Introduction
The Mormon cricket (MC) (Anabrus simplex Haldeman, 1852), a katydid of the family Tettigoniidae (Orthoptera), has one generation per year; and it is geographically restricted to the western USA (primarily Utah, Nevada, Wyoming, Idaho, Colorado, Montana, and Arizona) (Pfadt, Reference Pfadt1994). MC is a large flightless insect. The adults weigh up to 3 g and reach up to 50 mm in length (fig. 1a). These insects have the ability to migrate up to 80 km during their lifetime of approximately 6 months (MacVean, Reference MacVean1991; Pfadt, Reference Pfadt1994; Gwynne, Reference Gwynne2001; Simpson et al., Reference Simpson, Sword, Lorch and Couzin2006). Noisy populations (listen to Supplementary material 1) of this insect found on the western slope of the Rocky Mountains and westward tend to aggregate into migratory bands (fig. 1b, c, Supplementary material 2) that can extend up to 16 km in length in sagebrush-dominated rangeland (Lorch and Gwynne, Reference Lorch and Gwynne2000; Gwynne, Reference Gwynne2001; Bailey et al., Reference Bailey, Gwynne and Ritchie2005; Lorch et al., Reference Lorch, Sword, Gwynne and Anderson2005; Sword et al., Reference Sword, Lorch and Gwynne2005), and their feeding can cause significant damage to forage plants and cultivated crops (MacVean, Reference MacVean1991).
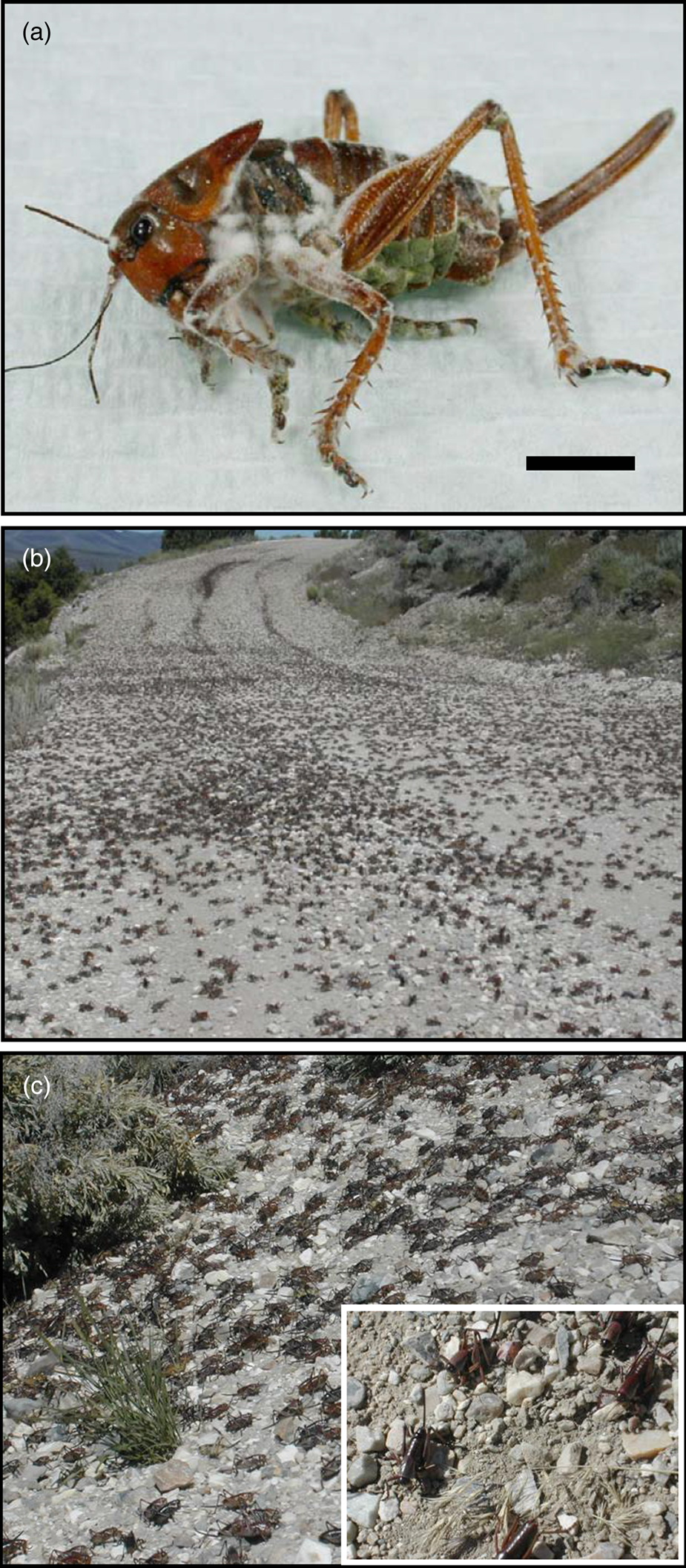
Figure 1. (a) Conidial production of M. acridum (ARSEF 324) on a dead A. simplex adult. Bar = 1 cm. (b) Migratory band of MCs in NE Utah in 2006. (c) Migratory band of MCs in NE Utah in 2006. Inset: Close-up of individuals in lower band.
MC bands were recorded as early as 1825 by trappers in Utah. The first record of serious damage occurred in 1848, the initial farming season in Utah by newly arrived Mormon settlers. The A. simplex insects were present in very large numbers and caused devastation to crops such as alfalfa, cucumbers, corn, beans, small grains, seeds, grasses, and vegetables (Hartley, Reference Hartley1970). Accordingly, the common name of this insect became ‘Mormon crickets’. The intervention of local seagull populations in June 1848, known to the Mormon pioneers as the ‘Miracle of the Seagulls’, greatly reduced MC populations that year (Hartley, Reference Hartley1970; Quinn, Reference Quinn1997; Schwarz, Reference Schwarz1998). More than 150 years later, this insect periodically still causes serious agricultural losses in Utah and nearby states. A monument in Salt Lake City's Temple Square commemorates the seagulls that protected desperately needed food and forage from the hoards of MCs in the late 1840s (Schwarz, Reference Schwarz1998). Because of their long, negative history with this insect, Western USA agriculturists react strongly to large MC populations and frequently seek advice and help from state and federal agencies for its control.
Fungal entomopathogens are viable agents for biological control of insects (Alston et al., Reference Alston, Rangel, Lacey, Golez, Kim and Roberts2005; Li et al., Reference Li, Alves, Roberts, Fan, Delalibera, Tang, Lopes, Faria and Rangel2010; Garrido-Jurado et al., Reference Garrido-Jurado, Resquín-Romero, Yousef-Naef, Ríos-Moreno and Quesada-Moraga2020; Rangel et al., Reference Rangel, Piedrabuena, Roitman and Messias2020). Metarhizium spp. fungi have been extensively studied and used in several countries for locust and grasshopper control (Bateman et al., Reference Bateman, Carey, Batt, Prior, Abraham, Moore, Jenkins and Fenlon1996; Bidochka et al., Reference Bidochka, St. Leger, Roberts, Goettel and Johnson1997; Blanford and Thomas, Reference Blanford and Thomas2001; Hunter et al., Reference Hunter, Milner and Spurgin2001; Lomer et al., Reference Lomer, Bateman, Johnson, Langewald and Thomas2001; Faria et al., Reference Faria, Magalhães, Alves, Schimidt, Tavares da Silva and Frazão2002; Enserink, Reference Enserink2004; Li et al., Reference Li, Alves, Roberts, Fan, Delalibera, Tang, Lopes, Faria and Rangel2010; Keyser et al., Reference Keyser, Fernandes, Rangel, Foster, Jech, Reuter, Black, Jaronski, Flake, Evans and Roberts2017). Entomopathogenic fungi have been field tested for MC control (Streett and Woods, Reference Streett, Woods, Branson and Redlin2001; Foster et al., Reference Foster, Jaronski, Reuter, Black, Schlothauer, Harper and Jech2011; Keyser et al., Reference Keyser, Fernandes, Rangel, Foster, Jech, Reuter, Black, Jaronski, Flake, Evans and Roberts2017). Microsporidian and nematode entomopathogens also have been tested for control of MC in laboratory and field studies (MacVean and Capinera, Reference MacVean and Capinera1991, Reference MacVean and Capinera1992). In the current study, several species of Metarhizium were tested in the laboratory for virulence to MCs. The goal of this study was to compare the virulence of selected isolates to different developmental stages of the MC. The selected Metarhizium isolates demonstrated high virulence against A. simplex.
