Introduction
The mealworm beetle, Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae) is a pest of stored products such as starches, food for cats and dogs, and pasta. This insect may also infest broken grains of Zea mays (L.) (Poales: Poaceae), Triticum aestivum (L.) (Poales: Poaceae), and Glycine max (L.) (Fabales: Fabaceae) (Punzo & Mutchmor, Reference Punzo and Mutchmor1980; Fazolin et al., Reference Fazolin, Estrela, Catani, Alécio and Lima2007; Cosimi et al., Reference Cosimi, Rossi, Cioni and Canale2009). The presence of T. molitor in stored grain and bran can contaminate food with fragments of the body, feces, and indirectly by saprophytic microorganisms causing loss of food quality (Loudon, Reference Loudon1988; Schroeckenstein et al., Reference Schroeckenstein, Meier-Davis and Bush1990; Barnes & Siva-Jothy, Reference Barnes and Siva-Jothy2000). This insect causes losses up to 15% of grains and flour production worldwide (Dunkel, Reference Dunkel1992; Flinn et al., Reference Flinn, Hagstrum, Reed and Phillips2003; Neethirajan et al., Reference Neethirajan, Karunakaran, Jayas and White2007).
Tenebrio molitor is controlled primarily with chemical insecticides, but this method has restrictions against stored product insects (Shaaya et al., Reference Shaaya, Kostjukovski, Eilberg and Sukprakarn1997), due to residual toxicity and insect resistance (Isman, Reference Isman2006), especially in countries with extensive cereal production for export and domestic consumption (Arthur, Reference Arthur1996; Shaaya et al., Reference Shaaya, Kostjukovski, Eilberg and Sukprakarn1997). Chemical control of this insect can be achieved by phosphine treatment; however, fumigants cannot kill the eggs of storage pests and several issues have been discussed in the employment of insecticides, such as residue, environment impact, and toxicity to humans (Arthur, Reference Arthur1996; Shaaya et al., Reference Shaaya, Kostjukovski, Eilberg and Sukprakarn1997; Isman, Reference Isman2006). Economic, social, and environmental concerns have caused a gradual change to reduce chemical control in starches and stored products (Arthur, Reference Arthur1996; Zettler & Arthur, Reference Zettler and Arthur2000; Isman, Reference Isman2006). More selective and biodegradable products, including ‘green pesticides’, can reduce the use of synthetic chemicals in warehouses (Isman, Reference Isman2000; Martínez et al., Reference Martínez, Plata-Rueda, Zanuncio and Serrão2015; Plata-Rueda et al., Reference Plata-Rueda, Martínez, Dos Santos, Fernandes, Wilcken, Soares, Serrão and Zanuncio2017).
Plant essential oils have favorable ecotoxicological properties (low toxicity to humans, degradation, and lower environmental impact), making them suitable to managing insects in organic farming (Chermenskaya et al., Reference Chermenskaya, Stepanycheva, Shchenikova and Chakaeva2010; Zanuncio et al., Reference Zanuncio, Mourão, Martínez, Wilcken, Ramalho, Plata-Rueda and Serrão2016). These oils are plants secondary metabolites and include alkaloids, amides, chalcones, phenols, flavones, lignans, neolignans, or kawapirones which are important in insect–plant relationships (Isman, Reference Isman2000; Martínez et al., Reference Martínez, Plata-Rueda, Zanuncio and Serrão2015). In this sense, essential oils represent an alternative for pest control as repellents, deterrent of oviposition and feeding, growth regulators, and toxicity to insects with low pollution and quick degradation in the environment (Chermenskaya et al., Reference Chermenskaya, Stepanycheva, Shchenikova and Chakaeva2010; Zanuncio et al., Reference Zanuncio, Mourão, Martínez, Wilcken, Ramalho, Plata-Rueda and Serrão2016; Plata-Rueda et al., Reference Plata-Rueda, Martínez, Dos Santos, Fernandes, Wilcken, Soares, Serrão and Zanuncio2017). Various studies have focused on the possibility of using plant essential oils for application to stored grain to control insect pests (Zapata & Smagghe, Reference Zapata and Smagghe2010; Stefanazzi et al., Reference Stefanazzi, Stadler and Ferrero2011; Jemâa et al., Reference Jemâa, Tersim, Toudert and Khouja2012).
