Introduction
Adult nutrition regulates insect reproductive potential (Carey et al., Reference Carey, Harshman, Liedo, Müller, Wang and Zhang2008; Gullan & Cranston, Reference Gullan and Cranston2014). Among macronutrients, proteins are essential for both male and female sexual maturation as well as for egg production (Fischer et al., Reference Fischer, O'Brien and Boggs2004; Grandison et al., Reference Grandison, Piper and Partridge2009; Kouloussis et al., Reference Kouloussis, Damos, Ioannou, Tsitsoulas, Papadopoulos and Nestel2017). Lipids and carbohydrates are also relevant as the former are involved in ovary development (Ziegler & Van Antwerpen, Reference Ziegler and Van Antwerpen2006; Arrese & Soulages, Reference Arrese and Soulages2010) and the latter are the main energy source (Teal et al., Reference Teal, Gavilanez-slone and Dueben2004; Cheng et al., Reference Cheng, Chen, Yi, Liang and Xu2014). Micronutrients, especially certain vitamins and minerals, also modulate fecundity (Geister et al., Reference Geister, Lorenz, Hoffmann and Fischer2008). Thus, the availability of the required nutrients in the adult food influences the reproductive potential of most insect species (Jervis et al., Reference Jervis, Boggs and Ferns2005, Reference Jervis, Ellers and Harvey2008; Bauerfeind et al., Reference Bauerfeind, Fischer, Hartstein, Janowitz and Martin-Creuzburg2007; Taylor et al., Reference Taylor, Perez-Staples, Weldon, Collins, Fanson, Yap and Smallridge2011). However, the extent of such impact will depend on species-specific factors such as adult lifespan, nutrients acquired during the immature phase, and the dynamics of gonadal development.
In order to get the optimum levels of each nutrient, insects regulate ingestion reaching an intake target from single or complementary sources (sensu Raubenheimer & Simpson, Reference Raubenheimer and Simpson1993). The ratio at which nutrients are consumed to maximize a given life-history trait (resource congruence) can be threatened if certain nutrient conditions the ingestion and metabolism of others (Bauerfeind & Fischer, Reference Bauerfeind and Fischer2005; Geister et al., Reference Geister, Lorenz, Hoffmann and Fischer2008; Lee et al., Reference Lee, Simpson, Clissold, Brooks, Ballard, Taylor, Soran and Raubenheimer2008). For example, protein regulates the intake of carbohydrates. In diets with high amounts of protein, carbohydrates are ingested below the optimum level, whereas in diets with low amounts of protein, carbohydrates are ingested in excess (Simpson & Raubenheimer, Reference Simpson and Raubenheimer1995; Nestel et al., Reference Nestel, Papadopoulos, Pascacio-Villafán, Righini, Altuzar-Molina and Aluja2016). Additionally, protein modulates lipid metabolism (Arrese & Soulages, Reference Arrese and Soulages2010). These processes are associated with the nutritional content of the individual and modulate the way in which resources are allocated (Leitão-Gonçalves et al., Reference Leitão-Gonçalves, Carvalho-Santos, Francisco, Fioreze, Anjos, Baltazar, Elias, Itskov, Piper and Ribeiro2017).
Among insect crop pests, the Tephritidae family comprises several species of great economic importance ranging from monophagous to extremely polyphagous (Christenson & Foote, Reference Christenson and Foote1960). In nature, adults feed on different sources such as honeydew, nectar, and bird drops (Aluja et al., Reference Aluja, Piñero, Jácome, Díaz-Fleischer, Sivinski, Aluja and Norrbom2000; Drew & Yuval, Reference Drew, Yuval, Aluja and Norrbom2000), suggesting they reach their optimum nutrient balance from complementary food sources. Given the great economic impact of these pests and the need to reduce insecticide use, several species are being suppressed with the sterile insect technique (SIT). SIT relies on the mass rearing and release of sterile males that compete against wild males for wild females (Knipling, Reference Knipling1959). Transfer of sterile sperm to fertile females results in no offspring and, consequently, in a reduction of the target population (Dyck et al., Reference Dyck, Hendrichs and Robinson2006). For species such as the South American fruit fly, Anastrepha fraterculus (Wiedemann), SIT implementation has been highly encouraged (Ortiz, Reference Ortiz1999). Since then, its taxonomic status, reproductive biology (Cladera et al., Reference Cladera, Vilardi, Juri, Paulin, Giardini, Gómez Cendra, Segura and Lanzavecchia2014; Hernández-Ortiz et al., Reference Hernández-Ortiz, Canal, Salas, Ruíz-Hurtado and Dzul-Cauich2015; Juárez et al., Reference Juárez, Devescovi, Břízová, Bachmann, Segura, Kalinová, Fernández, Ruiz, Yang, Teal, Cáceres, Vreysen, Hendrichs and Vera2015), sterilization doses (Allinghi et al., Reference Allinghi, Gramajo, Willink and Vilardi2007; Mastrangelo et al., Reference Mastrangelo, Parker, Jessup, Pereira, Orozco-Dávila, Islam, Dammalage and Walder2010), and mass-rearing protocols (Vera et al., Reference Vera, Abraham, Oviedo and Willink2007; Reference Vera, Oviedo, Abraham, Ruiz, Mendoza, Chang and Willink2014) have been extensively assessed. Mass-rearing protocols are among the major factors involved in SIT development and production of high-quality flies at the lowest cost is desirable. One of the parameters that rearing protocols seek to maximize is fecundity. In this sense, hydrolyzed yeast is the standard protein source for the adult diet at most tephritid mass-rearing facilities. However, this ingredient is one of the most expensive supplies (Nemny-Lavy & Nestel, Reference Nemny-Lavy and Nestel2014).
Cost reduction of adult diets can be achieved with cheaper yeast derivatives, other ingredients or by reducing the amount of hydrolyzed yeast without comprising fecundity. For example, Morelli et al. (Reference Morelli, Zamboni Costa, Martins Fagioni, Zamboni Costa, Do Nascimento, Meirelles de Azevedo Pimentel and Melges Walder2012) evaluated yeast extract supplemented with wheat germ in A. fraterculus adult diet with promising results. Likewise, Liendo et al. (Reference Liendo, Devescovi, Bachmann, Utgés, Abraham, Vera, Lanzavecchia, Bouvet, Gómez-Cendra, Hendrichs, Teal, Cladera and Segura2013), evaluated brewer's yeast, significantly cheaper than hydrolyzed yeast, as a pre-release protein supply for males. However, its impact on female fecundity remains unknown. In addition, other ingredients, such as wheat germ or its derivatives satisfy certain nutritional requirements of several insect species (Cohen, Reference Cohen2004). In fruit flies, wheat germ oil has been studied as a supply in the larval rearing medium (Chang et al., Reference Chang, Vargas, Caceres, Jang and Cho2006, Reference Chang, Coudron, Goodman, Stanley, An and Song2010, Reference Chang, Coudron, Goodman and Stanley2012, Chang & Vargas, Reference Chang and Vargas2007; Vera et al., Reference Vera, Oviedo, Abraham, Ruiz, Mendoza, Chang and Willink2014). However, the direct impact of incorporating wheat germ into the adult diet on female reproduction has been poorly investigated (Chang, Reference Chang2009; Morelli et al., Reference Morelli, Zamboni Costa, Martins Fagioni, Zamboni Costa, Do Nascimento, Meirelles de Azevedo Pimentel and Melges Walder2012). Cost-effective diets can also be obtained by determining the optimal protein:sucrose ratio. Low protein ratios result in low fecundity (Lee, Reference Lee2015), whereas high protein ratios maximize offspring and egg production (Lee et al., Reference Lee, Simpson, Clissold, Brooks, Ballard, Taylor, Soran and Raubenheimer2008; Fanson & Taylor, Reference Fanson and Taylor2012; Jensen et al., Reference Jensen, McClure, Priest and Hunt2015) but are disadvantageous in terms of adult survival (Fanson et al., Reference Fanson, Weldon, Pérez-Staples, Simpson and Taylor2009; Lee, Reference Lee2015). Most fruit fly mass-rearing facilities use a 1:3 (protein:sucrose) ratio for the adult diet. However, adopting this hydrolyzed yeast-based diet at this ratio without considering the nutritional requirements of each particular species may result in an unnecessarily expensive diet (Pascacio-Villafán et al., Reference Pascacio-Villafán, Williams, Sivinski, Birke and Aluja2015).