Materials and methods
The fungal isolates
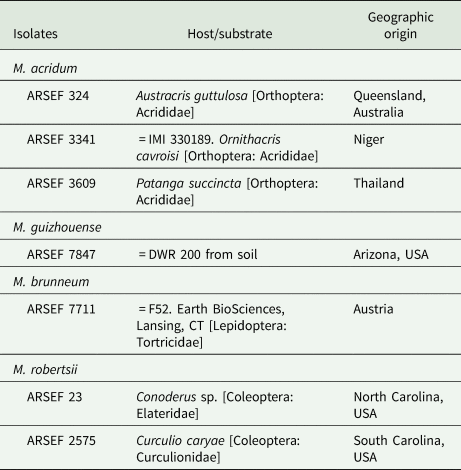
The isolates were obtained from the USDA-ARS Collection of Entomopathogenic Fungal Cultures (ARSEF); Robert W. Holley Center for Agriculture & Health, Cornell University Campus, Ithaca, NY, USA, and from Earth BioSciences, Lansing, CT, USA. In addition, DWR 200 ( = ARSEF 7847) was originally isolated from western USA soil in our laboratory by baiting with Galleria mellonella larvae and/or dodine selective medium (Zimmermann, Reference Zimmermann1986; Goettel and Inglis, Reference Goettel, Inglis and Lacey1997; Rangel et al., Reference Rangel, Dettenmaier, Fernandes and Roberts2010a). The insect hosts and geographical origins of the isolates are listed in table 1.
Table 1. Fungal isolates
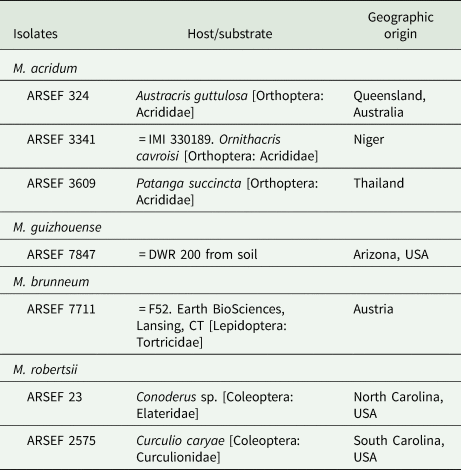
The isolate ARSEF 3341 of Metarhizium acridum ( = IMI 330189) is the active constituent of one of the biopesticides used in Africa for locust control (Lomer et al., Reference Lomer, Bateman, Johnson, Langewald and Thomas2001).
Isolate ARSEF 7711 (Metarhizium brunneum from Austria) was provided by Earth BioSciences, FairWeld, CT ( = Earth BioSciences F52; IMI 385045). It has been sold for insect control by Novozymes Biologicals Inc., Salem, VA (Bruck et al., Reference Bruck, Snelling, Dreves and Jaronski2005; Bruck and Donahue, Reference Bruck and Donahue2007).
The insect
MCs (A. simplex), at different stages of development, were collected in rangeland and surrounding areas in Tooele and Box Elder counties (UT) as well as the Curlew National Grasslands Reserve, Oneida county (ID). The field-collected insects were first held for 3–7 days in the laboratory and fed Flucker's® high calcium cricket feed (Flucker Laboratories, Baton Rouge, LA, USA), sagebrush leaves, sunflower seeds, and a variety of organically produced green leaves such as mixed baby greens, baby romaine salad, and baby spinach salad (Earthbound Farm®, CA, USA). In addition, high-protein dry fish food (TetraMin® Tetra Holding Inc., Blacksburg, VA, USA) and a gelled water source with calcium (Fluker's® Cricket Quencher, Baton Rouge, LA, USA) were provided. This gelled water prevents the insect from drowning. Cannibalization is a minor problem for MC with ready access to protein (Simpson et al., Reference Simpson, Sword, Lorch and Couzin2006), and most of the insects reached adulthood. The stage of insect development was determined by measurements of body and femur length (Pfadt, Reference Pfadt1994). Use of field collected insects was required because, despite numerous attempts by our and other research groups, no MC laboratory colonies have been successfully established.
Conidia production and inoculum preparation
The fungal isolates were grown on 23 ml potato dextrose agar (PDA) medium (Difco Laboratories, Detroit, MI, USA) supplemented with 1% yeast extract (1 g l−1) (Technical, Difco) in Petri dishes (polystyrene, 95 × 15 mm2, Fisherbrand®, Pittsburg, PA, USA) and incubated in the dark at 28 ± 1°C for 14 days. Conidia were harvested and suspended in 10 ml of 0.1% Tween 80 solution in 15-ml tubes (modified polystyrene, Corning®, USA). The suspensions were vortexed, and conidial concentrations estimated by hemocytometer counts. A 100 ml conidial suspension with concentration adjusted to 1 × 107 conidia ml−1 was prepared for each isolate. Suspensions of all isolates were used immediately for bioassay tests. The conidial viabilities of all suspensions used were high (usually ca. 95%).
Virulence assays (bioassays)
The immersion method was used to expose older MCs (instars 6 and 7 and adults of either sex) to conidial suspensions. The insects, in groups of six, were cold immobilized (ca. 5°C) (Gillespie et al., Reference Gillespie, Burnett and Charnley2000) for 1 h and dipped into the conidial suspension (1 × 107 conidia ml−1 in 0.1% Tween 80) for 15 s at ambient temperature. The control-insect group was dipped in Tween 80 (0.1%) solution. However, for small MCs (instar 2 to 5), due to high control mortality from the dipping method, the insects were sprayed to run-off with conidial suspensions or with Tween 80 (control). The dipping method is known to reduce the medium lethal time for Beauveria bassiana in Chilo partellus (Lepidoptera: Pyralidae) to 1 day vs. 3 days for the direct spray method (Tefera and Pringle, Reference Tefera and Pringle2003).
Immediately after treatment, the insects were transferred individually to cloth-net-covered glass jars with a layer of sterile white sand and provided with food as described above. The jars were covered with cloth netting and held in moisture glass chambers (1-cm of deionized H2O in each chamber) at 28 ± 1°C. The relative humidity of the moisture chambers was above 80%, as indicated by humidity indicator strips glued inside the jars (Humidicator paper HJH-650 Hydrion, Micro Essential Laboratory, New York, USA, that changes color in response to humidity). Mortality was assessed daily; dead insects were collected, surface sterilized in 5% Clorox ( = 0.25% sodium hypochlorite) for 10 min, and maintained in humid chambers. Development of Metarhizium on a cadaver was assessed to confirm mycosis. Thirty insects were used for each fungal treatment. The experiments were repeated at least two times, but most were repeated three times on three different dates, as shown in the figure captions. The varied number of repetitions in the different experiments was due to the variability in availability of field-collected insects.
Germination-speed assay
In an effort to explain the lower-than-expected virulence (speed to kill) of ARSEF 7847, the germination speed of this isolate was compared with that of ARSEF 2575 using the methods described elsewhere (Rangel et al., Reference Rangel, Braga, Flint, Anderson and Roberts2004). Conidia of both isolates were collected after 14 days of growth and immediately suspended at concentration of 1 × 105 conidia ml−1. The suspensions were vortexed, and 40 μl were inoculated (dropped, but not spread) on 4 ml of PDA medium in polystyrene Petri dishes (35 × 10 mm2). The plates were kept at 28°C in the dark. Plates were observed and scored for germination every 2 h from 2 to 28 h after conidial inoculation. This experiment was repeated three times with different cultures and on different dates.