The cinnamon, Cinnamomum zeylanicum Blume (Lauraceae) is a tropical evergreen tree and grows wild in Sri Lanka, Madagascar, India, and Indochina. The inner bark of the tree is used in ethno-medicine and flavoring for foods (Bakkali et al., Reference Bakkali, Averbeck and Averbeck2008). Different studies showed that extracts and constituents of C. zeylanicum have antimicrobial, insecticidal, and acaricidal properties (Yang et al., Reference Yang, Lee, Lee, Clark and Ahn2005; Fichi et al., Reference Fichi, Flamini, Zaralli and Perrucci2007; Shahverdi et al., Reference Shahverdi, Monsef-Esfahani, Tavasoli, Zaheri and Mirjani2007). Clove, Syzygium aromaticum (L.) Merr at Perry (Myrtaceae) is an evergreen tree and native from Indonesia. Owing to its biological activities, clove oil finds extensive use in medicine, food, cosmetic items, and pest control (Ho et al., Reference Ho, Cheng, Sim and Tan1994; Yoo et al., Reference Yoo, Han, Cho, Ha, Park, Nam and Lee2005; Jirovetz et al., Reference Jirovetz, Buchbauer, Stoilova, Stoyanova, Krastanov and Schmidt2006). Toxic effects of cinnamon and clove essential oils have been evaluated with success to control of agricultural pests (Regnault-Roger, et al., Reference Regnault-Roger, Hamraoui, Holeman, Theron and Pinel1993; Ho et al., Reference Ho, Cheng, Sim and Tan1994; Isman, Reference Isman2000). There are a variety of insecticides that have toxicological properties, deterrents, and repellents used for the control of T. molitor; however, essential oil of cinnamon and clove could be an alternative for the control in stored products. Identification of toxic compounds of cinnamon and clove is important in understanding toxicity as it relates to pest control. In this study, we hypothesized that cinnamon and clove essential oils and their constituents have insecticidal activity in T. molitor.
In this series of experiments, we evaluated the toxicity and repellency of cinnamon and clove essential oils and their main constituents against T. molitor, in order to contribute for the development of strategies for controlling this insect pest affecting an important source of food.
Materials and methods
Insects
Tenebrio molitor were obtained from the Laboratory of Biological Control of the Institute of Applied Biotechnology to Agriculture (BIOAGRO, Universidade Federal de Viçosa) in Viçosa, Minas Gerais State, Brasil. Adults of T. molitor were kept in plastic trays (60 cm long × 40 cm wide × 12 cm) and maintained at 25 ± 1°C, 70 ± 10% RH and 12 : 12 h L:D photoperiod. These insects were fed ad libitum with wheat bran (12% protein, 2% lipids, 75% carbohydrates, and 11% mineral/sugar), pieces of sugarcane, Saccharum officinarum (L.) (Poaceae) and chayote Sechium edule (Jacq.) Swartz (Cucurbitaceae). Sheets of paper were placed on the substrate to facilitate oviposition. Healthy larvae (last instar larval), pupae, and adults of T. molitor of 48 h old were chosen for the bioassays.
Essential oils
The essential oils of cinnamon, C. zeylanicum and clove, S. aromaticum were obtained from Ferquima Industry & Commerce Ltda. (Vargem Grande Paulista, São Paulo State, Brazil), produced in industrial scale by hydrodistillation drag of water vapor (Dapkevicius et al., Reference Dapkevicius, Venskutonis, Van Beek and Linssen1998).
Identification of essential oil compounds
Quantitative analyses of the cinnamon and clove essential oils were performed in quadruplicate using a gas chromatograph (GC-17A series instrument, Shimadzu, Kyoto, Japan) equipped with a flame ionization detector. The following chromatographic conditions were used: a fused silica capillary column (30 m × 0.22 mm) with a DB-5 bonded phase (0.25 µm film thickness); carrier gas N2 at a flow rate of 1.8 ml min−1; injector temperature 220°C; detector temperature 240°C; column temperature programmed to begin at 40°C (remaining isothermal for 2 min) and then to increase at 3°C min−1 to 240°C (remaining isothermal at 240°C for 15 min); injection volume 1.0 µl (1%w/v in CH2Cl2); split ratio 1 : 10; column pressure 115 kPa.
The compounds were identified using a gas chromatograph coupled with a mass detector GC/MS (GCMS-QP 5050A; Shimadzu, Kyoto, Japan). The injector and detector temperatures were 220 and 300°C, respectively. The initial column temperature was 40°C for 3 min, with a programmed temperature increase of 3°C min−1 to 300°C where it was maintained for 25 min. The split mode ratio was 1 : 10. One microliter of each essential oil containing 1% (w/v in dichloromethane) was injected; helium was used as the carrier gas with a flow rate constant of 1.8 ml−1 on the Rtx®−5MS capillary column (30 m, 0.25 mm × 0.25 µm; Bellefonte, PA, USA) using the Crossbond® stationary phase (35% diphenyl–65% dimethyl polysiloxane). Mass spectrometer was programmed to detect masses in the range of 29–450 Da with 70 eV ionization energy. Compounds were identified by comparisons of the mass spectra with those available in the NIST08, NIST11 library and Wiley Spectroteca Data Base (7th edition), and by the Retention indices.
