was significantly (F(2,16)=6.635, P=0.008) greater than total catch in traps baited with the binary AA+BN kairomone blend alone (Table 3).
Table 3 Influence of combining a sex pheromone (Ph) with a binary kairomone containing a blend of acetic acid (AA) and benzyl nitrile (BN) on catches of Spilonota ocellana moths in sticky white delta traps relative to their catches with sex pheromone or kairomone lures alone.

* AA co-lure is an 8-mL polypropylene vial with 3 mL of acetic acid in cotton balls sealed with a lid having a 3-mm aperture. In experiment 5A Ph and BN (10 mg) were on the same red septum and in experiment 5B they were on separate red septa.
† Mean male and total moth catch within an experiment followed by the same letters are not significantly different (Tukey’s honest significant difference test, α=0.05) following significant (P<0.05) analysis of variance. Female catches within each experiment are not significantly different (P>0.05) by analysis of variance.
In experiment 5B, catches of male S. ocellana in traps baited with a 0.01 mg female-equivalent sex pheromone lure were 5.3 times greater (F(2,22)=31.106, P<0.001) than male catches in traps baited with an AA+BN lure alone (Table 3). When 10 mg benzyl-nitrile lures were added to traps baited with separate 0.01 mg sex pheromone lures, but in combination with an acetic-acid co-lure, they caught 32% fewer male moths than traps baited with 0.01 mg of sex pheromone alone (Table 3). Also in experiment 5B, total moth catch in traps baited with separate lures containing AA+BN and sex pheromone, respectively, was significantly (F(2,22)=12.269, P=0.001) greater than total catch in traps baited with the AA+BN kairomone blend alone (Table 3).
In both experiments 5A and 5B, traps baited with AA+BN combined with sex pheromone of either dose (0.01 or 1.0 mg), on the same or separate septa, respectively, caught significantly more male S. ocellana than AA+BN alone (Table 3). Neither loading of sex pheromone, respectively, had any significant effect (experiment 5A: F(1,8)=1.501, P=0.255 and experiment 5B: F(1,11)=0.135, P=0.91) on catches of female S. ocellana with the AA+BN binary lures (Table 3).
In experiment 6, delta traps that had sticky-trap liners replaced weekly caught significantly more male (F(2,18)=5.694, P<0.017), female (F(2,18)=5.261, P=0.016), and total moths (F(2,18)=3.594, P<0.049) than two non-saturating-type bucket traps (Table 4). The presence of killing agents in experiment 7 had a significant effect on male (F(2,8)=8.853, P=0.009), female (F(2,8)=24.04, P<0.001), and total moth catch (F(2,8)=18.462, P=0.001) in Multipher-I traps (Table 4). Traps with a propylene glycol killing agent caught significantly more male (1.7 times), female (3.2 times), and total (2.4 times) S. ocellana than traps with Vapona (Table 4). In experiment 8, placing delta traps at different heights in the canopy had no significant effect on male (F(1,9)=0.618, P=0.442), female (F(1,9)=2.227, P=0.17), or total catches (F(1,9)=0.975, P<0.349) of S. ocellana (Table 4).
Table 4 Influence of various traps, killing agents and trap height on catches of Spilonota ocellana moths with binary lures of acetic acid (3 mL/3-mm open 8-mL polypropylene vial) and benzyl nitrile (10 mg/red rubber septum).
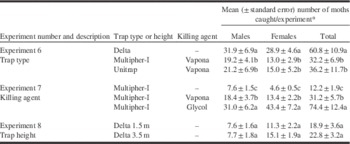
* Means within a column for experiments 6 and 7 followed by the same letter are not significantly (Tukey’s honest significant difference test, α=0.05) following significant (P<0.05) analysis of variance. Means within a column for experiment 8 are not significantly different (P>0.05) by analysis of variance.
Season-long weekly catches of S. ocellana in five organic apple orchards revealed a significant statistical interaction between the orchard plot treatment (control versus pheromone mating disruption) and the trap lures (pheromone versus kairomone) for both male (F(1,12)=8.110, P=0.016) and total moth catch (F(1,12)=8.307, P=0.015). This interaction occurs because traps baited with sex pheromone lures caught 10 times more male moths in control orchards than they did in pheromone-treated orchards, whereas kairomone-baited traps caught similar numbers of males across orchard treatments (Table 5). Given this interaction, moth catches in pheromone-baited versus kairomone-baited traps were compared in control and pheromone-treated plots separately. Sex pheromone-baited traps caught significantly (t=2.891, df=4, P=0.045) more male S. ocellana than kairomone-baited traps in control plots (Table 5), but in pheromone-treated plots, seasonal male catches with sex pheromone traps were not significantly different (t=0.918, df=4, P=0.411) than catches with kairomone-baited traps (Table 5).