Statistical analyses
The effect of treatment on mortality was assessed using a one-way analysis of variance (ANOVA) with a randomized complete block design. Mortality data were arcsine-square-root transformed prior to analysis to better meet assumptions of normality and homogeneity of variance. The mean of each treatment was compared to the mean of the control using Dunnett's test. Separate analyses were made for mortality on days 3, 4, and 5 for each of the four age classes (instars 2–3, instars 4–5, instars 6–7, and adults). Data analyses were generated using the MIXED procedure in SAS/STAT software, Version 9.1.3 of SAS System for Windows.
Results and discussion
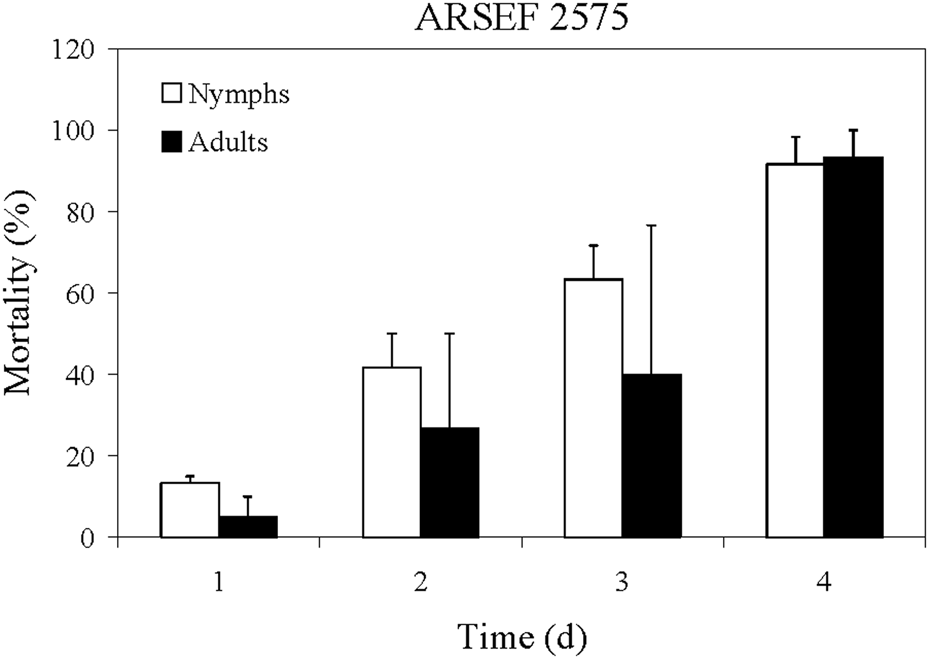
MC mortality after exposure to Metarhizium robertsii was remarkably fast in comparison with that reported for the desert locust, Schistocerca gregaria with M. acridum (ARSEF 3341) (Bateman et al., Reference Bateman, Carey, Moore and Prior1993, Reference Bateman, Carey, Batt, Prior, Abraham, Moore, Jenkins and Fenlon1996; Gillespie et al., Reference Gillespie, Burnett and Charnley2000; Hunt and Charnley, Reference Hunt and Charnley2011). For M. robertsii isolate ARSEF 2575 at concentration of 1 × 107 conidia ml−1, death of MCs started 1 day after conidial exposure, and by day 4 the mortality reached 100% (fig. 2). With the locust (S. gregaria), deaths caused by M. acridum (ARSEF 3341) started on day 3 after inoculation at conidial concentration of 3.75 × 107 conidia ml−1 and 100% mortality occurred on day 12 (Bateman et al., Reference Bateman, Carey, Batt, Prior, Abraham, Moore, Jenkins and Fenlon1996). In tests of additional isolates at 3.75 × 107 conidia ml−1, Bateman et al. (Reference Bateman, Carey, Batt, Prior, Abraham, Moore, Jenkins and Fenlon1996) found that the medium lethal time (LT50) was 4.8 days for the isolate ARSEF 2575 (referred to as isolate number I91 613 = ME1); however, for the isolates ARSEF 324 and ARSEF 3341, the LT50 were 3.7 and 4.4 days, respectively. Rapid MC mortality was also observed with B. bassiana in laboratory assays and 100% mortality occurred on day 6 at conidial concentration of 1 × 106 conidia ml−1 (Streett and Woods, Reference Streett, Woods, Branson and Redlin2001). Accordingly, the MC is very susceptible to M. robertsii ARSEF 2575. By comparison, we found that 100% of plum curculio larvae (Conotrachelus nenuphar) dipped in the ARSEF 2575 conidial suspension of 1 × 107 conidia ml−1 for 15 s were killed by 8 days post exposure (Alston et al., Reference Alston, Rangel, Lacey, Golez, Kim and Roberts2005).
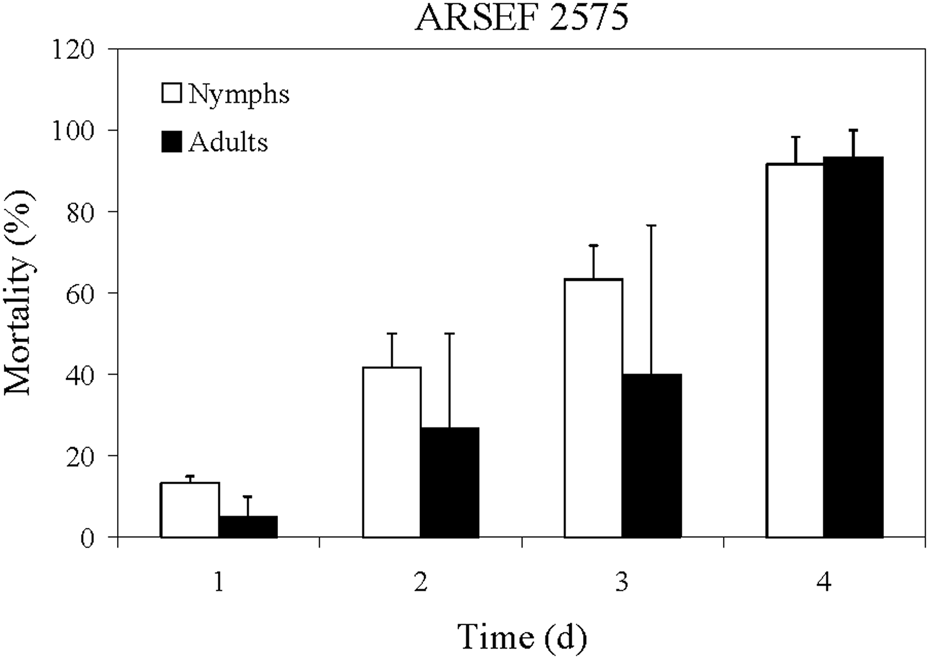
Figure 2. Mean percentage mortality of A. simplex nymphs (instars 2 and 3) and adults exposed to M. robertsii (ARSEF 2575) conidial suspensions (107 conidia ml−1). Error bars are standard error of two or three independent experiments.