Because A. fraterculus oogenesis takes place during the adult stage, mass-rearing facilities should provide the females a diet with a protein source that maximizes egg production at the lowest cost. Here we evaluated the effect of three yeast derivatives and the addition of wheat germ in the adult diet on A. fraterculus fecundity. Then, we evaluated the effect of decreasing the amount of the widely used protein source (hydrolyzed yeast) on ovary maturation, fecundity, and fertility. Later, we evaluated the effect of increasing the amount of the cheapest protein source (brewer's yeast) and wheat germ on the same variables. Finally, we explored the association between adult diets of different composition and the nutrient content of females and its possible correlation with female age and reproductive condition.
Materials and methods
Insects
Flies were obtained as pupae from the artificial rearing kept at IGEAF (CICVyA, INTA, Argentina). Emerging flies were sorted by sex. Males were housed in 12-l plastic containers (300 individuals per container) and fed on a solid diet containing hydrolyzed yeast and sucrose in a 1:3 ratio. Females were housed in 1-l plastic containers (15 individuals per container) and offered the corresponding diet (see below). Water was supplied in plastic containers with a moistened cloth. Both sexes were kept at 24 ± 2 °C, 70 ± 10% RH and a 12:12 h light: dark photoperiod.
Diets
Three experiments were performed (table 1) involving one of three yeast derivatives, hydrolyzed yeast (MP Biomedicals®, Ohio, USA), yeast extract (Bionis® YE MF, São Paulo, Brazil) and brewer's yeast (Calsa®, Tucumán, Argentina), in combination with sucrose and wheat germ (Gran Diet, Buenos Aires, Argentina) at different ratios. The amino acid profile of the ingredients, as well as other aspects of their chemical composition (table 2), was obtained from the suppliers.
Table 1. Yeast derivatives, sucrose (S), and wheat germ (WG) ratios in diets prepared with hydrolyzed yeast (MP Biomedicals®, HY), yeast extract (Bionis® YE MF, YE), or brewer's yeast (CALSA®, BY) according to the experiment.

Table 2. Content of amino acids, vitamins, and minerals for hydrolyzed yeast (MP Biomedicals®), yeast extract (Bionis® YE MF), brewer's yeast (CALSA®), and wheat germ denoted as HY, YE, BY, and WG, respectively.
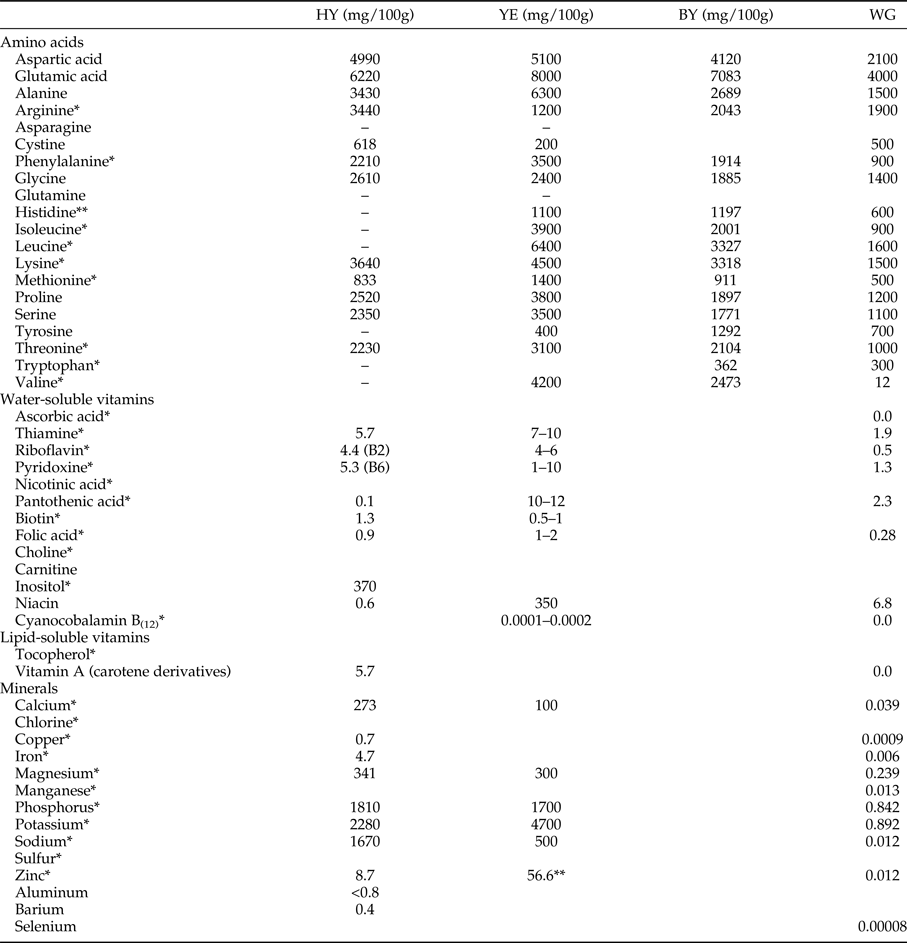
*Nutrients useful or essential to insects according to Cohen, 2004; **ppm.
Vitamin and mineral contents are not available for yeast extract and brewer's yeast.
Diet preparation and dispensing
Ingredients were blended with a mixer (Philips, HR 1364) and stored in a refrigerator until used. Daily, 3 g of diet were diluted in 30 ml of water and vigorously mixed to obtain a homogeneous blend. Small pieces of cotton were imbibed in one of the different diets and randomly assigned to one of the groups of females.
Experiments
Experiment I
Three yeast derivatives (hydrolyzed yeast, brewer's yeast, and yeast extract) were evaluated. Each one of them was combined either with sucrose or with sucrose and wheat germ (table 1). Newly emerged females (60) were provided a given diet for 12 days and then they were offered males to freely copulate. Once copulation finished, five mated females (of the same diet) were placed in 1-l plastic containers (five per diet) with the corresponding diet and water. The container was considered a replicate and sealed with a piece of voile cloth. A green colored agar disc was placed on top of the voile as oviposition substrate, which was replaced every 48 h throughout a period of 12 days. Eggs laid were counted under a stereoscopic microscope (10×). Female daily fecundity was estimated by dividing the number of eggs by the number of alive females in the container.