Table 5 Catches of Spilonota ocellana moths during experiment 9 (1 May to 15 September 2016) in sticky white delta traps baited with either a sex pheromone or a kairomone lure when deployed in five-paired untreated (control) and pheromone-treated mating-disruption plots.

n=5 paired orchard plots.
* Mating-disruption plots were treated with 750 Isomate®-LR/ESBM [LRESX22] twin-tube dispensers/ha.
† Sex pheromone lures contained 1 mg of a 99:1 blend of Z-8-14:OAc and Z-8-14:OH (McBrien et al. Reference McBrien, Gries, Gries, Borden, Judd, King and Slessor1991). Kairomone lures contained 10 mg of benzyl nitrile on a red septum and 3 mL of acetic acid in a 3-mm open 8-mL polypropylene vial.
‡ Paired means within an orchard plot treatment and column followed by the same letter are not significantly different by two-tailed paired t-tests (P<0.05).
Mean weekly male and female catches with kairomone-baited traps were similar in control and pheromone-treated orchards, respectively (Fig. 3). Mean weekly total catches of S. ocellana in traps baited with sex pheromone or AA+BN lures showed similar seasonal patterns in both untreated and pheromone-treated orchards (Fig. 3).
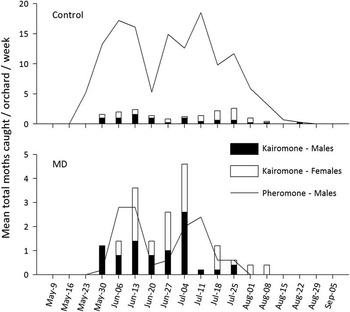
Fig. 3 Mean weekly catches of Spilonota ocellana in sticky white delta traps baited with sex pheromone (1 mg) or kairomone lures (3 mL of acetic acid in 3-mm open 8-mL polypropylene vial and a rubber septum with 10 mg of benzyl nitrile) when hung in five paired untreated (control) or pheromone-treated mating-disruption (MD)orchards in 2016. Note differences in y-axis scales on each graph panel. Mating-disruption plots were treated with 750 Isomate®-LR/ESBM [LRESX22] twin-tube dispensers/ha.
Discussion
Our studies have confirmed that a binary lure releasing acetic acid and benzyl nitrile attracts both male and female S. ocellana, and this combination lure is more attractive than either component alone. Experiment 1 clearly showed that 10 mg of benzyl nitrile alone is not attractive when released from a rubber septum. In this study benzyl-nitrile-baited traps caught no more moths than a blank trap, whereas acetic acid was confirmed to be a weak attractant (Knight et al. Reference Knight, Hilton, Basoalto and Stelinski2014). These findings are consistent with the role acetic acid often plays in the chemical ecology of many insects. Acetic acid alone attracts several groups of flies (Diptera: Drosophilidae and Tephritidae) (Keiser et al. Reference Keiser, Jacobson, Nakagawa, Miyashita and Harris1976; Zhu et al. Reference Zhu, Park and Baker2003; Becher et al. Reference Becher, Bengtsson, Hansson and Witzgall2010) and moths (Lepidoptera: Noctuidae, Pyralidae, and Tortricidae) (Landolt Reference Landolt2000, Reference Landolt2005; Knight et al. Reference Knight, Hilton, Basoalto and Stelinski2014). More frequently, however, acetic acid has been shown to enhance attraction of chemicals, including benzenoids, that exhibit low levels of activity when presented alone (Landolt Reference Landolt1998; Landolt et al. Reference Landolt, Suckling and Judd2007, Reference Landolt, Tóth, Meagher and Szarukán2013; Tóth et al. Reference Tóth, Szentkirályi, Vuts, Letardi, Tabilio, Jaastad and Knudsen2009; Becher et al. Reference Becher, Bengtsson, Hansson and Witzgall2010; Jones et al. Reference Jones, Horton, Mills, Unruh, Baker and Melton2015; El-Sayed et al. Reference El-Sayed, Knight, Byers, Judd and Suckling2016). The synergism between acetic acid and benzenoids often involves volatiles emitted by fruit and other sweet baits (e.g., 2-phenylethanol) and is explained by some authors as facilitating the orientation of insects that feed on fermenting materials (Zhu et al. Reference Zhu, Park and Baker2003; Landolt et al. Reference Landolt, Suckling and Judd2007; Becher et al. Reference Becher, Bengtsson, Hansson and Witzgall2010; Tóth et al. Reference Tóth, Landolt, Szarukán, Szólláth, Vitányi and Pénzes2012; Jósvai et al. Reference Jósvai, Koczor and Tóth2016). While a food-finding hypothesis of attraction might explain in part why so many different species of moths were caught in traps baited with acetic acid and 2-phenylethanol (El-Sayed et al. Reference El-Sayed, Knight, Byers, Judd and Suckling2016), field evidence that adult tortricids feed is lacking. Explaining the response of adult S. ocellana to acetic acid and benzyl nitrile as a food-finding response is problematic because to the best of our knowledge benzyl nitrile has not been found in fermenting pome fruits or sweet baits. Clearly, benzenoid compounds like benzyl nitrile and 2-phenylethanol are an interesting group of natural chemicals that elicit varied behaviours in different contexts, including flower feeding by some butterflies (Ômura et al. Reference Ômura, Honda and Hayashi1999). More work on feeding and host-selection behaviour in S. ocellana will be needed to understand its behavioural and ecological response to AA+BN.