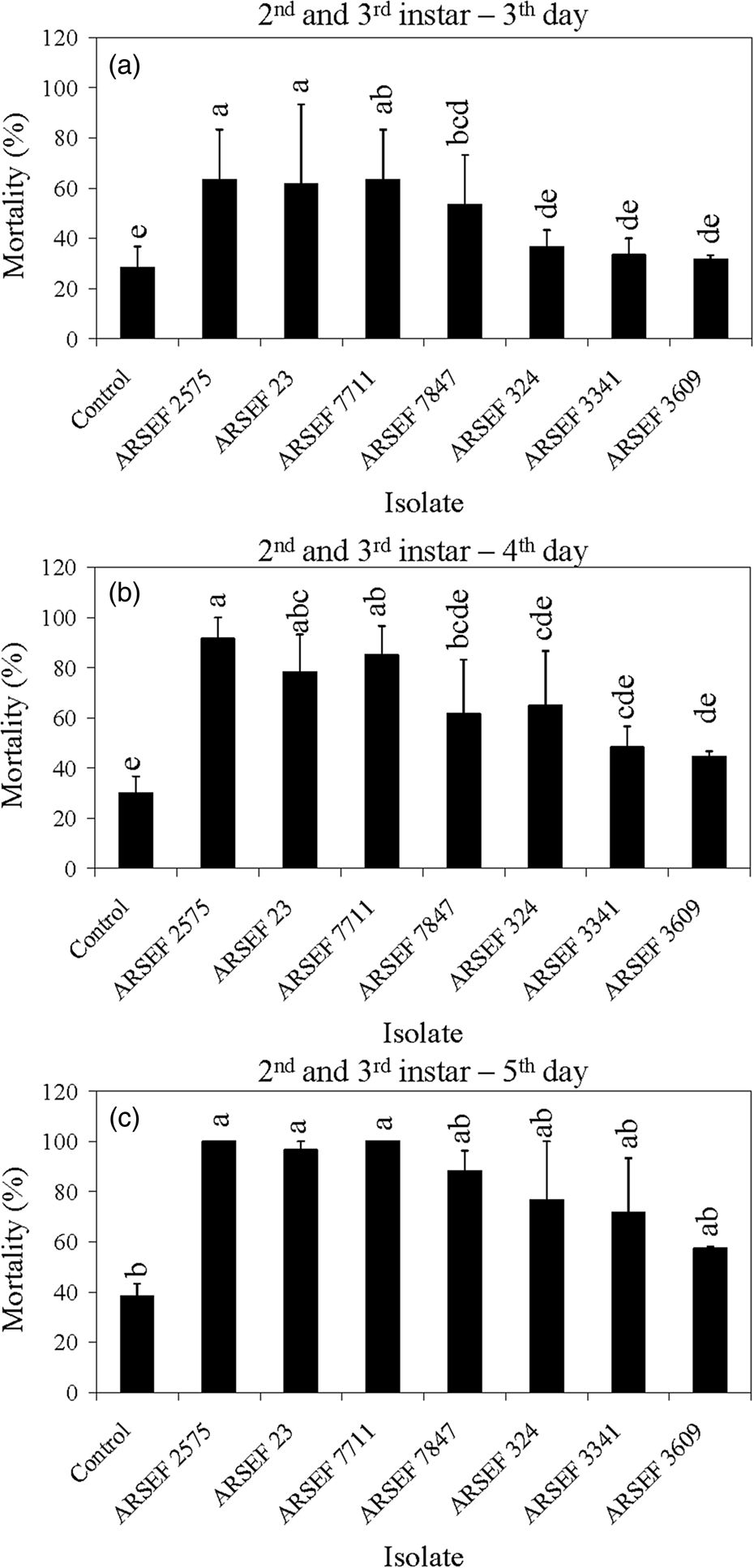
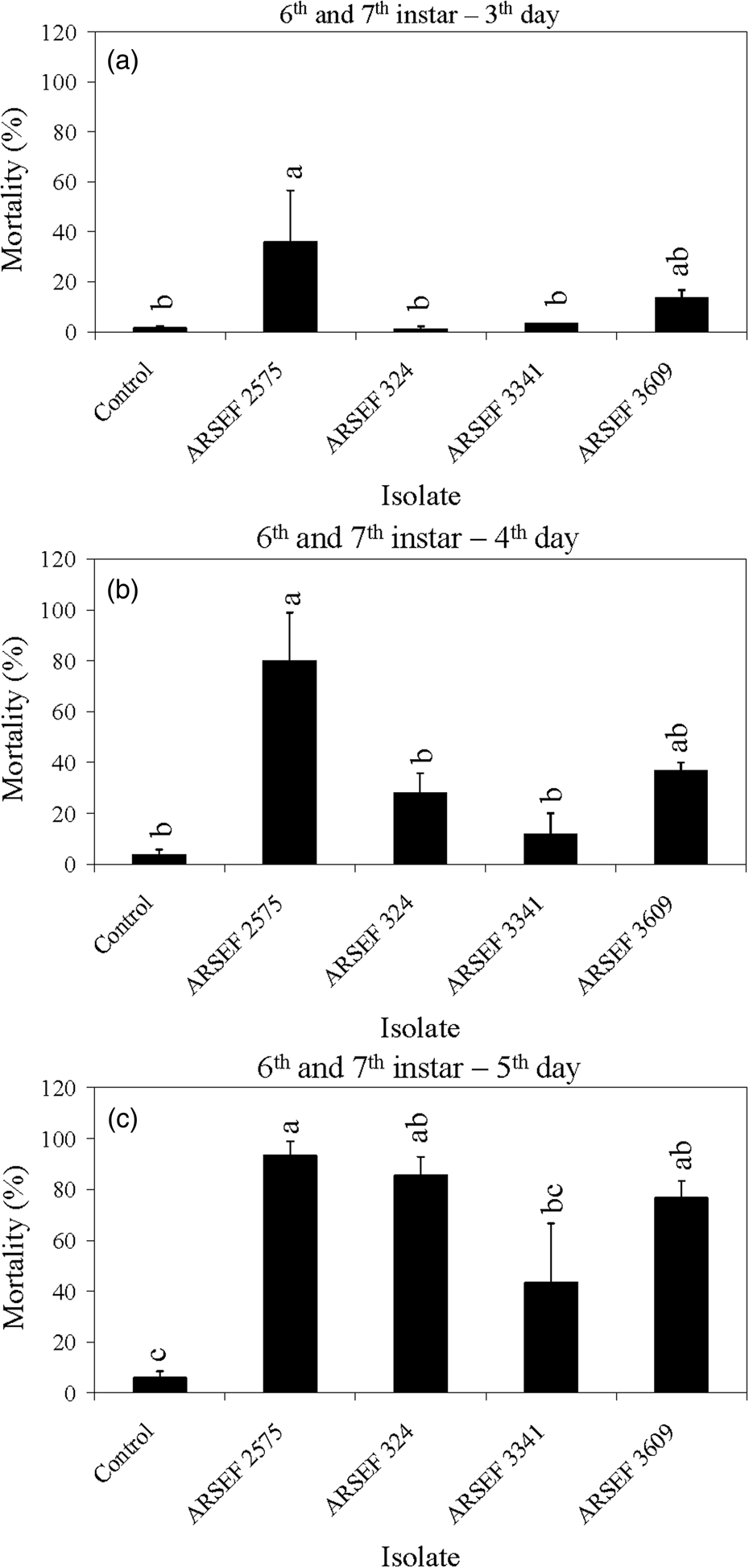
The mortality of MC nymphal instars 2–5 was faster with M. robertsii isolates (ARSEF 23, 2575) and M. brunneum (ARSEF 7711) than with M. acridum isolates (ARSEF 324, 3341, and 3609) (figs 3, 4). For larger nymphs (instars 6–7) and adults, the M. robertsii isolate ARSEF 2575 also killed faster than the M. acridum isolates (ARSEF 324, 3341, and 3609) (figs 5, 6). In contrast, M. robertsii (ARSEF 2575 = IMI I91 613) and M. acridum (ARSEF 3609 = IMI I91614) were similarly virulent to S. gregaria (Bateman et al., Reference Bateman, Carey, Batt, Prior, Abraham, Moore, Jenkins and Fenlon1996). The S. gregaria, however, were killed much faster by M. acridum ARSEF 3341 (total mortality at 10 days) than M. robertsii (ARSEF 2575) (total mortality at 17 days) (Hunt and Charnley, Reference Hunt and Charnley2011). However, when the locusts thermoregulated, M. robertsii killed faster than M. acridum (Hunt and Charnley, Reference Hunt and Charnley2011). This was possibly due to the production of destruxins by M. robertsii, but not by M. acridum. M. robertsii (ARSEF 23), as in Bischoff et al. (Reference Bischoff, Rehner and Humber2009) and Humber (Reference Humber2014), appears to possess much greater potential for the production of secondary metabolites than M. acridum (Gao et al., Reference Gao, Jin, Ying, Zhang, Xiao, Shang, Duan, Hu, Xie, Zhou, Peng, Luo, Huang, Wang, Fang, Wang, Zhong, Ma, St Leger, Zhao, Pei, Feng, Xia and Wang2011). Destruxin A (dtx A) produced by M. robertsii has been associated with the inhibition of the insect immune system (Hunt and Charnley, Reference Hunt and Charnley2011).