Experiment II
We evaluated the effect of adult diets with different ratios of hydrolyzed yeast (table 1) on the dynamic of ovary maturation, fecundity, fertility, and nutrient content. Ovary maturation was assessed as follows: groups of 12 females were distributed in 1-l plastic containers (15 per diet) with the corresponding diet over a period of 12 days in which 10 females per diet were dissected daily and the number of mature oocytes in each ovary recorded. Percentage of sexually mature females was estimated as follows: number of females with at least one mature oocyte (egg)/total number of analyzed females × 100. Fecundity was estimated following the methodology described in experiment I with the exception that eggs were collected for the first of 4 days of oviposition rather than during 12 days. To evaluate fertility (egg hatch), one sample of 20 eggs per replicate was taken (totalizing 100 eggs per diet) and placed in a moist chamber to record the number of unhatched eggs after 5 days. Nutrient content was determined for (1) newly emerged females, (2) 10-day-old sexually mature virgin females (same age at which their sisters copulated), and (3) 14-day-old mated females (after 4 days of laying eggs) following standard biochemical techniques. Protein content was determined with the Bradford method (Bradford, Reference Bradford1976) using Coomassie Brillant Blue G-250 reagent (Biopack®, Buenos Aires, Argentina). Lipid and carbohydrate contents were determined with the Van Handel method (Van Handel, Reference Van Handel1965). Total sugar and glycogen contents were measured with anthrone reagent (Cicarelli, Santa Fe, Argentina), whereas lipid content was measured with vanillin reagent (Sigma-Aldrich, St. Louis, USA). Optical density was measured on ZL-5100 Zeltee Spectrophotometer. Five replicates with 10 individuals each were evaluated.
Experiment III
We evaluated the effect of adult diets with different ratios of brewer's yeast and wheat germ (table 1) on the dynamic of ovary maturation, fecundity, fertility, and nutrient content. Procedures followed those of experiment II.
Data analysis
Data analysis involved finding the model for which the data best fitted. For count data, we first explored generalized linear models (GLMs) using the Poisson distribution with the logit link function for fecundity (number of eggs laid) and the binomial distribution with the logit link function for fertility (egg hatch). Model fit was assessed by examining the ratio of the deviance and its degrees of freedom. When deviance was far in excess for the degrees of freedom (more than 2.5), showing a poor fit of the model (Faraway, Reference Faraway2006; Di Rienzo et al., Reference Di Rienzo, Machiavelli and Casanoves2017b), linear models (LMs) were explored with non-transformed data. Standard model checks were used to verify normality (Q–Q plot) and homogeneity of standardized residuals (residuals’ plot) (Crawley, Reference Crawley2007). In cases of heteroscedasticity, the shape of the residual's plot allowed selecting the best variance function to apply to the model (Pinheiro & Bates, Reference Pinheiro and Bates2000). Multiple comparisons were performed with Di Rienzo, Guzmán & Casanoves (DGC) test (Balzarini et al., Reference Balzarini, Gonzalez, Tablada, Casanoves, Di Rienzo and Robledo2008). Analyses were performed with the R interface of Infostat (Di Rienzo et al., Reference Di Rienzo, Casanoves, Balzarini, Gonzalez, Tablada and Robledo2017a). We describe below the model chosen for each variable.
Fecundity. When GLMs using the Poisson distribution was built to analyze the number of eggs laid per female, deviance was far greater than the degrees of freedom (deviance/df = 649/24, 45/12, and 88/20 for experiments I, II, and III; respectively), showing over dispersion of the data. So, for experiment I we built a general LM with yeast derivative and the presence of wheat germ as fixed factors. Given the fan-shaped form of the residuals, deviation from heteroscedasticity was accounted with the power variance function [weights = varPower(.), reml R package]. For experiment II, we first built a LM with diet as fixed factor and residuals took the same shape as for experiment I. So, we applied the same variance function [weights = varPower(.), reml R package]. In experiment III, variances were homogeneous, and therefore a LM with no variance function was built to compare fecundity of females fed the different diets.
Ovary maturation. The impact of the diet on ovary maturation (experiments II and III) was evaluated in 2 ways. First, we determined the impact of the diet on the maturation time (MT) of the ovaries by estimating the time at which 50 and 95% of females had at least one mature oocyte in their ovaries (MT50 and MT95, respectively) by means of a dose–response analysis with POLO Plus V1 (LeOra Software, 2002–2003). Second, we compared the number of mature eggs in the ovary of females at the respective MT50 and MT95 obtained for each diet. To do so, we explored different models as described above. The GLMs with a Poisson distribution showed a poor fit in experiments II (deviance/df = 43/8 for MT50 and 375/17 for MT95) and III (deviance/df = 414/24 for MT50 and 469/43 for MT95). So, a LM was built for mature eggs at MT50 and MT95 in experiment II with diet as the fixed factor. In experiment III, a general LM was built for mature eggs at both MT50 and MT95, with diet as fixed factor and the varIdent variance function [weights = varComb(varIdent(form = ~1|Diet))] to correct heteroscedasticity.
Fertility. A GLM using the binomial distribution with the logit link function was used to analyze egg hatch in experiments II, with diet as a fixed factor. However, in experiment III deviance were far in excess for the degrees of freedom (deviance/df = 69/20), showing a poor fit of the model (Di Rienzo et al., Reference Di Rienzo, Machiavelli and Casanoves2017b). As a consequence, a general LM with diet as a fixed factor and the varIdent variance function [weights = varComb(varident(form = ~1|Diet))] to correct heteroscedasticity was built.
Nutrient content. A general LM was used for each nutrient (protein, lipid, carbohydrates, and glycogen) and each fruit fly age to evaluate the impact of the diet on nutrient content. Heteroscedasticity was corrected by the power variance function [weights = varPower(.), reml R package].
Results
Experiment I
The number of eggs laid per female differed according to the yeast derivative (F = 31.96; df = 2, 24; P < 0.001) and the addition of wheat germ in the diet (F = 9.23; df = 1, 24; P = 0.004), with no significant interaction between both factors (F = 1.58; df = 2, 24; P = 0.226) (fig. 1). Females given hydrolyzed yeast and yeast extract laid significantly more eggs (139.7 ± 14.3 for hydrolyzed yeast and 170.1 ± 26.0 eggs for yeast extract) than females given brewer's yeast (29.3 ± 7.9 eggs). The addition of wheat germ increased the number of eggs laid. Females fed on diets with wheat germ almost doubled the number of eggs laid by females fed diets without wheat germ (145.8 ± 17.7 vs. 80.3 ± 10.3 eggs per female).
