Introduction
The genus Cochliomyia Townsend is now considered to consist of four endemic new world Calliphoridae species, C. hominivorax (Coquerel, 1858), C. macellaria (Fabrícius, 1775), C. aldrichi (Del Ponte, 1938) and C. minima (Shannon, 1926) (FAO, 1993).
Cochliomyia hominivorax, commonly called the New World screwworm fly or primary screwworm, is the only species of the genus known to be of great economic importance. The larvae of this species are obligatory wound parasites of mammals and are only able to develop in nature on living tissues, causing severe myiasis in hosts (Guimarães & Papavero, Reference Guimarães and Papavero1999). The historical range of this insect extended from southern United States to Argentina, with seasonal fluctuations and with the greatest abundance in the Neotropical region; but it has been successfully eradicated from North and most of Central America by the sterile insect technique-SIT (IAEA/FAO, 2000; Wyss, Reference Wyss2000).
Cochliomyia macellaria, the secondary screwworm, is morphologically similar to C. hominivorax; and it is widely distributed and abundant in the new world, ranging from southern Canada to Argentina (Baumgartner & Greenberg, Reference Baumgartner and Greenberg1985; Dear, Reference Dear1985). Cochliomyia macellaria is a sarcosaprophagous species that develops primarily on carrion but are commonly confused with C. hominivorax in cases of myiasis because it tends to be a secondary invader. Old records of myiasis attributed to this species most certainly should be transferred to C. hominivorax (Guimarães & Papavero, Reference Guimarães and Papavero1999), but few cases of myiasis in humans by this species have been recorded (e.g. Smith & Clevenger, Reference Smith and Clevenger1986; Josephson & Krajden, Reference Josephson and Krajden1993). This species has also been reported to be a mechanical vector of human and animal diseases due to its synanthropic behaviour (Greenberg et al., Reference Greenberg, Varela, Bornstein and Hernandez1963).
Little is known about the habits or relative abundance of the other two species of the genus, C. aldrich and C. minima. They both have a restricted geographical distribution to some Caribbean islands and to Florida (Dear, Reference Dear1985). The immature stage of both species is undescribed and the flies are rarely considered, if at all, in identification guides to myiasis species (FAO, 1993). Adults are apparently attracted to fresh horse manure and carrion but a single case of C. minima myiasis has been reported in a dog in Puerto Rico (De León & Fox, Reference De León and Fox1980).
The taxonomic status of the species in the genus has been very controversial, mainly due to misidentification of and misapplications of names to C. hominivorax and C. macellaria. This, allied to the morphological similarity between the two species, their geographical overlap and the potential losses that C. hominivorax represents for cattle breeders, give great importance to the rapid and correct identification of these two species.
Different methods based on molecular markers for the characterization of the two species have been successfully investigated (Pomonis, Reference Pomonis1989; Taylor et al., Reference Taylor, Szalanski and Peterson1996; Litjens et al., Reference Litjens, Lessinger and Azeredo-Espin2001). But the current methods for species identification based on morphology are relatively restricted to specialists since, in adults, identification characters are mainly based on male genitalia, female basicostal coloration and pollinosity differences in the fifth tergite (FAO, 1993).
In addition to a correct identification of these species, a good knowledge of C. hominivorax biology can contribute to the design of control programs (IAEA/FAO, 2000; Krafsur, Reference Krafsur, Dyck, Hendrichs and Robinson2005). In the New World screwworm fly, molecular markers have also been used and have provided important information about population structure and genetic variability (e.g. Roehrdanz, Reference Roehrdanz1989; Infante-Vargas & Azeredo-Espin, Reference Infante-Vargas and Azeredo-Espin1995; Taylor et al., Reference Taylor, Szalanski and Peterson1996; Lyra et al., Reference Lyra, Fresia, Gama, Cristina, Klaczko and Azeredo-Espin2005, Torres et al., Reference Torres, Lyra, Fresia, Azeredo-Espin, Vreysen, Robinson and Hendrichs2007, Lyra et al., Reference Lyra, Klaczko and Azeredo-Espinin press). However, although phenotypic traits might provide interesting results that could not be obtained with neutral markers at the molecular level, there are no well-characterized morphological markers; and little is known about morphological variation in natural populations of C. hominivorax (Gagné & Peterson, Reference Gagné and Peterson1982; Richardson et al., Reference Richardson, Ellison and Averhoff1982; Azeredo-Espin, Reference Azeredo-Espin1987).