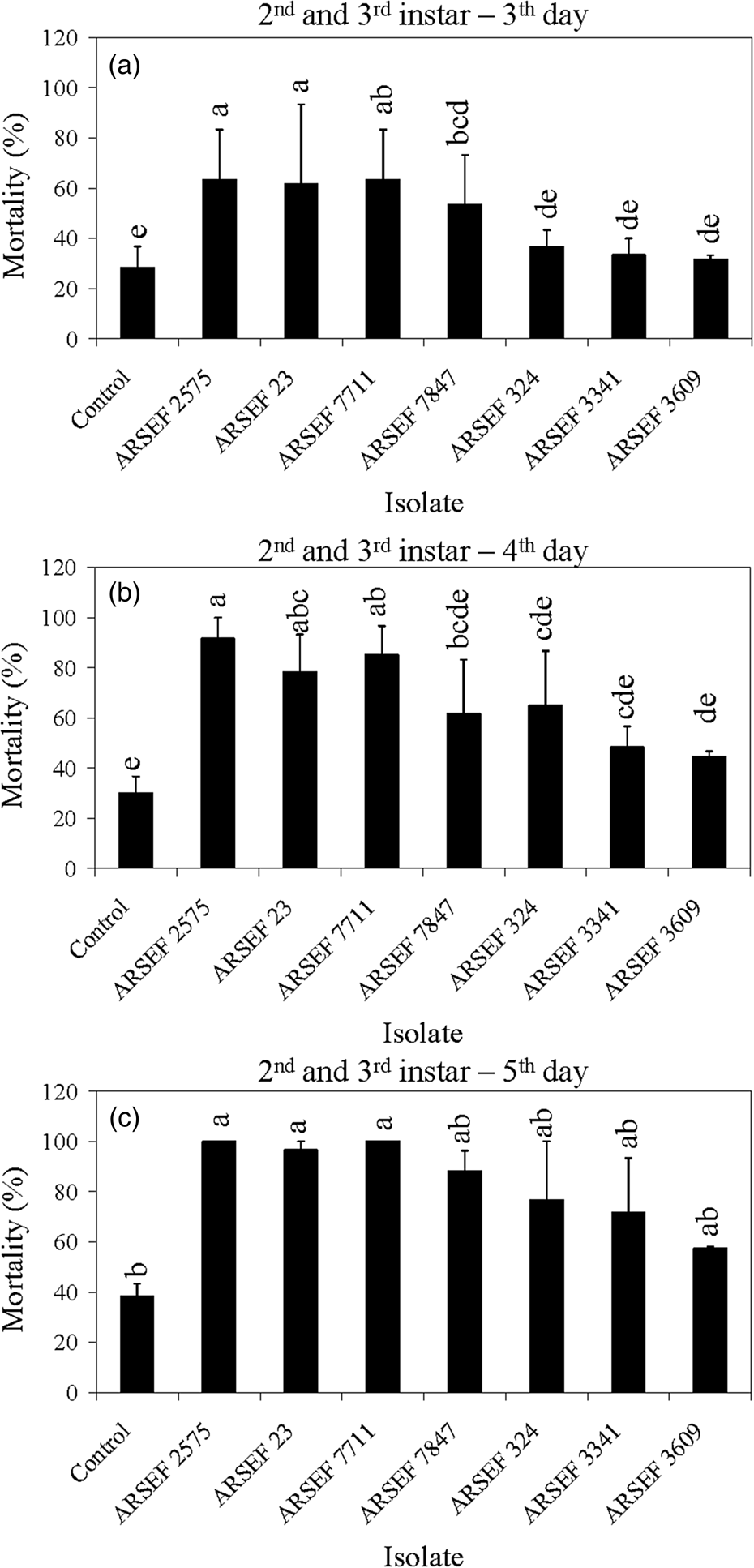
Figure 3. Mean percentage mortality of A. simplex nymphs (instars 2 and 3) exposed to M. acridum (ARSEF 324, 3341, and 3609), M. guizhouense (DWR 200 = ARSEF 7847), M. brunneum (F 52 = ARSEF 7711), and M. robertsii (ARSEF 23 and 2575) conidial suspensions (107 conidia ml−1). (a) Accumulated mortality on day 3; (b) accumulated mortality on day 4; (c) accumulated mortality on day 5. Error bars are standard error of two independent experiments. Graph bars with the same letters are not significantly different (P < 0.001; Dunnett's test following one-way ANOVA [see section ‘Materials and methods’]).

Figure 4. Mean percentage mortality of A. simplex nymphs (instars 4 and 5) exposed to M. acridum (ARSEF 324, 3341, and 3609), M. guizhouense (DWR 200 = ARSEF 7847), M. brunneum (F52 = ARSEF 7711), and M. robertsii (ARSEF 23 and 2575) conidial suspensions (107 conidia ml−1). (a) Accumulated mortality on day 3; (b) accumulated mortality on day 4; (c) accumulated mortality on day 5. Error bars are standard error of three independent experiments. Graph bars with the same letters are not significantly different (P < 0.001; Dunnett's test following one-way ANOVA [see section ‘Materials and methods’]).
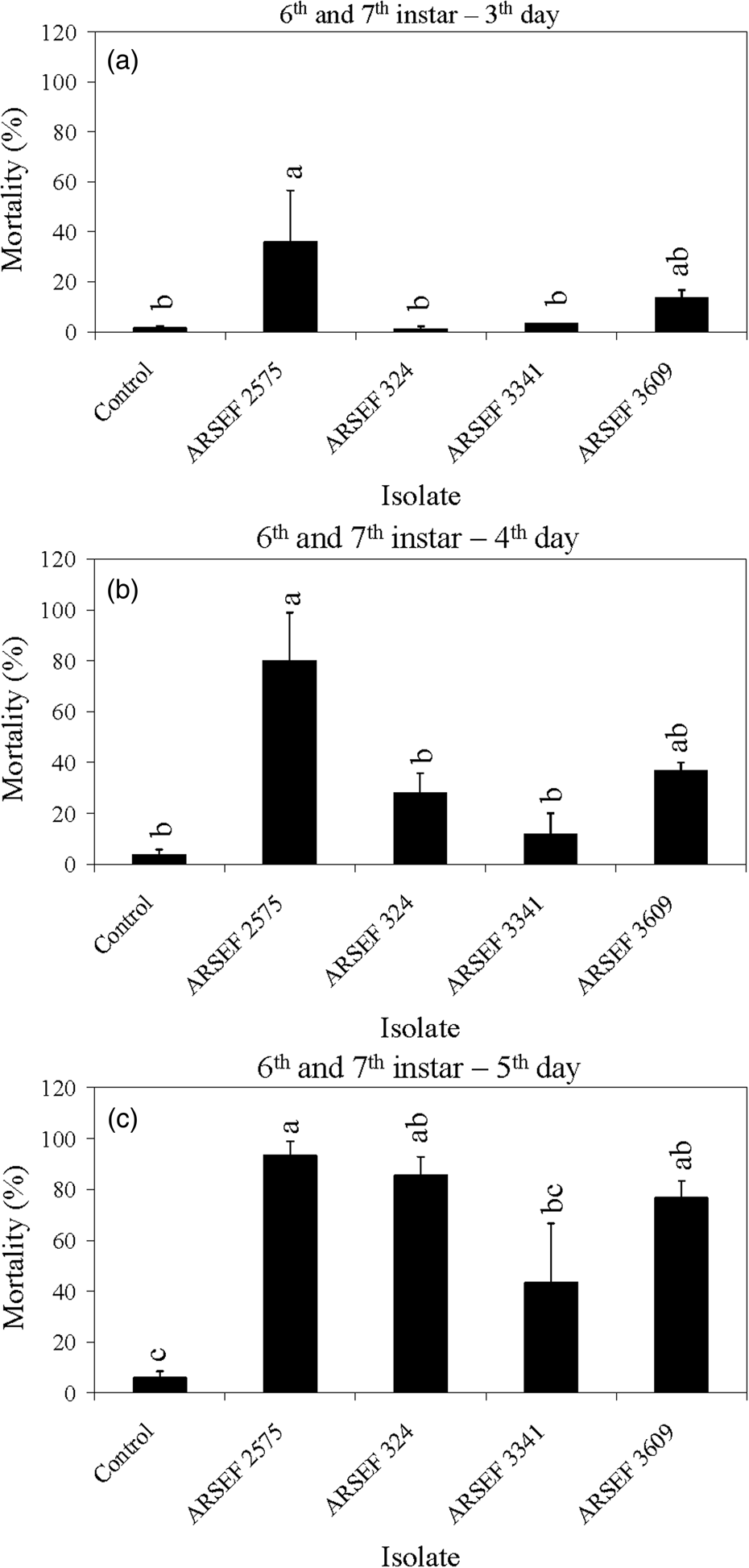
Figure 5. Mean percentage mortality of A. simplex nymphs (instars 6 and 7) exposed to M. acridum (ARSEF 324, 3341, and 3609), M. guizhouense (DWR 200 = ARSEF 7847), M. brunneum (F52 = ARSEF 7711), and M. robertsii (ARSEF 23 and 2575) conidial suspensions (107 conidia ml−1). (a) Accumulated mortality on day 3; (b) accumulated mortality on day 4; (c) accumulated mortality on day 5. Error bars are standard error of four independent experiments. Graph bars with the same letters are not significantly different (P < 0.001; Dunnett's test following one-way ANOVA [see section ‘Materials and methods’]).