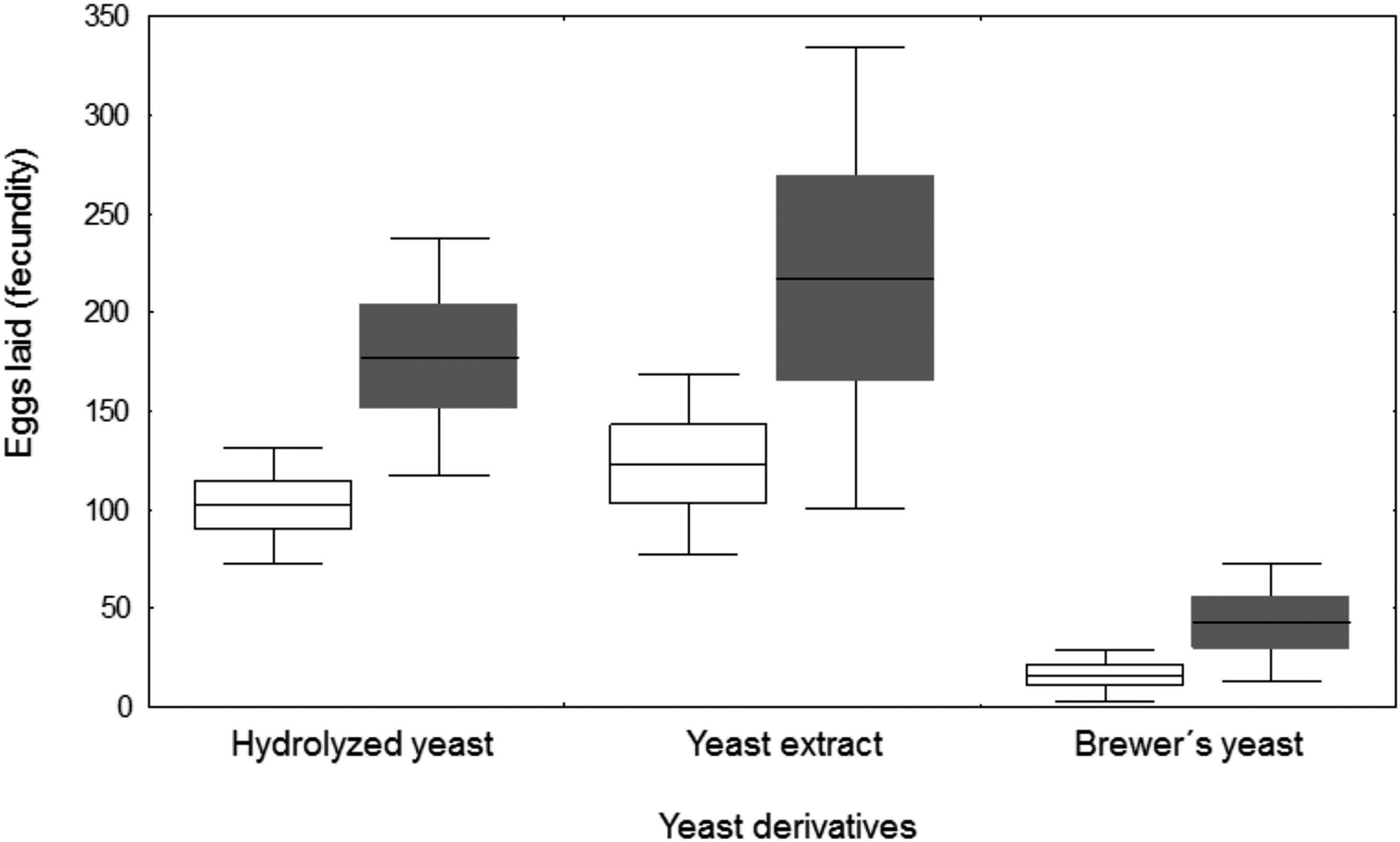
Fig. 1. Effect of yeast derivatives and the addition of wheat germ (gray) in the adult liquid diet on the number of eggs laid per female (fecundity) of Anastrepha fraterculus throughout a period of 12 days. Boxes denote means values ± SE and bars, means values ± SD.
Experiment II
MT and average mature eggs in the ovary
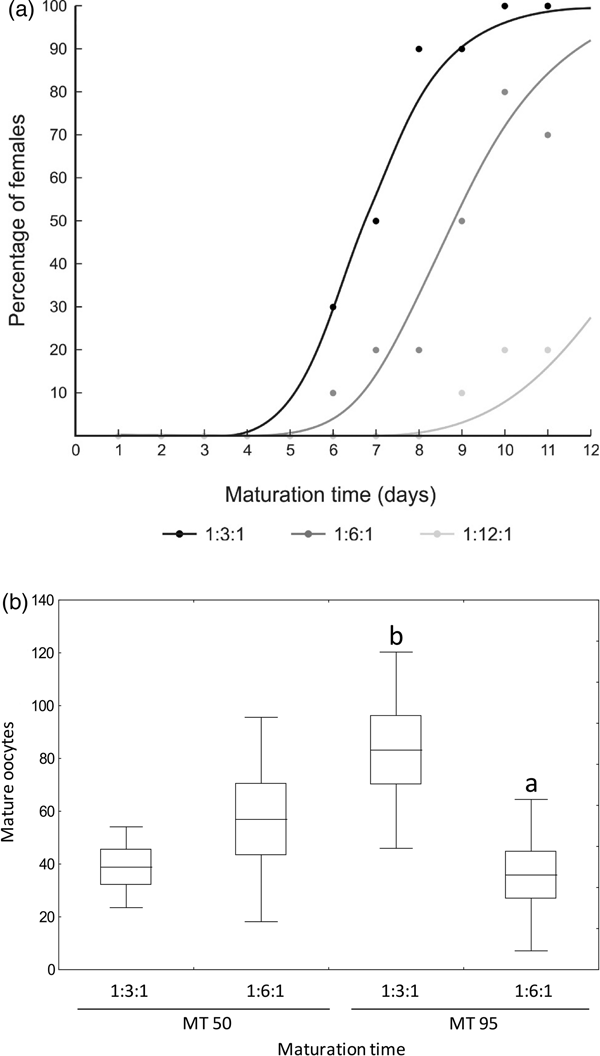
The age at which females showed mature eggs in their ovary changed according to the hydrolyzed yeast ratio provided to adults (fig. 2a). MT of females fed the concentrated (1:3:1) diet was lower than that of females fed the less concentrated (1:6:1) diet (table 3). Less than 20% of A. fraterculus females fed the yeast-reduced diet (1:12:1) showed mature eggs during the experimental period; therefore, this diet was not included in the following analysis. The average number of mature eggs in the ovary at MT50 was similar for 1:3:1 and 1:6:1 diets (F = 70.48; df = 1, 8; P = 0.870). However, the number of mature eggs at MT95 differed between diets (F = 11.52; df = 1, 17; P = 0.003). Females fed 1:3:1 diet showed more mature eggs in their ovary than females fed 1:6:1 diet (fig. 2b).
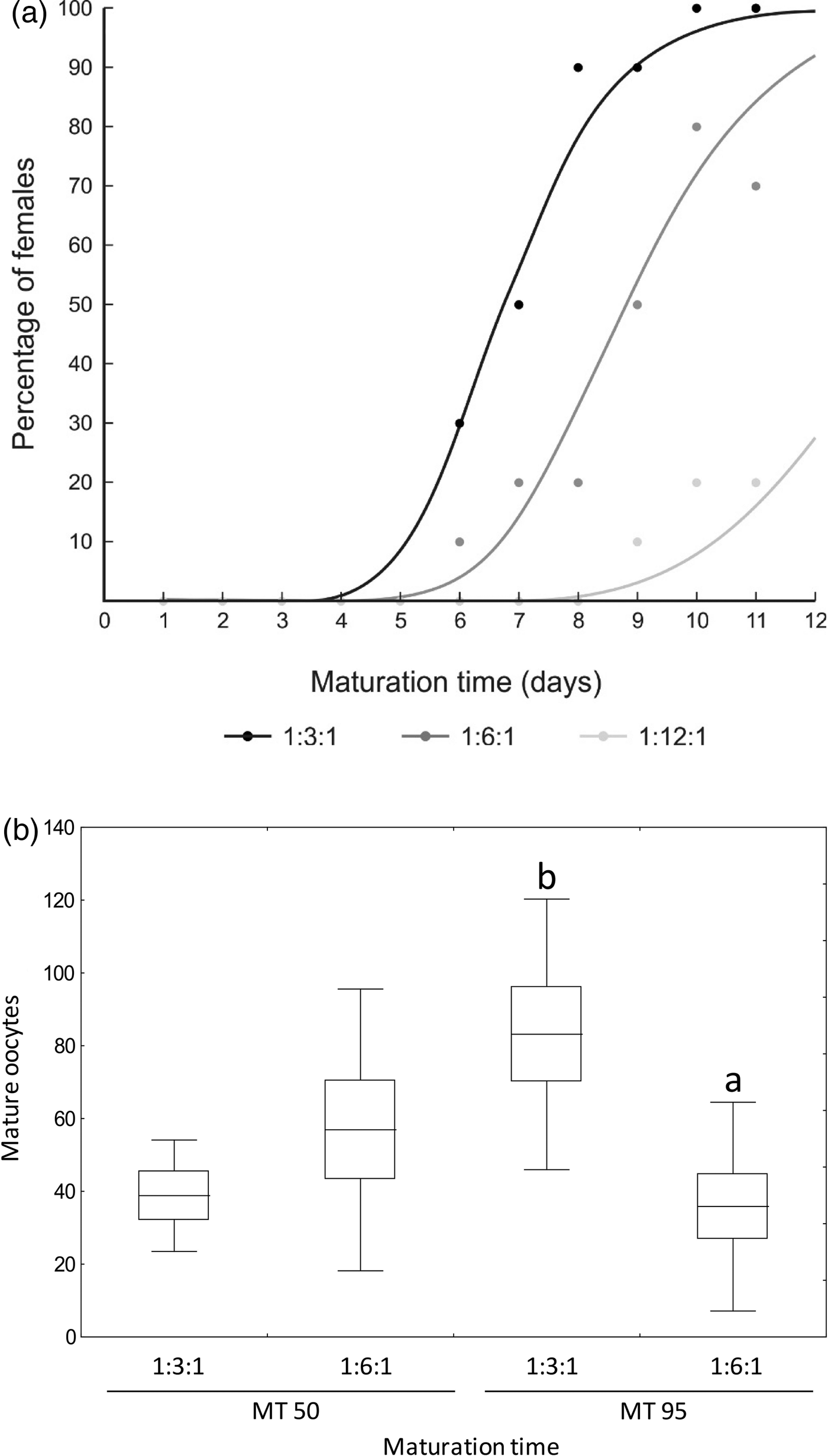
Fig. 2. Time when 50% (MT50) and 95% (MT95) of Anastrepha fraterculus females showed mature eggs (a), and average number of mature eggs in the ovary (b) of single females fed diets made with different hydrolyzed yeast (MP Biomedicals®), sucrose, and wheat germ ratios. See table 3 for statistical analysis with confidence limits of MT50 and MT95. Different letters for each maturation time in (b) denote significant differences between diets. Boxes denote means values ± SE and bars, means values ± SD.
