Introduction
The world is green, despite the astonishing abundance and diversity of herbivores with the capacity to consume all known plant tissues. Soon after Hairston et al. (Reference Hairston, Smith and Slobodkin1960) formally put forward hypotheses to explain why this is so, prolific debate populated ecological literature. Currently, ecologists tend to agree (Begon et al., Reference Begon, Townsend and Harper2006) that the world is green not only because herbivores are limited by predators (‘top-down’ forces), but also because plants present effective chemical and physical defences that make them inedible by herbivores (‘bottom-up’ forces: Murdoch, Reference Murdoch1966).
Expanding on Murdoch's ideas, Abe & Higashi (Reference Abe and Higashi1991) brought detritivores and decomposers to the scene, proposing that the world is green because consumers are either able to feed on cytoplasm (most herbivores) or on cell wall (detritivores and decomposers), with a marked scarcity of destructive consumers, i.e. generalists feeding on both cell components. According to Abe & Higashi (Reference Abe and Higashi1991), this scarcity of generalists, together with the abundance of cell-wall specialists, could be ‘[…]viewed as one cause preserving the green earth by reducing the consumption of the living part of plants while enhancing the decomposition of the dead part[…]’, thereby speeding up the return of nutrients to the living part.
Detritivores and decomposers, in fact, shift the ‘green world’ puzzle one step down, to encompass the soil food web and a further enigma – the ground is brown. Why is it so, given the existence of detritivores and decomposers with the numbers and ability speed up the turnover of dark-coloured soil organic carbon? Allison (Reference Allison2006) convincingly hypothesizes that the ground is brown because both bottom-up and top-down forces operate on detritivores and decomposers, impairing their effective processing of soil organic carbon (SOC) into carbon dioxide, hence keeping the ground brown because carbon is kept locked into soil.
Here, we explore such an idea using termites (Insecta: Isoptera) in a field experiment, investigating whether bottom-up and/or top-down forces can delay resource encounter (and hence, usage) by these insects. Our rationale is that such delays would prevent prompt release of CO2 from organic matter to the atmosphere, thereby contributing to enhanced amounts of humic complexes which confer the dark appearance of the ground. We base our reasoning on the fact that termites play a key role in release from dead organic matter in tropical soils. By feeding on the wide decomposing continuum from fresh litter to humus, termites can affect the entire dynamics of soil carbon, both directly, by digesting cellulose (Slaytor, Reference Slaytor, Abe, Bignell and Higashi2000), and indirectly, by breaking litter down, thereby easing microbes' action on otherwise unexposed surfaces of litter items. Wood-feeding termites and their microbiota, for instance, can oxidise ≈99% of the carbon they intake, releasing it mainly as CO2 (Slaytor, Reference Slaytor, Abe, Bignell and Higashi2000). Soil-feeding termites, meanwhile, are well known to feed on highly humified material and are even suspected to process complex polyaromatic components of soil organic matter (SOM) which have been previously modified by microorganisms (Brauman et al., Reference Brauman, Bignell, Tayasu, Abe, Bignell and Higashi2000). In short, we argue here that one of the reasons for the ground being brown (in the tropics) is that trophic controls prevent termites from processing all available litter and humus, the remaining material being left to form impervious dark-coloured humic complexes in the soil.
Hypotheses
Being one of the few groups of animals whose main diet consists of cellulose, termites could be thought to experience no shortage of food. In addition, as they forage within tunnels, most termite foragers could be considered to be released from strong predation risks. The corollary of these hypotheses is that termites would not be constrained by food types and status, readily occupying the first item they find in the field. Unoccupied resources would exist simply because termite foragers have not found them yet. If this is so, food processing by termites is not constrained by either bottom-up or top-down forces, which stands as our null hypothesis. An alternative idea is that termite foraging, albeit time-dependent due to the need to build tunnels/galleries, is also affected by any trait that would make a resource more worthwhile to explore than a competing one. According to this view, resources in larger amounts, presenting more nutrients and free from predation risks, would be the first to be occupied, providing evidence that trophic controls may contribute to delays in termite foraging and hence to the ground being brown.