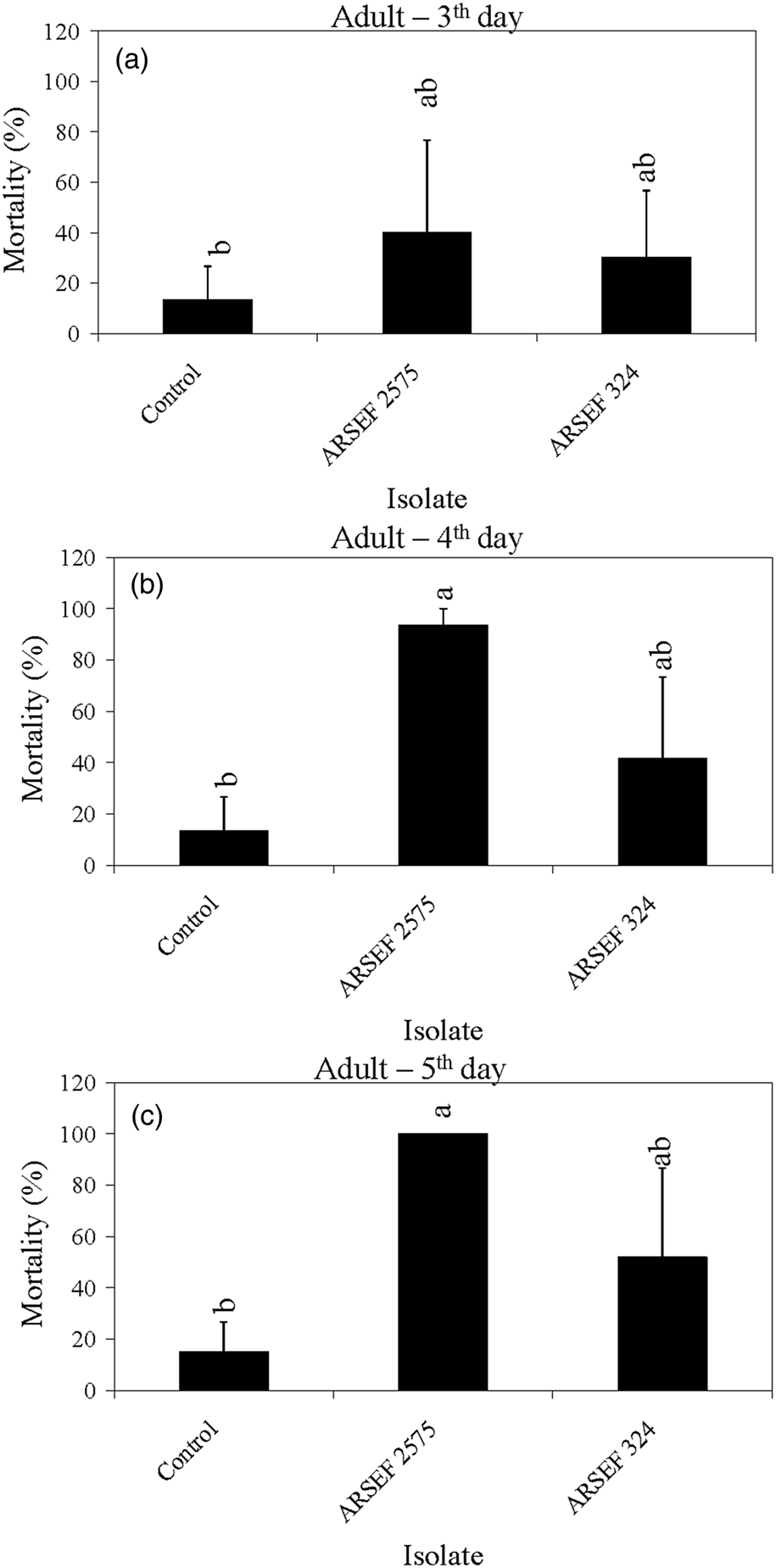
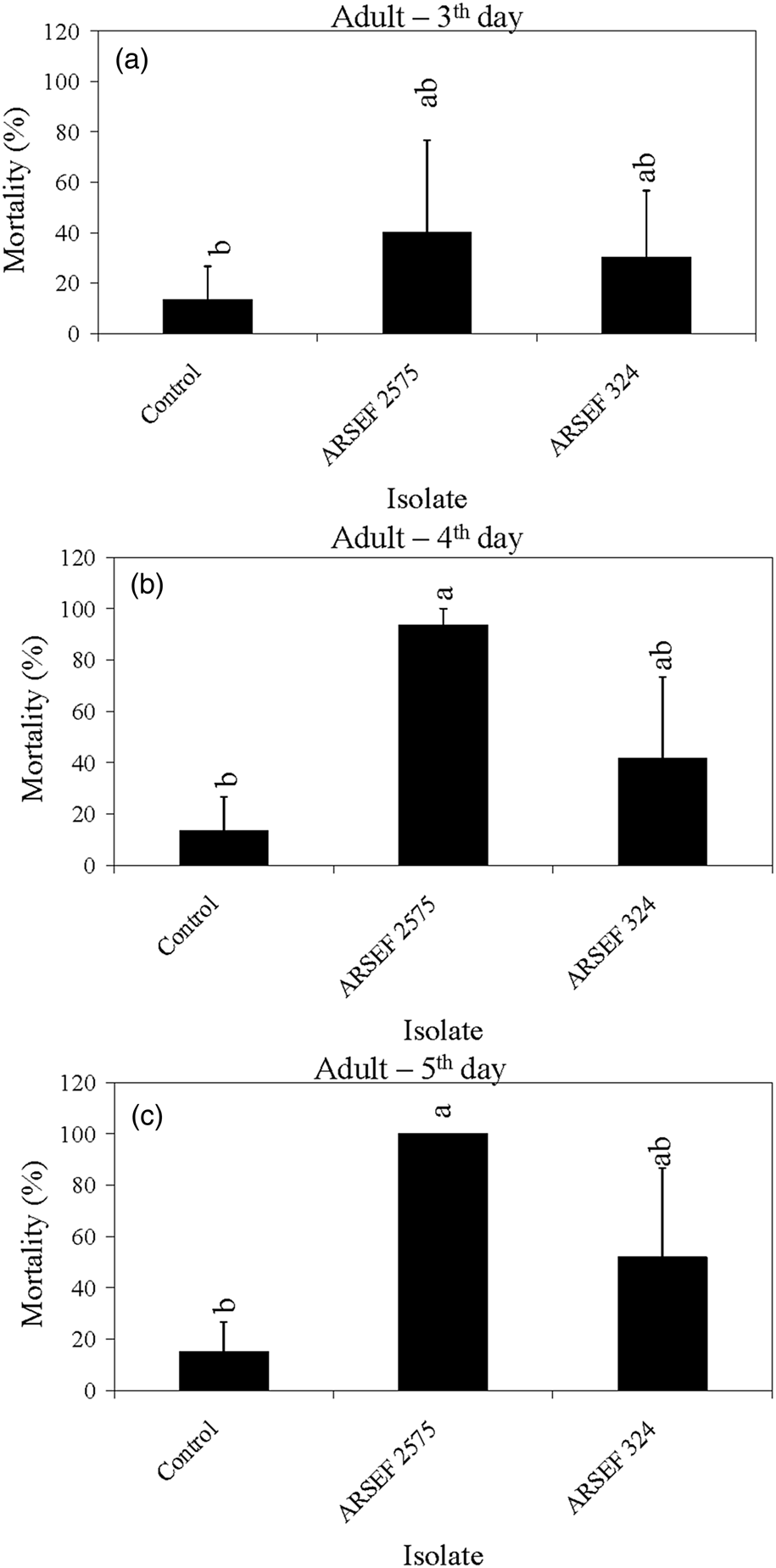
Figure 6. Mean percentage mortality of A. simplex adults exposed to M. acridum (ARSEF 324), and M. robertsii (ARSEF 2575) conidial suspensions (107 conidia ml−1). (a) Accumulated mortality on day 3; (b) accumulated mortality on day 4; (c) accumulated mortality on day 5. Error bars are standard error of two independent experiments. Graph bars with the same letters are not significantly different (P < 0.001; Dunnett's test following one-way ANOVA [see section ‘Materials and methods’]).