Table 3. Maturation time (MT50 and MT95) of Anastrepha fraterculus females fed adult liquid diet made with different hydrolyzed yeast (MP Biomedicals®, HY), sucrose (S), and wheat germ (WG) ratios and χ2 calculated by the maximum-likelihood method to assess model fit (P > 0.05).

CL, Confidence limits.
Different letters in a same column denote significant differences (P < 0.05) and values in brackets represent confidence intervals.
Fecundity and fertility
The number of eggs laid per females differed according to the hydrolyzed yeast ratio (F = 49.38; df = 2, 12; P < 0.001). Females fed a more concentrated diet (1:3:1) laid significantly more eggs than females fed 1:6:1 diet (fig. 3). The lowest fecundity values were recorded in females fed yeast-reduced diet (1:12:1) (fig. 3). Egg hatch of A. fraterculus females fed hydrolyzed yeast diet ranged from 65 to 100% and was not affected by the ratio of hydrolyzed yeast provided (F = 0.001; df = 1, 8; P > 0.999) (fig. 3).

Fig. 3. Fecundity (left) and fertility (right) of Anastrepha fraterculus fed adult liquid diets made with different hydrolyzed yeast (MP Biomedicals®, HY), sucrose (S), and wheat germ (WG) ratios. Different letters denote significant differences (DGC, α 0.05). Boxes denote means values ± SE and bars, means values ± SD.
Nutrient content
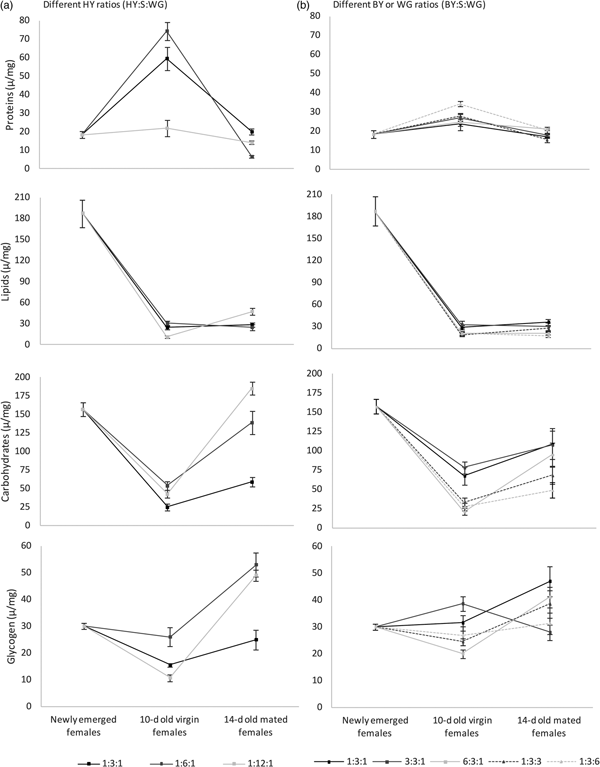
Nutrient content changed according to the hydrolyzed yeast:sucrose ratio provided in the diet (fig. 4a). Protein content of 10-day-old virgin females fed 1:3:1 and 1:6:1 diets was higher than that of females fed the 1:12:1 diet (F = 26.45; df = 2, 26; P < 0.001). For mated females, the protein was higher in females fed 1:3:1 diet compared with females fed 1:6.1 (F = 41.06; df = 2, 27; P < 0.001). These values were close to those showed by recently emerged females. Lipid content in 10-day-old virgin females changed according to the diet (F = 18.88; df = 2, 26; P < 0.001); with higher values in females fed 1:3:1 and 1:6:1 diets. In contrast, for 14-day-old mated females, higher lipid contents were recorded in females fed the protein-reduced 1:12:1 diet than the other diets (F = 7.17; df = 2, 27; P = 0.041). However, these values did not equal lipids of recently emerged females. Carbohydrate content was higher in 10-day-old virgin females fed the protein-reduced diets (1:6:1 and 1:12:1) (F = 8.04; df = 2, 26; P = 0.002). The same tendency was shown by 14-day-old mated females (F = 40.43; df = 2, 27; P < 0.001). However, carbohydrates showed relatively higher values that equaled and sometimes surpassed those of recently emerged females (fig. 4a). Glycogen content of 10-day-old virgin females was higher when females were fed the 1:6:1 diet, followed by females fed the concentrated 1:3:1 diet (F = 12.96; df = 2, 26; P < 0.001). For 14-day-old mated females, the lowest values of glycogen were recorded for females fed 1:3:1 diet (F = 18.45; df = 2, 27; P < 0.001).

Fig. 4. Average nutrient content per fly (μg mg−1) of Anastrepha fraterculus females fed adult liquid diet with different yeast derivative, sucrose (S), and wheat germ (WG) ratios. Analysis was performed separately for each yeast derivative: hydrolyzed yeast (MP Biomedicals®, HY) and brewer's yeast (CALSA®, BY), and female condition.
Experiment III
MT and average mature eggs in the ovary
All females fed on brewer's yeast diets exhibited matured ovaries. However, the time at which 50 and 95% of females showed mature eggs in their ovaries (MT50 and MT95) differed among diets (table 4). MT50 was lower for females fed brewer's yeast diet with more wheat germ (1:3:6) than the reference diet (1:3:1) (fig. 5a). Likewise, only females fed the 1:3:6 diet showed the lowest MT95. The average number of mature eggs in the ovary at MT50 did not differ among diets (F = 1.07; df = 4, 24; P = 0.391). However, the average number of mature eggs in the ovary at MT95 was significantly higher for females fed the most wheat germ-concentrated diet (1:3:6) than those fed the other diets (F = 6.23; df = 4, 43; P < 0.001) (fig. 5b).