Materials and methods
Study site
The experiment was carried out in the municipality of Coimbra (S20–50.049'×W42–47.452'; altitude 650 m above sea level), Minas Gerais State, Southeastern Brazil. The climate is wet subtropical, with a dry season between May and September, annual mean precipitation of 1400 mm and annual mean temperature of 19°C (Valverde, Reference Valverde1958). The study area is a grassland neighboring a small fragment (7 ha) of Brazilian coastal rainforest (‘Atlantic rainforest’) naturally separated from it by a 3-m-wide stream at the bottom of a valley. The experimental site presents a flat topography and is visually homogeneous regarding vegetation and soil. Both grassland and forest are immersed in a matrix composed of a mosaic of implanted pastures and small-scale agricultural fields.
Experimental procedure
The experiment aimed to check how fast termites are found on feeding (cellulosic) resources of varying quantity and nutrient content (i.e. bottom-up effects), and how predation risks (top-down) affect such an encounter rate. Cellulosic baits (unbleached, unscented, uncolored, single ply toilet paper rolls 12-cm tall×10-cm Ø weighing 100 g) were used to mimic feeding resources. Such baits are well known to be promptly accepted and consumed by termites from all guilds in the field (Dawes-Gromadzki, Reference Dawes-Gromadzki2003). Two 12×12 grids were set up. Baiting points within grids were placed 1 m apart, each point holding one of the four instances of a factorial combination of high or low resource quantity×high or low resource quality. These four treatments were placed systematically through the grid such that every treatment neighbored all the others from all possible locations, thereby assuring that each bait type had equal chances of being chosen by termites. Although such a grid design does not favour independence between bait locations, this interdependence was intentional as it allows equal chances of termite ‘choice’ (if it exists) for any bait type, as explained above. Baits on grid edges were not inspected; rather, they were installed to ensure that every baiting point was affected by the same number of neighboring baits (eight). Thus, the sampling area in each grid corresponded to 10×10 baits=100 baiting points.
Variation in resource quantity was simulated by supplying a baiting point with a single bait or three side-by-side triangularly disposed baits. Two pieces of expanded polystyrene with the same size and weight as the paper roll were allocated beside single baits (mimicking a 3-bait station) to ensure that shading and pressure on the soil surface were the same at all points. This was intended to limit differences in ‘thermal shade’, which are known to affect food location by termites (Ettershank et al., Reference Ettershank, Ettershank and Whitford1980). Variation in resource quality was simulated by adding nitrogen to the baits, which was based on the fact that termites are known to seek nitrogen-rich resources in the field (Shellman-Reeve, Reference Shellman-Reeve1994). This was achieved by ‘enriching’ baits with 100 ml of a 3% w/v water solution of NH4NO3, as used by Curtis & Waller (Reference Curtis and Waller1997) as the nitrogen source for termites in artificial diets. Baits, which were not nitrogen-enriched, received 100 ml of water.
Every week, termites and ants were collected under baits with the aid of entomological forceps. Baits were returned to their original location immediately after inspection/collection. Predation risks were estimated by recording the presence/absence of ants on the baits, since ants are known to be major predators of termites (e.g. Sheppe, Reference Sheppe1970; Leal & Oliveira, Reference Leal and Oliveira1995). A bait was classified as occupied only when it contained galleries and termites were actually found feeding on it. A bait was recorded as free from predation risk only if ants were never spotted on it throughout the whole experiment. Sampling took place in the warm-wet period of January to March 2004, from 8:00 to 12:00 h. The experiment was repeated, with a new set of baits, in the cool-dry period of April to June 2004, to allow for differences in seasonal patterns of foraging (Moura et al., Reference Moura, Vasconcellos, Araújo and Bandeira2006).
Statistical analyses
Data were taken as the number of days it took for termites to occupy baits, whether or not they were subsequently abandoned. For this, data were subjected to censored survival analysis with a Weibull distribution (Crawley, Reference Crawley2007), performed with survival package in R (R Development Core Team, 2006). Survival analysis, or failure time data analysis, means the statistical analysis of data where the response of interest is the time, t, from a well-defined time origin to the occurrence of some given event (end-point) (Martinussen & Scheike, Reference Martinussen and Scheike2006). In our specific case, the time origin is the moment of bait installation in the field, and the end-point is the first day termites were spotted on such bait. The analysis aimed to inspect whether resource traits would affect the time elapsed until a bait was first spotted with termites. Here, we are inspecting, therefore, the ‘survival’ of a bait in the field; its ‘death’ being considered to happen at the moment it was first found with termites. Similar uses of such an analysis for termites (but not for baits) can be found in DeSouza et al. (Reference DeSouza, Miramontes, Santos and Bernardo2001) and Miramontes & DeSouza (Reference Miramontes and DeSouza1996).