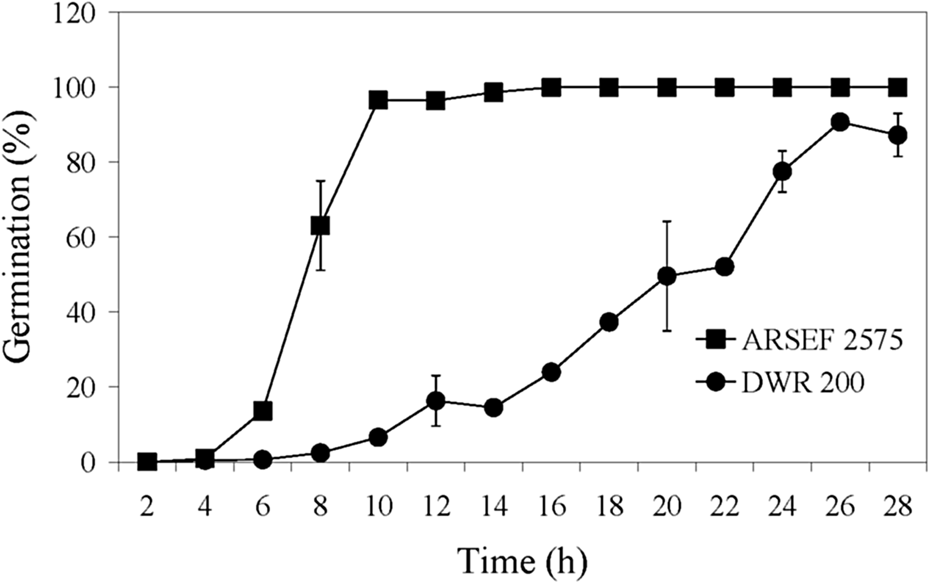
Isolate ARSEF 7847 (DWR 200), based on culture characteristics and amplified fragment length polymorphism analysis, is a M. anisopliae s.l. (Fernandes et al., Reference Fernandes, Keyser, Chong, Rangel, Miller and Roberts2010); however, according to sequence analysis of its elongation factor I-alpha (eEF1A), it is Metarhizium guizhouense (Roberts, unpublished). This isolate was relatively slow in killing MCs and was similar to M. acridum in this regard (figs 3, 4). The germination rate of isolate ARSEF 7847 (50% germination at 20 h) was much slower than ARSEF 2575 (50% germination at 7 h) (fig. 7), which may partially explain the slower mortality rate by ARSEF 7847. High germination rates correlate with high virulence in several entomopathogenic fungi (Altre et al., Reference Altre, Vandenberg and Cantone1999; Rangel et al., Reference Rangel, Alston and Roberts2008; Oliveira et al., Reference Oliveira, Braga and Rangel2018; Oliveira and Rangel, Reference Oliveira and Rangel2018).
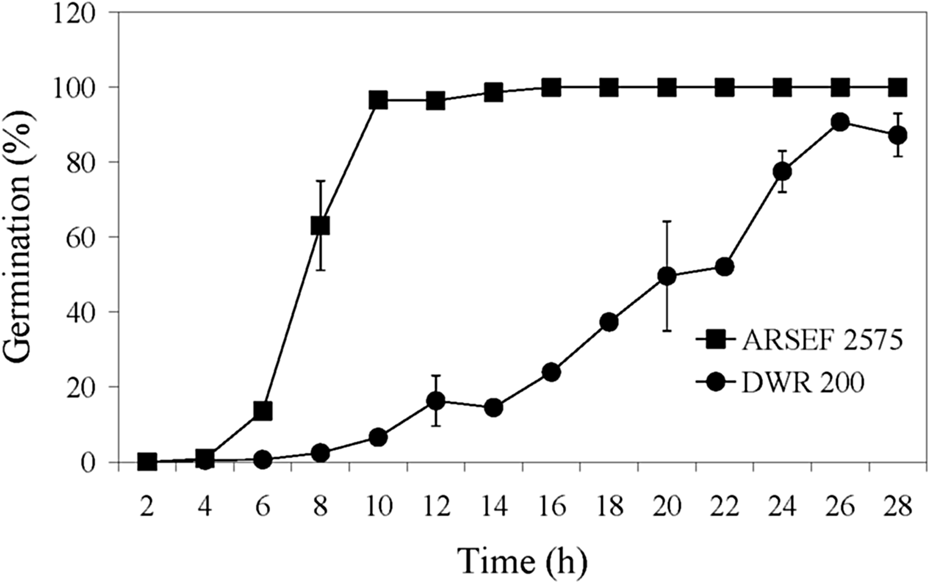
Figure 7. Germination speed of M. robertsii (ARSEF 2575) and M. guizhouense (DWR 200 = ARSEF 7847) conidia on PDAY medium at 28°C. Error bars are standard deviation of three independent experiments.
Differences in virulence of entomopathogenic fungi to insects of different ages has been reported, e.g. first-instar European corn borer (Ostrinia nubilalis) are more susceptible to B. bassiana than later larval stages (Feng et al., Reference Feng, Carruthers, Roberts and Robson1985). First-instar nymphs of MC were not available for our experiments, but the susceptibility to fungi of nymphal stages 2–7 and adult were similar (fig. 2).
Isolates ARSEF 2575 and ARSEF 324, M. robertsii and M. acridum, respectively, have the potential for high conidial production on their dead insect hosts in the laboratory (e.g. fig. 1a); however, various Utah MC populations from three different years sometimes demonstrated severely reduced conidial production on their cadavers, and conidial production was mostly inside the insect. The incidence of insect mycosis was higher for instar 6 and 7 nymphs than with adults and younger nymphs for all fungal isolates. Mortality from fungal infection without external conidial production on insects has been shown by other authors (Bateman et al., Reference Bateman, Carey, Batt, Prior, Abraham, Moore, Jenkins and Fenlon1996). Apparent non-mycosis mortality ( = without high outgrowth of fungus from cadavers) may indicate that pathogenesis includes the action of toxins (Bateman et al., Reference Bateman, Carey, Batt, Prior, Abraham, Moore, Jenkins and Fenlon1996). This lack of fungus emergence from dead hosts was also found with one M. anisopliae isolate [viz. ESC 1 ( = ARSEF 9591) from EcoScience (EPA registration no. 129056)] in a comparison of Abbott-corrected and mycosis-confirmed (conidial production on cuticle observed) mortality of C. nenuphar larvae (Alston et al., Reference Alston, Rangel, Lacey, Golez, Kim and Roberts2005).