Toxicity of essential oils
The essential oil efficacy was determined by calculating the lethal concentration (LC50 and LC90) values under laboratory conditions and conducted in triplicate. Six concentrations of cinnamon and clove essential oils besides the control (acetone) were adjusted in 1 ml of stock solution (essential oil and acetone): 1, 2, 4, 8, 16, and 32% (w/v). Aliquots were taken from the stock solution and mixed with acetone in 5 ml glass vials. Different concentrations of each essential oil were applied in 1 µl solution on the thorax of larva, pupa, and adult of T. molitor, using a micropipette. For each developmental stage, 50 insects were used per concentration and placed individually in Petri dishes (Ø 90 mm × 15 mm) with perforated cap for ventilation and an absorbent paper, fed with chayote and sugarcane ad libitum (larvae and adults) and maintained in the dark. The number of dead insects was counted after essential oil exposure for 48 h.
Toxicity of commercial compounds of two essential oils in T. molitor
Major constituents of the cinnamon and clove essential oils including eugenol, caryophyllene oxide, α-humulene, α-phellandrene, and α-pinene were tested for toxic effects and obtained commercially. Eugenol (purity 99%), caryophillene oxide (purity 99%), α-humulene (purity 96%), α-phellandrene (purity 85%), and α-pinene (purity 98%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Six different concentrations of the commercial compounds besides the control (acetone) were adjusted in 1 ml of stock solution (treatment and acetone) used to calculate the lethal LC50 and LC90 concentrations: 2, 4, 8, 16, 32, and 64 µg insect−1. For each treatment, aliquots were taken from the stock solution and mixed with acetone in 5 ml glass vials. Different concentrations of the each treatment were applied in 1 µl solution on the thorax of larva, pupa, and adult of T. molitor. Individuals were placed in Petri dishes and fed with natural diet (10 g wheat bran + 10 g sugarcane in the ratio 1 : 1). A total of 50 larvae, 50 pupae, and 50 adults were used for each concentration and mortality was evaluated for 48 h.
Comparison of the LT50 values of the essential oils and their major compounds
Toxicity of cinnamon and clove essential oils, and commercially obtained eugenol, caryophyllene oxide, α-humulene, α-phellandrene, and α-pinene at the calculated LC50 concentration was compared. Acetone was used as a control. For the LC50 of each compound, 1 µl was applied on the thorax of larvae, pupae, and adults of T. molitor using a micropipette. Insects were individualized in Petri dishes with wheat bran and sugarcane. A total of 240 larvae, 240 pupae, and 240 adults were used for each treatment, with a total of four replicates. Mortality was recorded every 6 h for 48 h, and estimated lethal time values for 50% mortality (LT50) were compared.
Repellency effects of the essential oils and their major compounds