The purpose of the present study is: (i) to provide a morphological analysis of wing size and shape variation of C. hominivorax and C. macellaria; (ii) to assess how suitable geometric morphometric methods are for identification of these species and for studies of population morphological variation; and (iii) to conduct a preliminary analysis of population variation in C. hominivorax.
Materials and methods
Data acquisition
Cochliomyia hominivorax were obtained as third instar larvae found in wounded sheep, dogs or cattle from 12 different localities, six in Brazil and six in Uruguay, totalling 476 individuals. A total of 119 individuals of C. macellaria were obtained as third instar larvae in carcasses of dead animals (horse and cattle) from three different localities, two from Brazil and one from Uruguay. Geographic locations of the sampled areas and the number of individuals analyzed are shown in table 1. All collections were carried out in the summer months between January 2003 and March 2005.
Table 1. Geographic locations of the sampled areas and number of analyzed individuals of Cochliomyia hominivorax and Cochliomyia macellaria (F, females; M, males).

Collected larvae were brought into the laboratory for species identification and allowed to pupate in sawdust. The pupae were maintained at constant temperature (25°C) and humidity (70%), and the adults were frozen 24 h after emergence. Sex identification was carried out after emergence, considering that in this genus, like some other Calliphoridae, males are holoptic or subholoptic, and females are dichoptic (Dear, Reference Dear1985).
The right wing of each fly was removed and mounted in water on a slide under a coverslip (Bitner-Mathé & Klaczko, Reference Bitner-Mathé and Klaczko1999). Wing images were captured with a video camera attached to a microcomputer and slide preparations were retained. TpsDig version 2.11 (Rohlf, Reference Rohlf2006) was used to obtain Cartesian coordinate data for 16 landmarks (fig. 1).
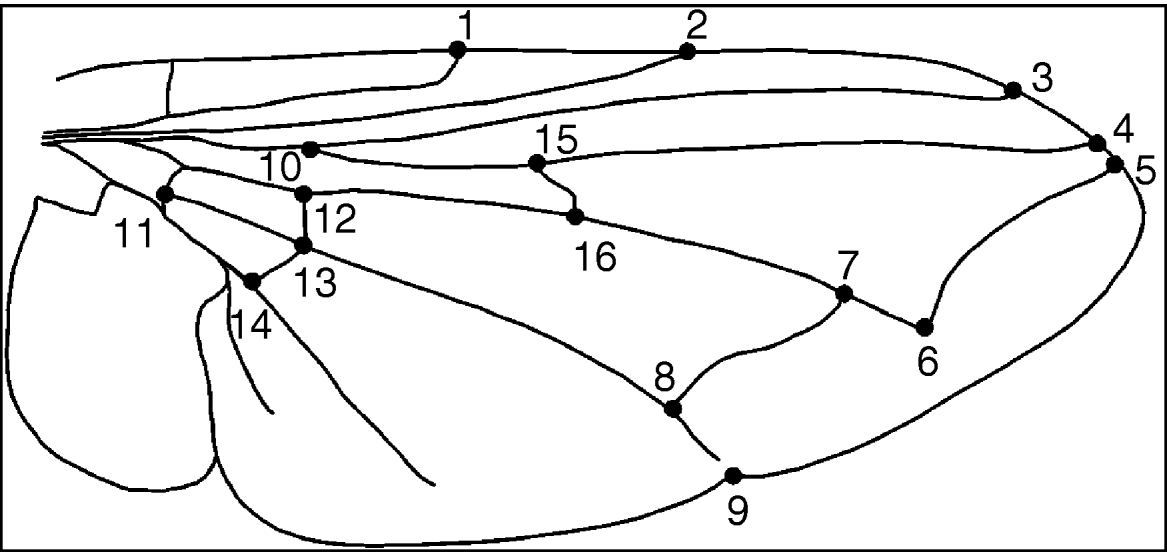
Fig. 1. Wing of Cochliomyia sp. showing landmarks.
Data analyses
Centroid size (CS) was used as an overall measure of wing size. This was calculated as the square root of the sum of the squared distances between the centroid and its landmarks (Bookstein, Reference Bookstein1991) using tpsRegr version 1.31 (Rohlf, Reference Rohlf2005a). Analysis of variance (ANOVA) was conducted to ascertain the effects of species, sex or locality on size.