The general model for this analysis followed the equation:
where S(t) is the accumulated proportion of baits occupied until time, t; the mean time, μ, is the time elapsed until 50% of the baits of a given resource type are found with termites; and α is the shape parameter for the survival curve. When α=1, the probability of finding a bait does not change as time elapses. If α<1 this probability reduces as time elapses, the converse happening when α>1.
Statistical analysis was used to check whether the resource traits, bait quantity (single or triple baits), bait quality (nitrogen added/not-added), and predation risk (absence/presence of ants), would affect the mean time, μ, spent until termites are found on the bait. The analysis began by estimating α for the whole dataset and proceeded with hypotheses testing. Under the null hypothesis for a given α, the mean time, μ, to find a bait does not differ between resource traits and, hence, time, t, alone explains S(t). Alternatively, if resource traits affect bait encounter by termites, a typical μ can be calculated for each resource trait, and histograms can be plotted to ease visualisation of the differences between bait types. This would be taken as evidence that its corresponding regulatory force (bottom-up or top-down) was in effect speeding up the decomposition process of some items at the expense of others, which would point to a contribution of this regulatory force to delay SOC processing and, ultimately, to the brown ground.
Modelling proceeded by building a full model, including all of the above parameters and their second and third order interactions, plus a blocking term (‘season’) to distinguish between the two runs of the experiment. To inspect redundancy of effects, various full models were created, composed of the same terms entered in a different order. Model simplification, was performed by backward term extraction, removing one term at a time. Terms returned to the model if their removal provoked a change of deviance with P<0.05.
Voucher specimens
Specimens of ants and termites were preserved in 80% alcohol, labelled and subsequently identified to species or morphospecies as appropriated. Termite identifications followed the literature (Constantino, Reference Constantino1999, and papers therein), being subsequently confirmed by comparison with the collection of the Termite Section of the Entomological Museum (UFVB) of the Federal University of Viçosa (UFV) (http://www.insecta.ufv.br/museu) where voucher specimens were deposited. Further confirmation of termite identities was kindly provided by R. Constantino from the University of Brasilia and A. Acioli from the Federal University of Amazonas. Ants were identified by comparison with the collection of the Community Ecology Laboratory of UFV.
Results
In the first half of the study period (January to March 2004, end of wet-warm season), rainfall attained a daily average of 10.2 mm, dropping to 1.9 mm in the second half (April to June 2004, beginning of the cool-dry season). Daily mean temperatures were 22.0 and 18.7°C, respectively.
Termites collected comprised ten species from seven genera and three subfamilies, all belonging to a single family (Termitidae) (table 1). Among those, at least seven species feed on soil and on highly humified wood (Apicotermitinae and Termitinae) and three (Nasutitermitinae) feed on litter and wood at initial stages of decomposition. Termites were easily spotted extracting fragments of the bait. Apicotermitinae termites frequently dug galleries on the lower end of the bait, whereas Nasutitermes jaraguae (Nasutitermitinae) conspicuously bore many galleries through the bait. Ants (Hymenoptera: Formicidae) comprised ten species from nine genera and four subfamilies, among which eight species are predators, generalists or omnivores and two species are fungus cultivators (table 2). On several occasions, ants were spotted preying on termites in the baits.
Table 1. Termites (Insecta: Isoptera: Termitidae) recorded in cellulosic baits disposed in a grassland bordering an ‘Atlantic forest’ relict in southeastern Brazil. Diet types are based on the genus and are in accordance with Araújo et al. (Reference Araújo, Galbiati and DeSouza2007). Soil feeders may sometimes be reported as ‘humivorous’.