Using membrane dispensers we confirmed that AA+BN is a more attractive binary lure for S. ocellana than AA+PET. In contrast, two sympatric apple leafrollers, Choristoneura rosaceana (Harris) and Pandemis limitata (Robinson) (Lepidoptera: Tortricidae), were caught significantly more often with AA+PET, than with AA+BN (El-Sayed et al. Reference El-Sayed, Knight, Byers, Judd and Suckling2016). Combining AA and these two benzenoid compounds into a single dispenser or trap could be useful for developing multispecies mass-trapping or monitoring systems for sympatric tortricid species. Experiments with Trécé membrane dispensers suggest this may be feasible. For example, a membrane dispenser containing an AA-BN binary mixture (TRE-1378) caught as many moths as did the AA+BN treatment with an acetic-acid co-lure+separate benzyl-nitrile lure (TRE-1381). More interestingly, traps baited with an AA+BN-PET ternary blend consisting of an acetic-acid co-lure and a membrane dispenser with a mixture of BN-PET (TRE-1379) caught more total moths than any other lure. These results are intriguing and further research combining multiple kairomone components into a single lure, if not trap, in an effort to develop multispecies trapping tools seems warranted.
Although our AA+BN lure is not fully optimised, we found no evidence to suggest that an AA+BN kairomone is as attractive as a sex pheromone lure (El-Sayed et al. Reference El-Sayed, Knight, Byers, Judd and Suckling2016). In this study we found AA+BN lure was significantly less attractive to male S. ocellana than a female-equivalent sex pheromone lure. This current result is surprising given our lure had a loading that was at least 100 times lower and likely less attractive (McBrien et al. Reference McBrien, Gries, Gries, Borden, Judd, King and Slessor1991), than the commercial lures used by El-Sayed et al. (Reference El-Sayed, Knight, Byers, Judd and Suckling2016). It should be noted that the apple orchard in which El-Sayed et al. (Reference El-Sayed, Knight, Byers, Judd and Suckling2016) conducted their 2015 study had been treated with Isomate-LR/ESBM pheromone dispensers in 2014 (G.J., unpublished data). When left in the orchard as they were, these pheromone dispensers can release a significant amount of S. ocellana sex pheromone the following year (Porcel et al. Reference Porcel, Sjöberg, Swiergiel, Dinwiddie, Rämert and Tasin2014). The sex pheromone catches (≈6.5 moths/trap) reported by El-Sayed et al. (Reference El-Sayed, Knight, Byers, Judd and Suckling2016) were similar to what we found in our pheromone-treated plots (Table 5). It seems likely that sex pheromone trap catches reported by El-Sayed et al. (Reference El-Sayed, Knight, Byers, Judd and Suckling2016) were partially disrupted by the presence of mating-disruption pheromone.
Host-plant volatiles can have both positive (Schmidt-Büsser et al. Reference Schmidt-Büsser, von Arx and Guerin2009; Varela et al. Reference Varela, Avilla, Anton and Gemeno2011; von Arx et al. Reference von Arx, Schmidt-Busser and Guerin2012) and negative effects (Reddy and Guerrero Reference Reddy and Guerrero2004) on the sex pheromone responses of male tortricids. We found that the addition of AA+BN to the sex pheromone of S. ocellana significantly reduced male moth catch relative to sex pheromone alone. Likewise, Knight et al. (Reference Knight, El-Sayed, Judd and Basoalto2017) found that both benzyl nitrile and 2-phenylethanol, but without acetic acid, significantly reduced catches of C. rosaceana in sex pheromone-baited traps. An elegant study on Spodoptera littoralis Boisduval (Lepidoptera: Noctuidae) showed that some herbivore-induced plant volatiles can suppress olfactory signalling pathways and inhibit sexual behaviours in male and female moths (Hatano et al. Reference Hatano, Saveer, Borrero-Echeverry, Strauch, Zakir and Bengtsson2015). All of these results contradict an argument that male S. ocellana respond to caterpillar-induced benzyl nitrile and 2-phenylethanol to increase mate finding. Alternatively, catches of males in AA+BN-baited traps may be a response to previously trapped females (El-Sayed et al. Reference El-Sayed, Knight, Byers, Judd and Suckling2016). Nevertheless, from an applied perspective, catches of male S. ocellana in traps baited with the ternary AA+BN+pheromone blend still caught more males than traps baited with AA+BN alone. This result, and more importantly, the fact that sex pheromone had no effect on catches of female S. ocellana in traps baited with AA+BN, means combining these different semiochemicals into a single dispenser or trap may be beneficial to mass-trapping or attract-and-kill technologies.