Partial warps and uniform component scores were calculated with the program tpsRelw version 1.42 (Rohlf, Reference Rohlf2005b) (no weights assigned to any of the landmarks), and the matrix obtained was then interpreted as the set of shape variables (Zelditch et al., Reference Zelditch, Swiderski, Sheets and Fink2004). Effects of species, sex or locality on shape were evaluated by multivariate analysis of variance (MANOVA) on partial warps and uniform components scores. In order to account for allometric effects, we performed a multivariate regression of shape variables on size (CS).
The matrix of shape variables was used for canonical variate analysis (CVA) to examine the pattern of among-species/-sexes variation in total shape space and to obtain a classification matrix. Regression analysis of shape variable on CVA roots (CVA1 and CVA2) was used to illustrate shape changes by constructing the thin plate spline distortion graphics.
Multivariate or linear regressions were used as an exploratory analysis to examine the association between C. hominivorax population traits and a geographic variable, latitude.
Linear regression of size in latitude, ANOVA and MANOVA were conducted in Minitab14 (© 2004 by Minitab Inc.). Multivariate regression of shape variables on size, latitude and CVA roots was performed in the tpsRegr 1.31 (Rohlf, Reference Rohlf2005a). Canonical variate analysis was conducted in Statistica for Windows (version 6.0).
Results
Interspecific variation
The ANOVA on the centroid size of the wings showed a highly significant difference, according to sex and species (table 2). In C. hominivorax, male wings were approximately 12% larger than in females: CSmales=753.06±3.56 (CI 95%: 746.02–760.09) and CSfemales=675.77±2.99 (CI 95%: 670.14–681.40). In contrast, in C. macellaria males were approximately 2% smaller than females: CSmales=690.85±2.32 (CI 95%: 686.46–695.23) and CSfemales=704.57±2.75 (CI 95%: 699.10–710.04). Wing size alone was not sufficient to reliably identify the two species, but the overlap between the groups was small.
Table 2. Results of ANOVA on centroid size and MANOVA on non-uniform components of partial warps for Cochliomyia hominivorax (Ch) and C. macellaria (Cm) and for C. hominivorax populations.

A significant variation was found between species and a striking sexual dimorphism for wing shape. Table 2 shows the results of MANOVA on wing shape for C. hominivorax and C. macellaria. In general, male wings were narrower than female wings in both species (fig. 2).

Fig. 2. Scatterplot of individual scores from canonical variate analysis. The amount of variation expressed for each CVA is in parentheses. Grids represent the consensus and negative and positive deformations over the CVA1 and CVA2. Groups: ChF (![]() ), C. hominivorax females; ChM (▪), C. hominivorax males; CmF (
), C. hominivorax females; ChM (▪), C. hominivorax males; CmF (![]() ), C. macellaria females; CmM (□); C. macellaria males.
), C. macellaria females; CmM (□); C. macellaria males.
Variation in wing shape was significantly correlated to size in both females and in C. hominivorax males (C. hominivorax females: R2=0.028, P<0.001; C. macellaria females: R2=0.042, P<0.001; C. hominivorax males: R2=0.031; P<0.001) but was not significant in C. macellaria males (C. macellaria males: R2=0.023, P=0.071). However only a small proportion (<5%) of shape variation was due to size, indicating a negligible allometric effect.
Canonical variate analysis (CVA) conducted on the shape variables showed that all individuals of C. hominivorax and C. macellaria (100% of samples, according classification matrix) were correctly classified according to sex and species. The first canonical root (CVA1) accounted for 77% of total variation and completely separated the sexes in C. hominivorax and most males and females in C. macellaria. The CVA2 accounted for 21% of total variation and could roughly separate the two species (fig. 2). The CVA3 accounted for the last 2% of total variation.
Cochliomyia hominivorax population variation
C. hominivorax populations were significantly differentiated for both wing size and shape. ANOVA and MANOVA results for effects of localities on size and shape are shown in table 2. Since sexual dimorphism was present in C. hominivorax, a linear regression was performed separately for each sex. We were not able to find any obvious geographic pattern for the variation observed. Figure 3 represents observed variation of size for males and females in populations. The regression of size on latitude was significant for both females and males, but the percentage of explained variation was very small (females: R2=0.043, P=0.01; males: R2=0.023, P=0.01). The results from multivariate regression of shape on latitude were very similar to those obtained for size (females: R2=0.016, P<0.001; males R2=0.009, P<0.001).