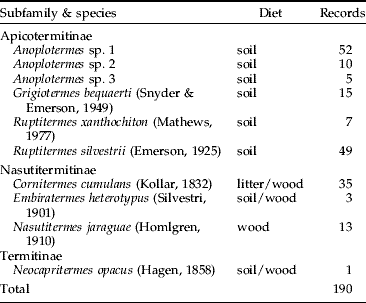
Table 2. Ant species (Hymenoptera: Formicidae) collected in cellulosic baits disposed to collect termites in a grassland bordering an ‘Atlantic forest’ relict in southeastern Brazil. Trophic groups are based on Brown Jr. (Reference Brown, Agosti, Majer, Alonso and Schultz2000).
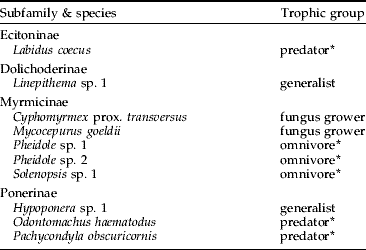
* denotes ants known to prey upon termites.
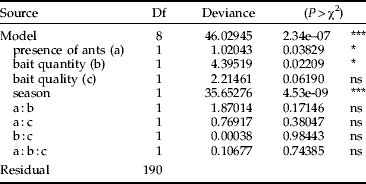
The minimal statistical model achieved (equation 2) presented α=1.26, indicating that the probability of finding termites on a bait increased as time elapsed. That is, termites were not promptly spotted on the bait; they started to be found only after some initial time lag. This model also shows that some resource traits (but not their interactions) did modify the time needed until the bait was first spotted with termites. That is, all baits took some initial time lag to be occupied by termites, but for some bait types such a time lag was shorter than for others. Therefore, we have evidence disproving our null hypothesis in favour of the alternative one, namely, that food types and status would constrain termite use of resources.
The time, μ, that baits of a given resource trait took to be occupied in the field by termites can be estimated by
where s refers to the season when the data was collected, q is bait quantity and r is predation risk (the resource traits referred to in the ‘Material and methods’ section). This μ value, when replaced in equation 1 above, gives the accumulated proportion S(t) of baits found with termites after a given time, t, has elapsed. To calculate loge μ (and hence μ for each resource trait), it is sufficient to replace the value 0 or 1 in equation 2, respectively, for q=single or triple baits; r=ants absent or present; s=warm-wet or cool-dry.
As a general pattern (fig. 1), resource use by termites was severely delayed during the warm-wet period by low bait quantity and by the presence of ants. Bait quality showed no significant effect, indicating that in the present case, nitrogen additions did not pose strong constraints on termite foraging. Baits took longer to be occupied in the first run of the experiment (warm-wet season) than in the subsequent period (cool-dry season). In both periods, single baits took longer to be occupied than triple baits. Accordingly, regardless of season and bait quantity, baits with ants took longer to be occupied by termites than baits without ants.

Fig. 1. Number of days (mean±SE), as a function of bait quantity (single or triple bait) and status (with or without ants), spent in the field until cellulosic baits are found to have termites for two consecutive seasons in the same year in a grassland bordering an ‘Atlantic forest’ relict in southeastern Brazil. Differences between treatments are significant, see table 3 (▪, with ants; ![]() , without ants).
, without ants).
Discussion
Trophic control in soil food webs is receiving increased attention in recent years, because it is becoming evident that top-down and bottom-up forces belowground may severely impact aboveground dynamics (Moore et al., Reference Moore, McCann, Setälä and de Ruiter2003). In fact, in temperate soils, whether carbon is kept in the soil or released as CO2 to the atmosphere will depend in large part on the interruption and/or delay of decomposer activity (Ekschmitt et al., Reference Ekschmitt, Kandeler, Poll, Brune, Buscot, Friedrich, Gleixner, Hartmann, Kastner, Marhan, Miltner, Scheu and Wolters2008). Such activity depends on (i) bottom-up forces, represented by the quality of the substrate and the rate of its encounter by decomposers (Ekschmitt et al. Reference Ekschmitt, Liu, Vetter, Fox and Wolters2005) and (ii) top-down controls upon microbes (Moore et al., Reference Moore, McCann, Setälä and de Ruiter2003). Detritivores, in turn, may also impact carbon flux since their action accelerates the decomposition of soil carbon (Fox et al., Reference Fox, Vetter, Ekschmitt and Wolters2006). It follows that the concurrent action of bottom-up and top-down controls upon detritivores and decomposers may delay litter and SOC processing, thereby contributing to the ground being brown (Allison, Reference Allison2006).