Four Petri dishes (12 × 1.5 cm) were used as an arena, connected to a central board with plastic tubes (diameter 2 cm) at an angle of 45°. The other dishes were distributed around them in equidistant distances and two plates were put together symmetrically opposed (fig. 1). Two hundred and forty individuals (120 larvae and 120 adults) of T. molitor were used in this experiment. In each assay replicate, 20 insects were released in the central board and the control group received sugarcane and chayote. A total of 5 µl of the estimated LC50 lethal concentration of each essential oil and toxic compounds were applied on absorbent filter paper (2 × 2 cm) placed in two opposite plates used as treatment, and two opposite ones with 5 µl of distilled water on absorbent filter paper represented the control. Four replicates per treatment and control were evaluated by the number of individuals per plate after 12 h calculating the repellency index (RI): RI = 2G/(G + P), where G is the percentage of insects in the treatment and P is the percentage of insects in control. Treatments were classified as neutral if the index was equal to one (1); repellent, higher than one (1), and; attractive lower than one (1).
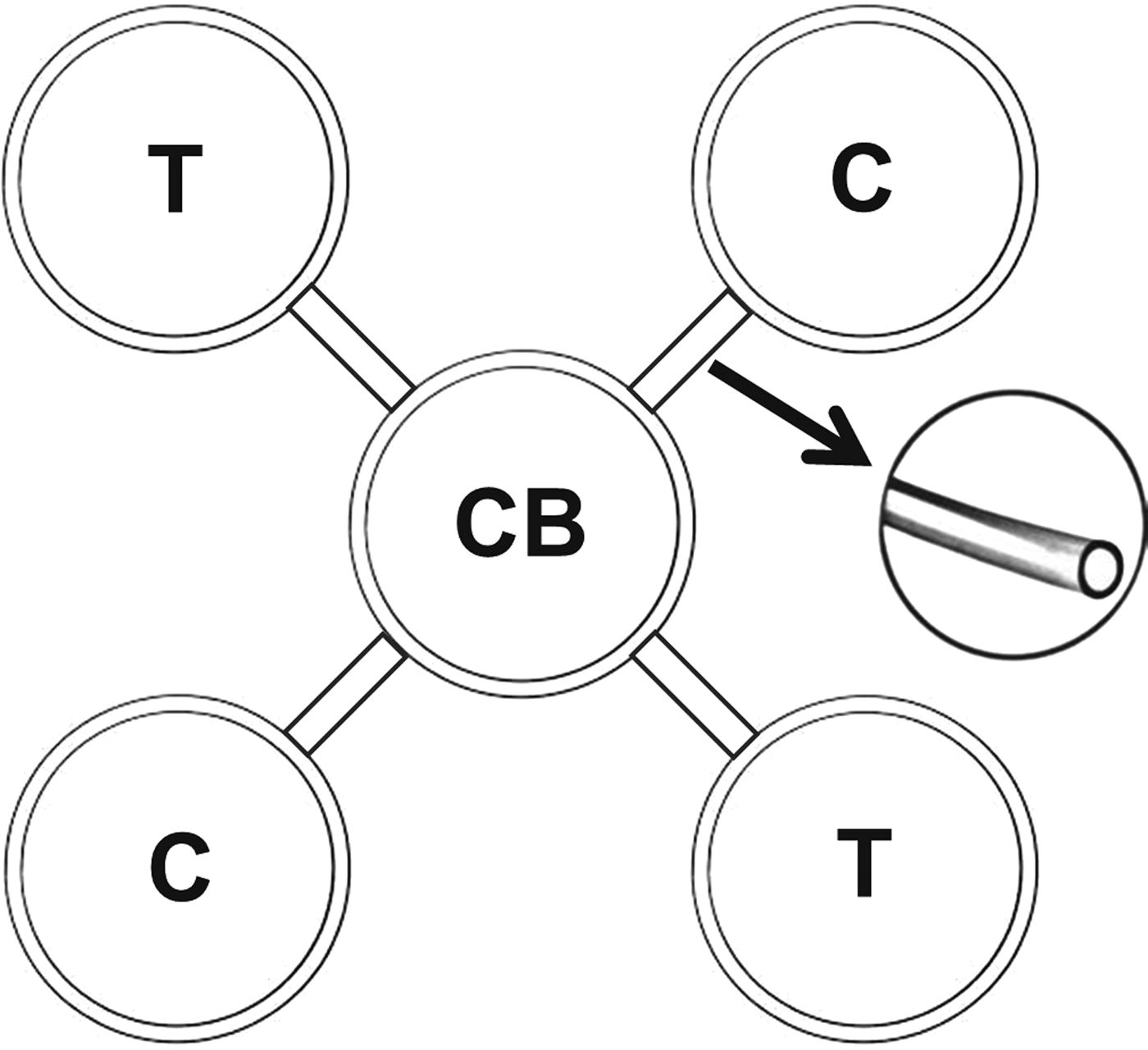
Fig. 1. Schematic drawing of four Petri dishes used as an arena, connected to a central board (CB) with plastic tubes at an angle of 45°. Treatment (T) and control (C) were distributed in equidistant distances and symmetrically opposed. Not drawn to scale.
Statistical analyses
Lethal concentration (LC50 and LC90) and their confidence limits for cinnamon and clove essential oils and compounds were determined by logistic regression in dose–response assays based on the concentration Probit-mortality (Finney, Reference Finney1964) using XLSTAT-PRO (v.7.5) program for Windows (XLSTAT, 2004). Student's t test was used for pairwise comparisons regarding lethal time effects in T. molitor using SAS User software (v. 9.0) for Windows (SAS, 2002).
Results
Identification of essential oil compounds
A total of 23 compounds were identified in the two essential oils, which accounted 96–97% of the total composition (fig. 2, table 1). The primary compounds of the essential oil of cinnamon were eugenol (10.19%), trans-3-caren-2-ol (9.92%), benzyl benzoate (9.68%), caryophyllene (9.05%), eugenyl acetate (7.47%), α-phellandrene (7.18%), α-pinene (6.92%), cinnamyl acetate (6.91%), safrole (5.51%), nerolidol (5.02%), terpinolene (4.35%), cinnamaldehyde (4.12%), linalol (2.87%), β-pinene (2.43%), terpineol (2.11%), α-copaene (1.96%), myrcene (1.95%), and camphene (1.31%). For clove essential oil, the primary compounds were eugenol (26.64%), caryophyllene (23.73%), caryophyllene oxide (17.74%), 2-propenoic acid (11.84%), α-humulene (10.48%), γ-cadinene (4.85%), and humulene oxide (4.69%).

Fig. 2. Gas chromatogram profiles of peak retention of compounds of the cinnamon and clove essential oils. (a) Cinnamon: α-pinene (1), camphene (2), β-pinene (3), myrcene (4), α-phellandrene (5), terpinolene (6), trans-3-caren-2-ol (7), linalol (8), α-terpineol (9), cinnamaldehyde (10), safrole (11), eugenol (12), copaene (13), caryophyllene (14), cinnamyl acetate (15), eugenyl acetate (16), nerolidol (17), benzyl benzoate (18). (b) Clove: 2-propenoic acid (1), eugenol (2), caryophyllene (3), α-humulene (4), γ-cadinene (5), caryophyllene oxide (6), humulene oxide (7).
Table 1. Chemical composition of cinnamon and clove essential oil.

MM, molecular mass; RI, relative intensity; R i, retention indices; R t, retention time; m/z, molecular weight.