Evaluating different traps is important for developing trapping systems using kairomones because little is known about olfactory-guided search of female moths or their response and willingness to enter traps. Some moth species, like Synanthedon myopaeformis (Borkhausen) (Lepidoptera: Sesiidae), are reluctant to enter kairomone-baited bucket traps (Tóth et al. Reference Tóth, Landolt, Szarukán, Szólláth, Vitányi and Pénzes2012) that are otherwise highly effective when baited with sex pheromone (Judd and Eby Reference Judd and Eby2014). Catches of S. ocellana in kairomone-baited bucket traps were significantly lower than similarly baited delta traps, but their aversion was not complete. Delta traps are suitable for monitoring S. ocellana with kairomones but the need to regularly replace sticky liners makes them impractical for mass trapping. Both of the non-saturating traps tested in this study could be useful for mass trapping male and female S. ocellana. To maximise their effect these non-saturating traps need a killing agent. Propylene glycol was a superior killing agent to Vapona, but unfortunately even the food-grade material we used is not acceptable under current organic regulations (www.omri.org).
One of our main objectives for developing kairomone-based traps for S. ocellana was to provide a tool for monitoring females within the context of pheromone mating disruption. Unfortunately, seasonal catches of S. ocellana in AA+BN-baited delta traps in untreated and pheromone-treated plots were disappointingly low compared with catches in all of the lure and trap-development experiments. This result likely reflects population differences among orchards chosen for various trials, but there is concern that our AA+BN lures did not attract enough female moths to allow managers to track their seasonal phenology or immigration into pheromone-treated orchards. However, AA+BN lures provided remarkably similar catches of male and female S. ocellana in untreated and pheromone-treated apple plots. The combined catches of males and females with AA+BN lures were higher than male catches with sex pheromone in the pheromone-treated orchards. Weekly total catches with AA+BN lures versus sex pheromone lures revealed that in nine of the 11 weeks when S. ocellana moths were caught in pheromone-treated plots, catches with AA+BN were equal to or greater than catches with sex pheromone. At no time did the AA+BN lures fail to catch moths when sex pheromone traps caught moths. Given a 1:1 sex ratio in catches with AA+BN, total moth catch can probably be used to develop useful thresholds or measure immigration threats.
Development of kairomone-based trapping technologies for management of S. ocellana and a suite of sympatric tortricid moths in organic apples looks promising. Discovery that acetic acid synergises aromatic benzenoids produced by caterpillar feeding on apples leaves (El-Sayed et al. Reference El-Sayed, Knight, Byers, Judd and Suckling2016; Giacomuzzi et al. Reference Giacomuzzi, Cappellin, Khomenko, Biasioli, Schutz and Tasin2016), has already uncovered effective lures for many leaf-feeding tortricid species in Europe, New Zealand, and North America. For S. ocellana specifically, mass trapping with AA+BN requires development of long-lasting commercial release devices, with the possible addition of related aromatic compounds like 2-phenylethanol, and an organically acceptable killing agent or trap. Future work should also explore whether volatiles from microbial fermentation, other than acetic acid, might synergise these aromatic compounds or other host–plant volatiles.
Acknowledgements
The authors thank Mark Gardiner and Kandace Zurowski-Tiffin for technical assistance and their preparation of lures. Tamara Richardson was instrumental in locating organic orchards for the mating-disruption trials. The authors thank Ron Schneider, Godfrey Sellmer, and Andrea Turner for allowing to conduct research in their orchards. Thanks are also due to Bill Lingren, Trécé Incorporated, Adair, Oklahoma, United States of America, for donating some of the dispensers used in the studies. This project was supported with partial funding from the Washington Tree Fruit Research Commission, Wenatchee, Washington, United States of America.