Fig. 3. Graphic of centroid size (mean CS±SE) vs. latitude for Cochliomyia hominivorax (Ch) populations. ○, the mean CS for females; ▪, the mean CS for males.
Discussion
Interspecific variation and sexual dimorphism
Differences between males and females are common in animals. Since Darwin's (Reference Darwin1871) first investigations, these differences have been the subject of discussion by numerous evolutionary biologists (e.g. Maynard Smith, Reference Maynard Smith1978; Andersson, Reference Andersson1994; Fairbairn, Reference Fairbairn1997). Sexual dimorphism has been studied in relation to physiology, ecology and behavior. The two major explanations for its evolution are intra-specific niche divergence and natural (and/or sexual) selection (Lande, Reference Lande1980; Shine, Reference Shine1989; David et al., Reference David, Gibert, Mignon-Grasteau, Legout, Pétavy, Beaumont and Moreteau2003).
In insects, females are usually larger than males (e.g. Huey et al., Reference Huey, Moreteau, Moreteau, Gibert, Gilchrist, Ives, Garland and David2006), probably due to the fecundity advantage for larger females (but see David et al., Reference David, Gibert, Mignon-Grasteau, Legout, Pétavy, Beaumont and Moreteau2003). A larger male size is rare and is believed to be favored in cases of competition among males, such as fight for females or territory, or when they carry females during nuptial flight (Andersson, Reference Andersson1994; David et al., Reference David, Araripe, Bitner-Mathé, Capy, Goñi, Klaczko, Legout, Martins, Vouidibio, Yassin and Moreteau2006a).
In C. macellaria, we found that females are larger than males and that this size difference may be interpreted as a fecundity advantage, as in some Drosophila species. On the other hand, C. hominivorax males are significantly larger than females, suggesting the existence of selective pressures probably related to male behaviour. Even though adult flies of C. hominivorax are often difficult to observe in nature, Guillot et al. (Reference Guillot, Brown and Broce1978) and Krafsur (Reference Krafsur1978) were able to observe an aggressive behaviour in field C. hominivorax males, showing that they are territorial and have a striking behaviour towards screwworms, and even towards other species of small insects.
The observation of larger males than females would agree with adaptive interpretations mentioned previously although other factors are probably related to the dimorphism. For example, different sexual behavior of females in the two species may be considered, since C. hominivorax females mate only once (Crystal, Reference Crystal1967), a fact that could reinforce sexual selection and increase competition among the males of this species. Furthermore, to our knowledge, there is no record of monandry for C. macellaria in the literature.
Results reported here also found a significant sexual dimorphism for wing shape in C. hominivorax and C. macellaria (males have narrower wings when compared to females in both species). Sexual shape dimorphism is poorly understood, and it is believed to arise from ecological causes or natural selection, that is, the adaption of each sex to different ecological niches (Shine, Reference Shine1989; Andersson, Reference Andersson1994; Bonduriansky, Reference Bonduriansky2006). Although there is no direct evidence for the functional significance of shape variation in the species analyzed, it seems reasonable to suppose that the differences in foraging habitats and behavior, reproductive activities and flight agility or resistance may influence the shape dimorphism observed, as has been proposed for other species (e.g. Sivinski & Dodson, Reference Sivinski and Dodson1992; Bonduriansky, Reference Bonduriansky2006).
The observed sex dimorphism in C. hominivorax is consistent with a hypothesis that males engage in combat, either for territory or for females, when larger males would be favoured. However, more detailed studies of social behaviour and life history are needed to understand the meaning of the variation found and to confirm and/or generalize these tentative conclusions.
Wing morphometry as a tool for species identification
Geometric morphometrics methods applied to wing venation have been successfully used in previous studies to discriminate between different insect species (Dujardin et al., Reference Dujardin, Le Pont and Baylac2003; Houle et al., Reference Houle, Mezey, Galpern and Carter2003; Villemant et al., Reference Villemant, Simbolotti and Kenis2007; Hatadani & Klaczko, Reference Hatadani and Klaczko2008; Ludoski et al., Reference Ludoski, Francuski, Vujic and Milankov2008). The results described herein demonstrate that wing morphometrics is a simple and reliable method for differentiating C. hominivorax and C. macellaria samples, since canonical variate analysis of wing shape variables presented a 100% good match for sexes and species. Our results suggest that the partial warp scores could be used as the basis of a test to distinguish C. hominivorax and C. macellaria to monitor the expansion and control of C. hominivorax throughout infested and non-infested countries.