Toxicity of essential oils
Mortality of T. molitor was obtained with 16 and 32% (w/v) of each essential oil and two different lethal concentration levels were estimated by Probit (χ2, P < 0.0001) (table 2). The LC50 and LC90 values indicated that cinnamon essential oil was the most toxic to T. molitor larvae (LC50 = 30.4 µg and LC90 = 55.1 µg; χ2 = 99.76, df = 5), followed by clove oil (LC50 = 35.1 µg and LC90 = 67.2 µg; χ2 = 60.43, df = 5). In contrast, clove essential oil was most toxic to T. molitor pupae with LC50 = 6.45 µg and LC90 = 14.6 µg (χ2 = 60.43, df = 5) followed by cinnamon oil with LC50 = 10.7 µg and LC90 = 20.9 µg (χ2 = 74.74, df = 5). Clove essential oil was the most toxic to T. molitor adults with LC50 = 21.6 µg and LC90 = 44.5 µg (χ2 = 12.39, df = 5) and cinnamon oil with LC50 = 29.8 µg and LC90 = 56.03 µg (χ2 = 17.81, df = 5). Mortality was always <1% in the control.
Table 2. Lethal concentrations of cinnamon and clove essential oil against different developmental stages of Tenebrio molitor after 48 h exposure.

EO, essential oil; IS, insect stage; LC50 and 90, lethal concentration causing 50 and 90% mortality; EV, estimated value; CI, confidence interval; χ2, chi-squared value for the lethal concentrations and fiducial limits based on a log scale with significance level at P < 0.001.
Toxicity of commercial compounds of two essential oils in T. molitor
The toxicity of commercially obtained eugenol, caryophyllene oxide, α-humulene, α-phellandrene, and α-pinene in T. molitor was estimated by Probit (χ2, P < 0.0001) and evaluated at different concentrations (table 3). To larva stage, dose–response bioassays showed results with eugenol with LC50 = 9.15 (7.84–10.7) μg and LC90 = 18.9 µg (16.4–22.6), followed by caryophyllene oxide with LC50 = 9.21 (7.59–11.1) μg and LC90 = 22.9 (19.7–27.9) μg, α-pinene with LC50 = 14.02 (12.1–16.3) μg and LC90 = 27.16 (23.7–32.0) μg, α-humulene with LC50 = 15.2 (13.2–17.8) μg and LC90 = 30.9 (26.8–36.8) μg, and α-phellandrene with LC50 = 17.09 (14.7–20.1) μg and LC90 = 34.6 (29.9–41.6) μg. The LC50 and LC90 values indicated that eugenol was toxic to pupae with LC50 = 13.4 (11.6–15.5) μg and LC90 = 26.1 (22.9–30.6) μg, followed by caryophyllene oxide with CL50 = 14.6 (12.5–17.2) μg and LC90 = 30.9 (26.7–37.1) μg, α-pinene with LC50 = 17.5 (15.1–20.8) μg and LC90 = 35.5 (30.6–43.0) μg, α-phellandrene with LC50 = 18.7 (16.3–21.8) μg and LC90 = 35.2 (30.7–41.9) μg, and α-humulene with LC50 = 21.4 (18.5–25.4) μg and LC90 = 40.4 (34.6–49.2) μg. To adult stage, increased mortality was observed following application of caryophyllene oxide with LC50 = 25.4 (21.9–30.4) μg and LC90 = 54.5 (46.3–67.2) μg, followed by eugenol with LC50 = 26.6 (23.2–31.4) μg and LC90 = 53.7 (46.1–65.2) μg, α-phellandrene with LC50 = 27.5 (24.3–31.9) μg and LC90 = 51.6 (44.9–61.2) μg, α-pinene with LC50 = 29.9 (26.1–35.4) μg and LC90 = 56.4 (48.4–68.5) μg, and α-humulene with LC50 = 31.8 (27.9–37.4) μg and LC90 = 56.7 (48.9–68.4) μg. Mortality was always <1% in the control.
Table 3. Lethal concentrations of the cinnamon and clove oil constituents on different developmental stages of Tenebrio molitor after 48 h exposure.

IS, insect stage; LC50 and 90, lethal concentration causing 50 and 90% mortality; EV, estimated value; CI, confidence interval; χ2, chi-squared value for the lethal concentrations and fiducial limits based on a log scale with significance level at P < 0.001.