The mortality in controls was somewhat high in some experiments, reaching 40% mortality on the fifth day post-treatment for nymphs of instar 2 and 3 (fig. 3). High control (untreated) mortality in the laboratory may occur with field-collected insects, usually due to pre-existing conditions such as poor nutrition, pesticide exposure, or the presence of other pathogens. Mortality near 40% of insects in the control treatment has also been recorded in other studies (Wright et al., Reference Wright, Raina and Lax2005; Ansari et al., Reference Ansari, Brownbridge, Shah and Butt2008). For example, 44% of alates of the Formosan subterranean termite (Coptotermes formosanus) had died in the control treatment 4 days after treatment application (Wright et al., Reference Wright, Raina and Lax2005). In the current study, crickets of instar 4 to adult survived well in the control treatment with mortality below 15% (figs 4–6). For all age groups, cricket mortality in the control treatment was always less than mortality caused by M. robertsii or M. acridum. The results indicate that most of the M. robertsii isolates were more virulent (in terms of speed of kill) than the M. acridum isolates against MCs, but all isolates tested eventually killed 100% of the MCs exposed to them. M. brunneum (ARSEF 7711), registered by US EPA as a myco-insecticide, was similar to two M. robertsii isolates (ARSEF 23 and ARSEF 2575) in virulence to instar 2–3 and 4–5 MC nymphs (figs 3, 4). Isolate ARSEF 7711 also is highly virulent to other pest-insect species [e.g. the cabbage maggot, Delia radicum (Diptera: Anthomyiidae)] (Bruck et al., Reference Bruck, Snelling, Dreves and Jaronski2005).
Comparative ranking of the ten isolates examined here by time-to-kill bioassays remained consistent in a study of these and other isolates evaluated by hazard ratios (Cox proportional hazards regression model) (Keyser et al., Reference Keyser, Fernandes, Rangel, Foster, Jech, Reuter, Black, Jaronski, Flake, Evans and Roberts2017).
High mortality in laboratory bioassays, however, often has not translated to high levels of pest control in the field (Sosa-Gomez and Moscardi, Reference Sosa-Gomez and Moscardi1998; Streett and Woods, Reference Streett, Woods, Branson and Redlin2001). Despite slower MC mortality with the M. acridum isolates in the laboratory, high levels of MC kill eventually were obtained with these isolates; and, importantly, the M. acridum isolates all had considerably higher conidial tolerance to heat (45°C) and UV-B radiation (Moore et al., Reference Moore, Bridge, Higgins, Bateman and Prior1993; McClatchie et al., Reference McClatchie, Moore, Bateman and Prior1994; Rangel et al., Reference Rangel, Braga, Anderson and Roberts2005; Rangel et al., Reference Rangel, Butler, Torabinejad, Anderson, Braga, Day and Roberts2006; Souza et al., Reference Souza, Azevedo, Lobo and Rangel2014; Dias et al., Reference Dias, Araújo, Pupin, Ferreira, Braga and Rangel2018; Rangel and Roberts, Reference Rangel and Roberts2018) as well as mycelial thermotolerance (Rangel et al., Reference Rangel, Fernandes, Dettenmaier and Roberts2010b) than any of the M. robertsii isolates.
Conclusions
Both M. acridum and M. robertsii have good potential for MC control, with M. acridum isolates having the distinct advantage due to their host specificity limited to Orthoptera (e.g. locusts and MCs and excluding non-target beneficial insects).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485321000663
Acknowledgments
We are grateful to Susan Durham for the statistical analyses; to Stefan Jaronski, USDA-ARS, Sidney, MT for suggestions on handling Mormon crickets. This research was supported by grants from the Utah Department of Agriculture and Food; USDA-APHIS Cooperative Research Agreement 13-8130-044-CA; and by the Community/University Research Initiative of Utah State University. D.E.N.R. was supported by the National Council for Scientific and Technological Development (CNPq) of Brazil for PhD fellowship GDE 200382/2002-0 and productivity fellowship PQ1D 308436/2014-8 and 302100/2018-0 and grant supports from the São Paulo Research Foundation (FAPESP) 2010/06374-1 and 2013/50518-6. We sincerely thank the Fulbright Foundation for a fellowship for H.G.B. and H.G.G of the Philippines to train in insect pathology in our USU laboratory.
Conflict of interest
The authors declare none.
Dedication
The lead author Drauzio E. N. Rangel and his wife Alene Alder-Rangel dedicate this study to his dear PhD advisor, adoptive father, and friend Dr. Donald W. Roberts, who is also an author of this article.
Insect pathology lost an extremely important scientist on 2 May 2021 when Dr. Donald W. Roberts (1933–2021) passed away at 88 due to natural causes. Originally from Phoenix, Arizona, USA, Don was inspired to study insect diseases as a teenager when he first saw nucleopolyhedrosis virus infecting caterpillars in cotton fields.
When studying for his B.S. at Brigham Young University (1957), a mycology teacher, Dr Kent McKnight, sparked his fascination in combining the disciplines of entomology and mycology. He then obtained his M.S. at Iowa State University (1959), working on the chemical and biological control of Evora hemidesma. For his PhD from the University of California at Berkeley (1964), he studied under the father of insect pathology, Edward Steinhaus. Don's PhD dissertation focused on destruxins of Metarhizium anisopliae.
Don spent most of his career (1965–1997) at the Boyce Thompson Institute (BTI) in New York where he helped build the world-renowned Insect Pathology Resource Center. Don's enthusiasm for insect pathology helped convert other scientists at BTI to take up the study of various insect pathogens. Early in his career, Don conducted pioneering research on the entomopoxvirus Amsacta moorei and fungal pathogens Coelomomyces and Metarhizium.
Although Don and his group worked with virtually every type of insect pathogen, his favorite was always fungal insect-pathogens, especially Metarhizium anisopliae. Don's group conducted basic, applied, and molecular studies on this fungus, making it a premier model. Fittingly, when M. anisopliae was divided into several new species in 2008, the type that he had studied most was named Metarhizium robertsii in honor of Don.
Don was a passionate promoter of insect pathology and biological control world-wide. He journeyed to 64 countries promoting biological control, making presentations, conducting research, and collecting pathogens, especially in the developing world. In these travels, Don collected fungi and contributed significantly to the ARSEF fungal collection in Ithaca, NY. He also supervised research projects in India, Nigeria, the Philippines, and Brazil. Don's research was pivotal for the early development of Brazil as a leader in biological control of insects using fungal pathogens.
Don retired from BTI, and at the age of 65, became a Research Professor for the Department of Biology at Utah State University, where he remained for 22 years (1997–2019). He focused on the effects of solar UV radiation and heat on entomopathogenic fungi. He had a large grant to investigate using insect-pathogenic fungi to control Mormon crickets. Don delved into a completely new subject initiating a research program on the parasite that causes Whirling Disease in fish. Part of the reason for this Utah location was to be closer to his children and grandchildren.
He is a founding member of the Society for Invertebrate Pathology (SIP), and this Society has always been very close to his heart. He attended and presented at almost every SIP meeting from the first in 1968 until 2017. He took a leading role in organizing the annual SIP meetings in 1983, 1984, and 2009. He served in a multitude of ways including chairing various committees as well as serving as Vice President (1986–88), President (1988–90), and Treasurer (1990–92). He was honored to present the SIP Founders lecture in 1982 and was recognized as the Founders Lecture Honoree in 2009. His life stories are detailed in a new book (Alder-Rangel, Reference Alder-Rangel2021).
Don's greatest legacy will be the people that he trained. With the help of his wife Mae, he took excellent care of his staff on a personal and professional level; earning the nickname ‘Uncle Don.’ A talented storyteller, he enjoyed using stories about his own adventures as parables to teach others about both science and life. All of his personal and professional activities have been the direct outgrowth of his deeply held belief that it is his duty to help improve the lives of other people. He was a very supportive person that encouraged his family, those who had the privilege to work with him, and many others to explore the world and make it a better place. In this spirit of service to and love for all, and in his professional dedication, Don was a role model worthy of emulation.
Don was also the star of the dance floor and a talented photographer. Donald Roberts leaves behind his wife Mae Roberts, two children, and three grandchildren, as well as innumerable scientific descendants that will pass on his passion for insect pathology.