A major advantage of a wing shape test is the ease and speed of collecting test material. Data collection for wing shape analysis is both relatively cheap (requiring only a digitized image) and faster than DNA-based markers; several hundred wings can be digitized and analyzed on the same day (e.g. Houle et al., Reference Houle, Mezey, Galpern and Carter2003). It can also be easily collected from both live individuals and long-dead specimens. However, since the landmarks used in this study were distributed over the entire wing area, the major disadvantage of a wing shape test is that it requires non-damaged wings.
Cochliomyia hominivorax population variation among localities
Most of the literature on dipteran wing morphology variation concentrates on the genus Drosophila, in which latitudinal clines have been observed for body size in several species (e.g. Imasheva et al., Reference Imasheva, Bubli and Lazebny1994; Bitner-Mathé et al., Reference Bitner-Mathé, Peixoto and Klaczko1995; Gilchrist et al, Reference Gilchrist, Huey, Balanya, Pascual and Serra2004; Collinge et al., Reference Collinge, Hoffmann and McKechnie2006; David et al., Reference David, Legout and Moreteau2006b). Although alternative explanations have been proposed (Santos et al., Reference Santos, Cespedes, Balanya, Trotta, Calboli, Fontdevila and Serra2006), it is generally believed that the selective pressures acting on the formation of these clines are due to, or related to, temperature variation along latitude transects (Imasheva et al., Reference Imasheva, Bubli and Lazebny1994). Phenotypic plasticity occurs in the same direction of temperature and is expected to produce larger sizes in flies developing under lower temperatures than in those growing under higher temperatures (Partridge et al., Reference Partridge, Barrie, Fowler and French1994; Imasheva et al., Reference Imasheva, Moreteau and David2000; Pétavy et al., Reference Pétavy, Moreteau, Gibert, Morin and David2001; David et al. Reference David, Legout and Moreteau2006b). In fact, in various papers, about 50% of the total variation can be explained by a regression on latitude (James et al., Reference James, Azevedo and Partridge1995; Gilchrist et al., Reference Gilchrist, Huey, Balanya, Pascual and Serra2004).
Cochliomyia hominivorax is an obligate parasite of endothermic animals, and all larval stages develop in an almost constant environment inside the host. Hence, if temperature variation in developmental time of flies is one hypothetical explanation for size variation, it should not be surprising that we find little correlation between wing size and latitude, since latitude would probably influence size through environmental temperature, which is kept relatively constant during the larval stages development of C. hominivorax. Nonetheless, there is still a residual correlation that explains about 2 to 4% of the total variation. The comparison to previous studies is difficult because most of them focus on species whose larval development is influenced by variations in local temperature. Drosophila larvae often feed on rotting materials (such as fruit or cacti) and are subject to most of the environmental temperature fluctuations and also depend on larval crowding conditions.
Nevertheless, there are shape and size differences among populations that are not related to latitude. Due to its larval habit, we should expect little variation in developmental temperature or crowding conditions for C. hominivorax, regardless of its geographical location. Thus, we suggest that other aspects, such as seasonality (e.g. humidity and precipitation) and host characteristics, such as host nutritional aspects and body temperature, would have to be considered to understand the size and shape variation in this species. Naturally, we cannot ignore the possible effect of genetic drift and historic phenomena.
Based on the preliminary results presented here, we suggest that wing variation is a good morphological marker for studying population variation in C. hominivorax. Nevertheless, although our results showed morphological differences between C. hominivorax populations, they should be considered carefully when studying population structure. In order to address questions such as how much of the variation is due to plasticity or genetic differences, or how seasonality, temperature and/or host characteristics affect morphological variation, we need to conduct more detailed, specific and controlled experiments.
Acknowledgements
The authors thank R.A. Rodrigues for valuable technical and laboratorial assistance, M.S. Couto for maintaining the screwworm colonies and P. Fresia for help in collecting the samples from Uruguay. M.L.L. was supported by a fellowship from Fundação de Amparo à Pesquisa do Estado de São Paulo FAPESP (grants 01/12528-2 and 03/13598-0). This work was supported by grants from FAPESP, CNPq and the International Atomic Energy Agency (IAEA, grant 11822/RO).