Comparison of the LT50 values of the essential oils and their major compounds
Larvae, pupae, and adults of T. molitor applied with LC50 concentration of cinnamon and clove essential oils vs. toxic compounds showed lethal effects at different time points (fig. 3). However, LT50 values showed that clove oil took longer to kill insects than the cinnamon essential oil. At a high LC50 concentration of cinnamon oil, eugenol took longer to kill the larvae (t = 5.47, P < 0.001), pupae (t = 3.98, P < 0.001), and adults (t = 3.96, P < 0.001) with LT50 values of 32.6 ± 0.86, 28.3 ± 0.18, and 44.6 ± 0.85 h, respectively (fig. 3a–c); α-phellandrene took less to kill the larvae (t = 5.51, P < 0.001), pupae (t = 4.17, P < 0.001), and adults (t = 4.88, P < 0.001) with LT50 values of 45.6 ± 0.52, 45.6 ± 0.17, and 49.7 ± 0.11 h, respectively (fig. 3d–f); and α-pinene took longer to kill the larvae (t = 4.45, P < 0.001), pupae (t = 3.96, P < 0.001), and adults (t = 5.16, P < 0.001) with LT50 values of 47.7 ± 0.27, 48.8 ± 0.43, and 48.9 ± 0.79 h, respectively (fig. 3g–i).

Fig. 3. Lethal time of essential oils and toxic compounds on Tenebrio molitor after 48 h topical applied with a LC50: cinnamon oil (a–i) and clove oil (j–r) (control insects were applied with water). Control (●), cinnamon/clove essential oil (□), eugenol, caryophyllene, α-humulene, α-phellandrene, and α-pinene (▲).
In clove oil, eugenol took longer to kill the larvae (t = 4.59, P < 0.001), pupae (t = 3.71, P < 0.001), and adults (t = 5.08, P < 0.001) with LT50 values of 30.1 ± 0.25, 26.7 ± 0.56, and 40.3 ± 0.37 h, respectively; caryophyllene took less to kill the larvae (t = 3.93, P < 0.001), pupae (t = 7.31, P < 0.001), and adults (t = 5.13, P < 0.001) with LT50 values of 40.3 ± 0.45, 24.4 ± 0.89, and 36.7 ± 0.34 h, respectively; and α-humulene took longer to kill the larvae (t = 3.85, P < 0.001), pupae (t = 3.91, P < 0.001), and adults (t = 4.16, P < 0.001) with LT50 values of 47.5 ± 0.45, 46.1 ± 0.53, and 43.6 ± 0.36 h, respectively.
Repellency effects of the essential oils and their major compounds
The larvae of T. molitor RI by cinnamon essential oil and compounds with concentrations estimated for the LC50 values differ between them (table 4). Eugenol was the repellent (RI = 1.10 ± 0.05), while α-phellandrene (RI = 0.89 ± 0.08), α-pinene (RI = 0.87 ± 0.09), and cinnamon oil (RI = 0.83 ± 0.04) were attractant. The RI for adults of T. molitor differed with the concentration of the cinnamon essential oil and compounds, using the estimated LC90 values (table 4). Eugenol was the most repellent (RI = 1.10 ± 0.07), followed by that of the cinnamon oil (RI = 1.06 ± 0.03), while α-phellandrene (RI =0.90 ± 0.05), and α-pinene (RI = 0.87 ± 0.04) were attractant.
Table 4. Repellency index of two essential oils and toxic compounds to level LC50 application on larvae and adults of Tenebrio molitor.

Treatments were classified as neutral if the index was equal to one (1); repellent if higher than one (1); and attractive if lower than one (1).
The larvae of T. molitor RI by clove essential oil and compounds with concentrations estimated for the LC50 values differ between them (table 4). Caryophyllene oxide was repellent (RI = 1.06 ± 0.04), while eugenol (RI = 0.98 ± 0.09), clove oil (RI = 0.84 ± 0.05), and α-humulene were attractant (RI = 0.74 ±0.04). The RI for adults of T. molitor differed with the concentration of the clove essential oil and compounds, using the estimated LC90 values (table 4). The clove essential oil (RI = 1.10 ± 0.07) was repellent, while caryophyllene oxide (RI = 1.01 ± 0.06) was neutral. The compounds eugenol (RI =0.99 ± 0.08) and α-humulene (RI = 0.84 ± 0.05) were attractant.
Discussion
Toxicity and repellency of two essential oils and their compounds against the mealworm beetle, T. molitor were determined from the bioassays in the laboratory conditions. Cinnamon and clove essential oils caused substantial mortality and repellency in larva, pupa, and adult stages. The best results were obtained with concentrations of 16 and 32% in T. molitor as reported for other stored grain pests according to the concentration of these products (Zettler & Arthur, Reference Zettler and Arthur2000; Haddi et al., Reference Haddi, Oliveira, Faroni, Guedes and Miranda2015). The susceptibility of stored pest products such as Sitophilus oryzae Linnaeus (Coleoptera: Curculionidae) and Callosobruchus chinensis Linnaeus (Coleoptera: Chrysomelidae) by cinnamon, and Sitophilus zeamais Motschusky (Coleoptera: Curculionidae) and Tribolium castaneum Hersbt (Coleoptera: Tenebrionidae) by clove may vary with the method of exposure (contact or fumigation) (Ho et al., Reference Ho, Cheng, Sim and Tan1994; Kim et al., Reference Kim, Roh, Kim, Lee and Ahn2003; Correa et al., Reference Correa, Faroni, Haddi, Oliveira and Pereira2015).