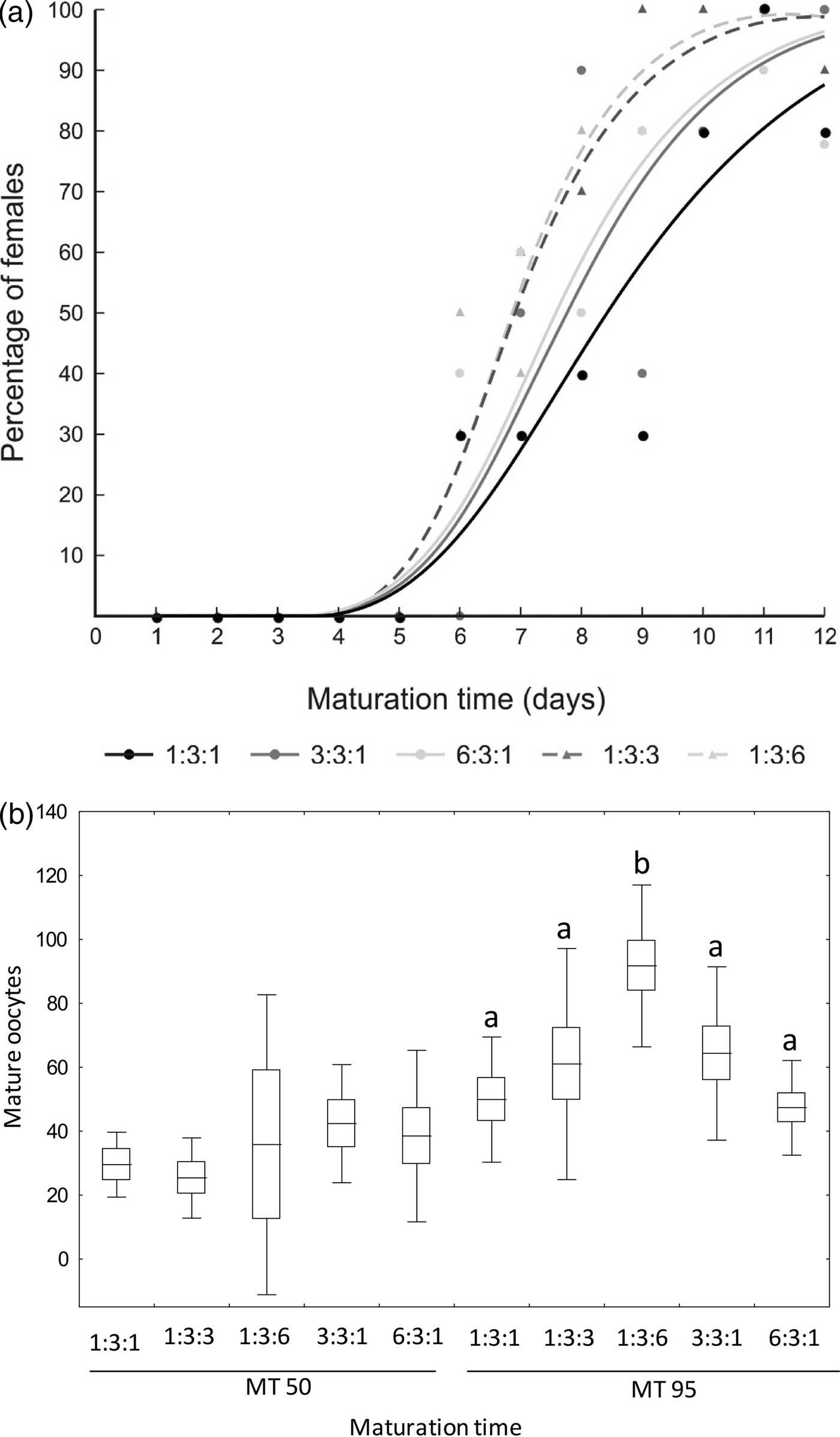
Fig. 5. Time when 50% (MT50) and 95% (MT95) of Anastrepha fraterculus females showed mature eggs (a) and average number of mature eggs in the ovary (b) of single females fed diets made with different brewer's yeast (CALSA®), sucrose and wheat germ ratios. See table 4 for statistical analysis with confidence limits of MT50 and MT95. Different letters for each maturation time in (b) denote significant differences between diets (DGC, α 0.05). Boxes denote means values ± SE and bars, means values ± SD.
Table 4. Maturation time (MT50 and MT95) of Anastrepha fraterculus females fed adult liquid diet made with different brewer's yeast (CALSA®, BY), sucrose (S), and wheat germ (WG) ratios.

Different letters in the same column denote significant differences (P < 0.05) and values in brackets represent the limits of confidence intervals (CL).
Fecundity and fertility
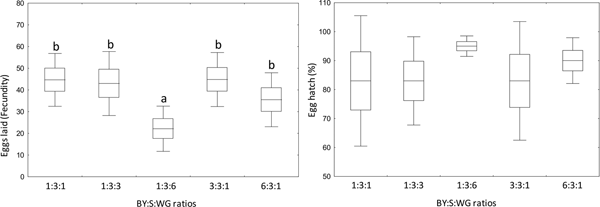
The number of eggs laid per female differed according to the diet (F = 2.97; df = 4, 20; P = 0.045) with fewer eggs laid by females fed brewer's yeast diet containing high ratio of wheat germ (1:3:6) compared with the other diets (fig. 6). On average, more than 83% of egg hatched when females fed brewer's yeast diet, with no significant differences among them (F = 1.65; df = 4, 20; P = 0.2) (fig. 6).
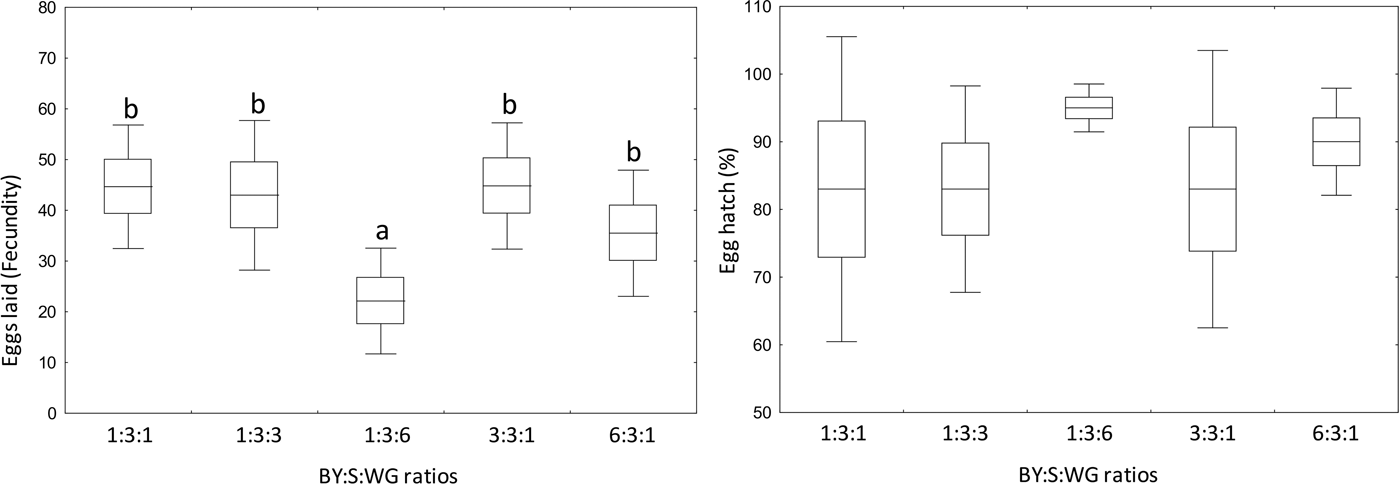
Fig. 6. Fecundity (left) and fertility (right) of Anastrepha fraterculus fed adult liquid diets made with different brewer's yeast (CALSA®, BY), sucrose (S), and wheat germ (WG) ratios. Different letters denote significant differences (DGC, α 0.05). Boxes denote means values ± SE and bars, means values ± SD.