While stressing the notion of detritivores and decomposers as crucial for the fate of carbon in tropical terrestrial ecosystems, our results seem to conform closely to the hypothesis above. Termites in the field were found occupying certain food types much earlier than others. More specifically, predation risks severely delayed bait occupation by termites; and, under the same predation risk, the smaller the bait, the longer it took to be occupied (table 3, fig. 1). This could be driven by previous evaluation by termites of the value of exploring a given resource and/or by predation upon and removal of termites in ant-occupied baits. No matter the mechanism, a combination of top-down and bottom-up forces seems to be playing a key role in this process.
Table 3. Analysis of deviance table for the survival model used to check whether resource traits would affect the promptness in which a bait is found having termites, more than time alone would do. Modelling proceeded under Weibull distribution; full details are given in the ‘Material and methods’ section.
ns P>0.05;
* P<0.05; *** P<0.01.
We argue that such results would pose trophic controls upon termites as an important bottleneck for the carbon cycle. After all, apart from releasing carbon directly as CO2 (Slaytor, Reference Slaytor, Abe, Bignell and Higashi2000) and exposing carbon to microbes by disassembling plant debris, termites also unlock carbon directly to the atmosphere as CO2 (Martius et al., Reference Martius, Fearnside, Bandeira and Wassmann1996), which emphasises their role as carbon processors in ecosystems. Besides being effective wood and litter processors, termites are also able to ingest mineral-containing soil horizons, as well as highly humified organic litter material (Brauman et al., Reference Brauman, Bignell, Tayasu, Abe, Bignell and Higashi2000), thus promoting further processing of SOC. Therefore, by impairing the effective use of resources by termites, top-down and bottom-up forces would limit prompt transformation in CO2 of all carbon that is available as dead organic matter, thereby enhancing the pools of humic complexes in the soil. That is, rather than readily exploring the first item they found in the field, termites would favour items that are larger and risk-free. As a consequence, carbon present in smaller or risky items remains locked in organic form in the soil, enhancing the pools of humic complexes rather than being released to the atmosphere. Such a reasoning is supported by the findings by Hedlund & Henderson (Reference Hedlund and Henderson1999) that termites (in the laboratory) will vary their rate of consumption with the size of their food, i.e. they eat small food more slowly.
The relative strength of trophic controls
A closer look into the final statistical model (equation 2) reveals some interesting patterns, if we compare the impact of its numerical estimates on the mean time (μ) taken to find termites on a bait. To ease comprehension, we invite the reader to check the reasoning below against fig. 1 along with equation 2. Firstly, the effects of season were much more marked than the effects of bait quantity and predation risk, as season (s) contributed to 1.191 units to logeμ, a value that is more than twice as big as the values for bait quantity, q, and predation risk, r. It is worth noting that the seasons reported here differed a lot in terms of rainfall (daily averages: 10.2 mm in warm-wet season and 1.9 mm in cool-dry season) but seemed similar in terms of temperature (daily mean temperatures: 22.0 and 18.7°C, respectively). In addition, we could not detect any visual differences in litter accumulation (hence, resource offer) between seasons. All this might indicate that what we saw as seasonal effects could be mostly attributable to rainfall, which is in line with previous findings by Jones & Gathrone-Hardy (Reference Jones and Gathrone-Hardy1995) and Cabrera & Rust (Reference Cabrera and Rust1996), who reported that rainfall impairs termite activity. We warn, however, that this is mostly speculative, since the experiment was not aimed to inspect seasonal effects; and, hence, no proper replication was set up for seasons.