Different concentrations of the cinnamon and clove essential oils showed toxic effects on larva, pupa, and adult of T. molitor 48 h after topical application. The dose–response bioassay confirmed toxicity against T. molitor, reaching a 90% mortality rate. Increasing concentrations of cinnamon and clove essential oil on different insects have shown immediate toxic responses within 12 h of application (Ho et al., Reference Ho, Cheng, Sim and Tan1994; Choi et al., Reference Choi, Lee, Choi, Park and Ahn2003; Kim et al., Reference Kim, Roh, Kim, Lee and Ahn2003; Lee et al., Reference Lee, Kim, Choi and Ahn2008). Comparing the contact toxicity of cinnamon and clove essential oils on developmental stages of T. molitor, the pupa was significantly more susceptible followed by adult and larva. The LC50 of cinnamon and clove essential oils of pupa (10.7 and 6.45 µg insect−1, respectively), adult (29.8 and 21.6 µg insect−1, respectively), and larva (30.4 and 35.1 µg insect−1, respectively) indicate that small quantities of these essential oils are toxic in all stages of development of this insect.
The chemical composition of cinnamon and clove essential oils revealed 23 compounds detected, identified, and quantified in terms of relative percentages. In particular, eugenol, trans-3-caren-2-ol, benzyl benzoate, caryophyllene, eugenyl acetate, α-phellandrene, and α-pinene were the main compounds that were detected in cinnamon oil and eugenol, caryophyllene, caryophyllene oxide, 2-propenoic acid, and α-humulene from clove oil. The results are in accordance with those of previous reports on monoterpenes obtained in essential oils (Simić et al., Reference Simić, Soković, Ristić, Grujić-Jovanović, Vukojević and Marin2004; Chaieb et al., Reference Chaieb, Hajlaoui, Zmantar, Kahla-Nakbi, Rouabhia, Mahdouani and Bakhrouf2007; Goni et al., Reference Goni, Lopez, Sánchez, Gómez-Lus, Becerril and Nerín2009). Monoterpenes, with sesquiterpenes, are the main constituents of essential oils extracted from plants, including fruits, vegetables, spices, and herbs (Loza-Tavera, Reference Loza-Tavera1999). They are products of the secondary metabolism of plants, although specialized classes occur in some animals and microorganisms (Banthorpe et al., Reference Banthorpe, Charlwood and Francis1972). Monoterpenes are a class of terpenes that consist of two isoprene units, with molecular formula of C10H16, and may be linear (acyclic) or contain rings. Defensive role in plants of simple monoterpenes have been demonstrated as for more complex compounds (Lerdau et al., Reference Lerdau, Litvak and Monson1994). In this study, the main compounds of cinnamon and clove essential oils are monoterpenes and may provide a solution to protect plants or stored products against insect attack.
Chemical compounds of cinnamon and clove essential oils demonstrated toxic activity on different developmental stages of T. molitor. Eugenol have stronger contact toxicity in larvae (LC50 = 9.15 µg), pupae (LC50 = 13.4 µg), and adult (LC50 =26.6 µg), than caryophyllene oxide in larvae (LC50 = 9.21 µg), pupae (LC50 = 14.6 µg), and adult (LC50 = 25.4 µg), followed by α-pinene in larvae (LC50 = 14.0 µg), pupae (LC50 = 17.5 µg), and adult (LC50 = 29.9 µg); α-phellandrene in larvae (LC50 = 17.0 µg), pupae (LC50 = 18.7 µg), and adult (LC50 = 27.5 µg); and α-humulene in larvae (LC50 = 15.2 µg), pupae (LC50 = 21.4 µg), and adult (LC50 = 31.8 µg). Eugenol, caryophyllene oxide, α-humulene, α-phellandrene, and α-pinene have been highly toxic to C. chinensis, Heliothis virescens (F.) (Lepidoptera: Noctuidae), S. oryzae, S. zeamais (Coleoptera: Curculionidae), and T. castaneum (Ho et al., Reference Ho, Cheng, Sim and Tan1994; Huang et al., Reference Huang, Ho, Lee and Yap2002) at different developmental stages as well as other insects (Gunasena et al., Reference Gunasena, Vinson, Williams and Stipanovic1988; Huang et al., Reference Huang, Ho, Lee and Yap2002; Park et al., Reference Park, Lee, Choi, Park and Ahn2003). Our results showed that T. molitor was more susceptible in the pupal stage followed by larvae and adults exposed to eugenol, caryophyllene oxide, α-humulene, α-phellandrene, and α-pinene. One possible explanation for the developmental stages difference is that efficacy may be affected by the penetration of the cinnamon and clove compounds into the body and the ability of the insect to metabolize these compounds.