Nutrient content
Nutrient content changed according to the diet (fig. 4b). Protein content was the highest for 10-day-old virgin females fed brewer's yeast diet with the highest amount of wheat germ (F = 5.17; df = 4, 45; P = 0.002). For mated females, proteins were the highest when females fed brewer's yeast 6:3:1 and 1:3:6 diets (F = 4.43; df = 4, 45; P = 0.004). Lipid content in 10-day-old virgin females changed according to the diet (F = 3.22; df = 4, 45; P = 0.021) with the highest values recorded in females fed 1:3:1 and 3:3:1 brewer's yeast diets. For 14-day-old mated females, the highest values were also recorded for females fed 1:3:1 and 3:3:1 brewer's yeast diets as well as those fed 1:3:3 (F = 5.04; df = 4, 45; P = 0.002). Carbohydrate content was the lowest in 10-day-old virgin females fed brewer's yeast diet with increasing both protein and wheat germ (F = 9.96; df = 4, 45; P < 0.001). Carbohydrates in 14-day-old mated females was also the lowest when females fed brewer's yeast diet with high wheat germ ratio (1:3:3 and 1:3:6) (F = 3.90; df = 4, 45; P = 0.008). Glycogen content of 10-day-old virgin females was the lowest when they fed diets with increasing both protein and wheat germ ratios (F = 7.23; df = 4, 45; P < 0.001). For 14-day-old mated females, the lowest values were recorded for females fed 1:3:6 and 3:3:1 brewer's yeast diets (F = 3.15; df = 4, 45; P = 0.023).
Discussion
Our study evaluated the effect of three yeast derivatives as well as the addition of wheat germ in the adult diet on A. fraterculus female reproductive parameters. Females fed hydrolyzed yeast and yeast extract attained the highest fecundity levels and those fed brewer's yeast the lowest. The addition of wheat germ in the adult diet improved fecundity regardless of the yeast derivative considered. Reducing hydrolyzed yeast:sucrose ratios in the diet, negatively affected fecundity and ovary maturation. Increasing the amount of brewer's yeast in the diet did not improved fecundity. The percentage of egg hatch was not affected by the diet showing that A. fraterculus females resigned fecundity but not fertility when fed a yeast-reduced diet. Nutrient contents of females were also affected by the diet provided. Protein and carbohydrates showed a complementary pattern as yeast:sugar ratios changed in the diets; while lipids were affected mainly by the age in which the flies were evaluated. We discuss the relevance of these results for mass rearing.
The effect of protein on ovary maturation and fecundity has been well documented for fruit flies (Jácome et al., Reference Jácome, Aluja and Liedo1999; Perez-Staples et al., Reference Perez-Staples, Prabhu and Taylor2007; Taylor et al., Reference Taylor, Perez-Staples, Weldon, Collins, Fanson, Yap and Smallridge2011; Papanastasiou et al., Reference Papanastasiou, Nakas, Carey and Papadopoulos2013). For A. fraterculus, we found that yeast extract showed similar values than hydrolyzed yeast not only when wheat germ was included in the diet, as seen by Morelli et al., (Reference Morelli, Zamboni Costa, Martins Fagioni, Zamboni Costa, Do Nascimento, Meirelles de Azevedo Pimentel and Melges Walder2012), but also when wheat germ was not included. This could be attributed to the similar chemical composition of the two yeast derivatives (table 2). The low performance of females fed the brewer's yeast-based diet is in concordance with the low mating success reported for males of this species fed on the same diet (Liendo et al., Reference Liendo, Devescovi, Bachmann, Utgés, Abraham, Vera, Lanzavecchia, Bouvet, Gómez-Cendra, Hendrichs, Teal, Cladera and Segura2013) and could reflect the lack, or insufficient amount, of certain nutrients as well as to certain specific amino acids. Leucine, glycine, phenylalanine, and methionine have been shown to be associated with insect reproduction (Grandison et al., Reference Grandison, Piper and Partridge2009; Levin et al., Reference Levin, McCue and Davidowitz2017). Glycine and phenylalanine contents were the lowest in brewer's yeast, whereas methionine was in similar amounts in brewer's yeast and hydrolyzed yeast but higher in yeast extract (table 2) providing different amounts of these key amino acids in each of the three yeast derivatives evaluated. In spite of this, we cannot rule out the effect of other nutrients present at different ratios in the three yeast derivatives. For example, the effect of dietary yeast on the lifespan of Bactrocera tryoni not necessarily results from its protein content and instead depends on certain micronutrients such as minerals, vitamins, and cholesterol (Fanson & Taylor, Reference Fanson and Taylor2012). Then, differences in the type and concentrations of lipids, certain vitamins or minerals could be responsible for the differential effect of the yeast derivatives evaluated. This highlights the need for caution when interpreting the effect of dietary yeast as the effect of protein (Fanson & Taylor, Reference Fanson and Taylor2012).
The impact of wheat germ on fecundity was evident regardless of the yeast derivative. As far as we know, this is the first report evaluating the direct impact of incorporating wheat germ into the adult diet of fruit flies. Yet, the addition of wheat germ oil in the larval diet increases pupal recovery as well as flight ability, egg production, and egg hatch of the emerged adults in Bactrocera dorsalis (Chang & Vargas, Reference Chang and Vargas2007); and enhances the expression of genes encoding enzymes needed for energy production when provided in the larval diet of B. dorsalis (Chang et al., Reference Chang, Coudron, Goodman, Stanley, An and Song2010). Wheat germ contains certain fatty acids, which serves as metabolic fuel in the moth Manduca sexta when provided in the adult diet (Levin et al., Reference Levin, McCue and Davidowitz2017). Wheat germ also provides amino acids that contribute to oviposition in Drosophila (Grandison et al., Reference Grandison, Piper and Partridge2009) representing an additional protein source. In our experiment, the larval diet in which the individual grew contained wheat germ. Then, the effect of adding wheat germ in the adult diet may be even higher in cases where wheat germ is not provided in the larval diet.
When we explored the possibility of decreasing the amounts of hydrolyzed yeast, we found a significant reduction in fecundity and ovary maturation even at a 1:6:1 (yeast:sucrose:wheat germ) ratio. We expected this reduction at the 1:12:1 ratio but not at the 1:6:1 ratio since previous studies in A. fraterculus suggested 1:5 (yeast:sucrose, without wheat germ) as the optimal consumption ratio (Oviedo et al., Reference Oviedo, Nestel, Papadopoulous, Ruiz, Prieto, Willink and Vera2011). Such difference can be explained by the age at which the flies were evaluated. Oviedo et al., (Reference Oviedo, Nestel, Papadopoulous, Ruiz, Prieto, Willink and Vera2011) recorded fly consumption during the pre-oviposition phase, whereas we evaluated females during the oviposition phase. Results suggest that yeast requirements for A. fraterculus females during the reproductive stage are higher than those required during the maturation and pre-oviposition stage. In contrast to females, males fed a poor (1:12) or an enriched (1:3) hydrolyzed yeast diet exhibited the same sexual performance (Liendo et al., Reference Liendo, Devescovi, Bachmann, Utgés, Abraham, Vera, Lanzavecchia, Bouvet, Gómez-Cendra, Hendrichs, Teal, Cladera and Segura2013), suggesting that protein requirements are different between sexes. Different nutritional requirements between females and males were already reported in other species of fruit flies (Nestel et al., Reference Nestel, Papadopoulos, Pascacio-Villafán, Righini, Altuzar-Molina and Aluja2016 and references herein) posing the question whether providing a single fixed diet ensures the highest fecundity at the laboratory and mass-rearing facilities.