The estimates for bait quantity and for predation risk (respectively, q and r in equation 2) present very similar values (0.448 and 0.470), denoting that their impact on the mean time to find a bait is of comparable magnitude, with predation risk being slightly more important than bait quantity. In other words, bottom-up and top-down effects seem to have operated with similar strengths. This is easily spotted in fig. 1, where single-baits-without-ants and triple-baits-with-ants take about the same time to be found with termites, in both seasons. Such results are supported by previous reports, which have shown (i) that termite foraging can be severely limited by predation risk from ants (Gonçalves et al., Reference Gonçalves, Reis-Jr, DeSouza and Ribeiro2005), (ii) that termites abandon less and consume more larger baits as opposed to smaller ones (Evans & Gleeson, Reference Evans and Gleeson2006), or even (iii) that termites tend to explore longer food items which are not under predation risk (Korb & Linsenmair, Reference Korb and Linsenmair2002).
Surprisingly enough, bait quality (=nitrogen additions to the bait) did not affect the mean time until bait occupancy (P=0.0619; table 3) which could be related to the ability of termites to fix nitrogen (Yamada et al., Reference Yamada, Inoue, Wiwatwitaya, Ohkuma, Kudo and Sugimoto2006).
Perhaps, more interestingly, the final statistical model did not include any interaction term, which would imply the independence of all factors studied. That is, despite differences between seasons, the general pattern does not change, termites seem to avoid smaller items that are under predation risk no matter the season (fig. 1). This seems to reinforce the idea that while climate determines the intensity of foraging activity of termites, trophic controls are responsible for ‘fine tuning’ such activities, determining where and how foraging would proceed.
Conclusion
The evidence gathered here for tropical soil food webs seems to support the idea that decomposers and detritivores, in general, and termites, in particular, may connect the green world to the brown ground, as depicted in fig. 2. As plants shed their dead parts naturally, decomposers act upon them, unlocking SOC and minerals. These would be ultimately reused by plants, thereby closing the cycle. Herbivores speed up the process, producing additional plant debris out of living plant tissue far quicker than natural shedding would. However, delays are imposed in this process by predators (Hairston et al., Reference Hairston, Smith and Slobodkin1960) and plant defences (Murdoch, Reference Murdoch1966), which secure the failure of herbivores in destroying plants and lead to the green world. Detritivores act upon such debris and speed up decomposition processes by breaking litter into smaller portions and exposing surfaces which, otherwise, would stay for much longer out of the reach of decomposers. Termites take an important role in this process, due to their ability to process plant matter directly into CO2 (as a decomposer would do), coupled with their well-known action as detritivores. Again, as evidenced by our results, predators and food unsuitability delay the action of termites, thereby establishing an important bottleneck to the system. Such delays increase residence times of carbon in soil, hence contributing to the increment of pools of humic complexes, hence making the ground brown. Additionally, reprocessing of SOC into compounds impervious to enzymatic action of decomposers (including perhaps termites, especially soil-feeding ones), prevents SOC from re-entering soil food web, “making the ground more brown than the world is green” (Allison, Reference Allison2006).

Fig. 2. Pathways connecting the green world to the brown ground and back, combining reasonings of Hairston et al. (Reference Hairston, Smith and Slobodkin1960) and Murdoch (Reference Murdoch1966) for the green world, with hypotheses of Allison (Reference Allison2006) for the brown ground. See text for details.
Acknowledgements
Many thanks to two anonymous referees whose comments led to important improvements on the manuscript. We thank Sam Elliot and Arne Janssen for invaluable discussion and English language revision. S. Allison kindly clarified the text, adjusting our reasoning to his original hypothesis. A. Pallini, B. Freymann, R. Krüger and J. Louzada provided useful insights; R. Constantino and A. Acioly confirmed termite identifications; Danival Souza identified the ants; C. Galbiati, J.G. Rocha and G.S. Brunow helped in field work. Maurinho L. dos Santos kindly allowed the use of the field site. O. DeSouza was supported by a fellowship (#306081/2007-5) from Brazilian National Council for Research (CNPq) and A.P.A. Araújo by a Capes PhD grant, respectively. This work was partially funded by Fapemig, CNPq, Capes. All computational work was performed using free software (GNU-Linux/Debian, LATEX, XEmacs, Inkscape, R, OpenClipArt, among others). This is contribution #40 of the Termitology Lab at Federal University of Viçosa, Brazil (http://www.isoptera.ufv.br).