The mechanisms of toxic action of monoterpenoids such as eugenol, caryophyllene oxide, α-humulene, α-phellandrene, and α-pinene are unknown. However, the insects exposed to the toxic compounds displayed altered locomotion activity, and muscle contractions in legs and abdomen that were observed at high concentrations in LC50 test. In some individuals, the paralysis was constant with concentrations near the LC50 without recovery signs. Paralysis and muscle contractions in individuals of T. molitor at LC50 followed by death can be explained by the toxic effect in the nervous system. Different studies have shown that neurotoxic effects of insects exposed to monoterpenoids can cause blockade of octopamine receptor binding sites (Livingstone et al., Reference Livingstone, Harria-Warrick and Kravitz1980; Enan, Reference Enan2001). In this context, octopamine induces hyperextension of the legs and abdomen by increasing the frequency of excitatory postsynaptic potentials received by the appropriate abdominal motor neurons (Livingstone et al., Reference Livingstone, Harria-Warrick and Kravitz1980; Enan, Reference Enan2001). Octopamine has a broad spectrum of biological roles in insects, acting as a neurotransmitter, neurohormone and circulating neurohormone neuromodulator (Orchard, Reference Orchard1982; Kostyukovsky et al., Reference Kostyukovsky, Rafaeli, Gileadi, Demchenko and Shaaya2002; Farooqui, Reference Farooqui2007). In insects, there is some evidence suggesting a role in neuromuscular transmission (Candy, Reference Candy1978; Whim & Evans, Reference Whim and Evans1988), but is not to say that octopamine is the neuromuscular transmitter, but rather that it may possess a modulating influence on nerve–muscle interaction. The presence of the monoterpenoids in cinnamon and clove essential oils may be responsible for the neurotoxic effect in T. molitor and may cause rapid mortality as reported to S. zeamais with eugenol, H. virescens with caryophyllene oxide, C. chinensis with α-humulene, α-phellandrene, and α-pinene causing hyperactivity, hyperextension of the legs and abdomen and rapid knock-down effect or immobilization (Gunasena et al., Reference Gunasena, Vinson, Williams and Stipanovic1988; Huang et al., Reference Huang, Ho, Lee and Yap2002; Park et al., Reference Park, Lee, Choi, Park and Ahn2003).
The compounds of cinnamon and clove essential oils and their constituents induced mortality in larva, pupa, and adult of T. molitor within a short period of time. The LT50 of T. molitor applied with LC50 eugenol was approximately 30, 26, and 40 h; caryophyllene oxide was 40, 24, and 36 h; α-humulene was 54, 46, and 43 h; α-phellandrene was 45, 45, and 49 h; and α-pinene was 47, 48, and 48 h for larva, pupa, and adult, respectively. Toxic compounds affect multiple regions of the insect body over a period of time, ranging from 1 to 20–40 h for death. In this period, the necrotic areas were increasing progressively on the insect body. The comparative effects on T. molitor between two essential oils and toxic compounds were observed at various time points. An essential oil of quick action should be preferred for protection of products stored to be able to prevent feeding and avoid or reduce damage by insect pests (Isman, Reference Isman2006; Chermenskaya et al., Reference Chermenskaya, Stepanycheva, Shchenikova and Chakaeva2010; Martínez et al., Reference Martínez, Plata-Rueda, Zanuncio and Serrão2015).
The repellency test indicated that eugenol and caryophyllene oxide had a greater effect on T. molitor behavior, while cinnamon and clove essential oils had little effect. Odor produced from volatile compounds was repulsive to larvae and adults of T. molitor and was observed during early hours after exposition. In exposure to vapors, the volatile substances enter with the air insects inhale through their spiracles as part of their respiratory process (Wasserthal, Reference Wasserthal1996). The substances are transported to different tissues through the network of tracheas and tracheoles, thus reaching their site of action. Monoterpenes eugenol, caryophyllene oxide, α-humulene, α-phellandrene, and α-pinene are majority compounds and toxic to insect pests of stored products such as Acanthoscelides obtectus Say (Coleoptera: Chrysomelidae) (Jumbo et al., Reference Jumbo, Faroni, Oliveira, Pimentel and Silva2014) and T. castaneum (Padin et al., Reference Padin, Ringuelet, Bello, Cerimele, Re and Henning2000). Our results suggest that cinnamon and clove essential oils and their constituents eugenol, caryophyllene oxide, α-humulene, α-phellandrene, and α-pinene have high activities of behavioral deterrence against T. molitor, as evaluated by the behavioral responses of larvae and adults to different odor sources and the number of insects repelled, indicating their potential to the pest control in stored products.
This study showed the potential of cinnamon and clove essential oil and main compounds to control the T. molitor in starches and stored products. In order to prevent or retard the development of insecticide resistance, the toxicity and repellency effects of two essential oils and toxic compounds on T. molitor show that they can be used individually or in mixture for the management of populations. The lethalities of eugenol, caryophyllene oxide, α-humulene, α-phellandrene, and α-pinene on T. molitor may have advantages by their mode of action on this insect. These findings show that the compounds of two essential oils are a potential source of insecticidal compounds and warrants further investigation.
Acknowledgements
This research was supported by ‘Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)’, and ‘Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG)’. To Asia Science for correction and English editing this manuscript.