Increasing the amount of brewer's yeast in the diet was not sufficient to improve fecundity to levels obtained with hydrolyzed yeast or yeast extract-based diets, suggesting that brewer's yeast by itself does not provide the required nutrients to attain high levels of egg production. Nevertheless, females fed brewer's yeast-based diets produce fertile eggs. The addition of wheat germ to brewer's yeast diets increased the number of eggs in the ovary, but not fecundity. Differences between a number of mature eggs in the ovary and fecundity were also reported for C. capitata by Chang (Reference Chang2004) who argued that ‘amino acids might affect the morphogenetic process in reproduction or the energy production during oviposition’. In our case, as brewer's yeast or wheat germ ratios increased, sucrose proportion decreased and this maybe restricted the energy available for oviposition even when females had the required nutrients to produce eggs. This could explain the low fecundity found. However, this interpretation needs some caution since, as mentioned above, we found certain differences in the amino acids content between the three diets. Maybe eggs start maturing but failed to complete development and therefore they accumulated in the ovaries. Alternatively, it could be argued that brewer's yeast may also lack certain lipids essential for oviposition as brewer's yeast is considered a lipid-poor yeast (Blagovićk et al., Reference Blagović, Rupčić, Mesarić, Georgiú and Marić2001). This is in congruence with Fanson & Taylor (Reference Fanson and Taylor2012) who concluded that cholesterol is essential for B. tryoni fecundity. Then, the low fecundity observed may be the combination of the lack or low amounts of specific amino acids or lipids.
Nutrient content of A. fraterculus females varied according to the adult diet provided and mating status. Protein and carbohydrates content showed a complementary pattern. Protein content decreased when hydrolyzed yeast decreased in the diet and carbohydrate content increased. The protein trend could be the result of resource allocation determined by a nutritional threshold of proteins required for females to lay eggs (Kaspi et al., Reference Kaspi, Mossinson, Drezner, Kamensky and Yuval2002). Females fed the 1:6:1 hydrolyzed yeast diet reached the nutritional threshold and laid eggs, but were unable to recover the protein lost during egg production. In contrast, females fed the 1:12:1 hydrolyzed yeast diet did not reach the nutritional threshold and consequently produced and laid few eggs (or none in some cases), and in turn did not experience such a marked reduction in their protein content. Females fed the 1:3:1 hydrolyzed yeast diet covered their protein requirements and had, therefore, the highest protein content even after undergoing loses associated with egg production. Protein (or amino acid) levels drive fruit fly feeding decisions (Oviedo et al., Reference Oviedo, Nestel, Papadopoulous, Ruiz, Prieto, Willink and Vera2011; Leitão-Gonçalves et al., Reference Leitão-Gonçalves, Carvalho-Santos, Francisco, Fioreze, Anjos, Baltazar, Elias, Itskov, Piper and Ribeiro2017) explaining the carbohydrate content trend. Females fed a yeast-enriched diet (hydrolyzed yeast 1:3:1) reduced their consumption to avoid ingesting too much protein and therefore showed low carbohydrates levels, whereas females fed yeast-reduced diets (1:6:1 and 1:12:1) consumed carbohydrates well above their nutritional requirements to prioritize reaching the optimum levels of other nutrients (most likely protein) needed to maximize their reproductive output. In addition, females fed hydrolyzed yeast 1:3:1 were reproductively active and thus allocated energy to reproduction. Lipids content showed a different pattern with a substantial drop in emergence, irrespectively of the diet. This drop was evidenced also in C. capitata (Nestel et al., Reference Nestel, Galun and Friedman1985) suggesting that lipids acquired during the larval stage are readily available for fruit fly adults, which use this energy reserves without any need to restore them. Notably, when females fed hydrolyzed yeast-based diets, the effect on lipid content changed according to the female condition. Lipid content was higher in 10-day-old virgin females fed 1:3:1 and 1:6:1 diets, whereas 14-day-old copulated females showed the highest lipid content when fed the more diluted diet (1:12:1). This trend was also recorded for females fed the brewer's yeast-based diet (1:3:1). Considering that protein ingestion favors lipid accumulation in the adult's fat body (Orr, Reference Orr1964) and lipids are involved in ovary development of fruit flies (Jácome et al., Reference Jácome, Aluja, Liedo and Nestel1995; Canavoso et al., Reference Canavoso, Jouni, Karnas, Pennington and Wells2001), then 10-day-old virgin females offered a yeast-enriched adult diet (1:3:1 and 1:6:1) are expected to show the highest lipid content. Fourteen-day-old mated females showed the opposite probably because of reproduction loses (Aluja et al., Reference Aluja, Birke, Guillén, Díaz-Fleischer and Nestel2011), suggesting a much complex interaction between lipid ingestion, lipid content, and reproduction (Hansen et al., Reference Hansen, Flatt and Aguilaniu2013).
In conclusion, the type of yeast provided in the adult diet has great influence on certain reproductive parameters of A. fraterculus females. Although the experimental approach used do not allow to discern if the effects on reproductive performance of A. fraterculus were a function of individual diet components or a combination of these, our study provides valuable information in the search of cost-effective adult diets at fruit fly mass-rearing facilities. We extended the results from Morelli et al. (Reference Morelli, Zamboni Costa, Martins Fagioni, Zamboni Costa, Do Nascimento, Meirelles de Azevedo Pimentel and Melges Walder2012) showing the good performance of yeast extract as a low-cost protein source. In addition, wheat germ emerges as a relevant component of the adult diet even when the diet is based on hydrolyzed yeast or yeast extract. Therefore, mass-rearing facilities should not underestimate the use of this low-cost ingredient. Brewer's yeast is also a very low-cost alternative thus, research is recommended to evaluate its possible adoption in the adult diet. Besides, our results encourage studies on the impact of certain amino acids, such as glycine, leucine, and phenylalanine, or other nutrients such as vitamins and minerals on A. fraterculus female performance. Our study also provides novel baseline information to understand the role of nutrition in A. fraterculus female fitness. Being A. fraterculus a holometabolous insect, nutrients acquired during the larval stage are expected to be available for the adults to be used for their metabolism and reproduction. Concurrently, adults can meet their nutritional needs as result of the combination of larval-derived reserves and adult feeding (Kaspi et al., Reference Kaspi, Mossinson, Drezner, Kamensky and Yuval2002; Nestel et al., Reference Nestel, Papadopoulos, Pascacio-Villafán, Righini, Altuzar-Molina and Aluja2016). Larval diet was equal for all adult diets evaluated here and seems to have cover lipid needs since recently emerged adults showed high lipids content compared with sexually mature adults. However, proteins showed a different pattern. The impact on fecundity was striking in those diets with low yeast derivative–sugar ratios. In other words, females were not able to compensate high protein deficiencies in the adult diet with reserves gained at the larval stage, reinforcing the relevant role of adult environment for female performance of fruit flies in general (Jácome et al., Reference Jácome, Aluja, Liedo and Nestel1995, Reference Jácome, Aluja and Liedo1999; Perez-Staples et al., Reference Perez-Staples, Prabhu and Taylor2007) and A. fraterculus in particular. The advantages of providing complementary nutritional sources also deserve special attention. Although this topic was not the focus of our study, our results suggest that nutrient optimization seems easier to reach if adults are provided nutritional complementary sources rather than a single diet, particularly when the nutritional requirements vary according to the sex, age, and reproductive status of the individuals.
Acknowledgements
We thank Osvaldo Arce for statistical advice. Funds were provided by Secretaría de Ciencia, Arte e Innovación Tecnológica, UNT, and the International Atomic Energy Agency (IAEA).