Introduction
Amaranth is a plant with a high nutritional value, whose nutrients are concentrated in leaves and grains (Santiago et al., Reference Santiago, Tenbergen, Vélez-Jiménez and Cardador-Martínez2014). It is used in human and animal products as well as forage, silage and green manure. It has potential industrial uses in cosmetology and biodegradable plastics (Paredes-López, Reference Paredes-López2018). Amaranth is, however, attacked by a complex of lepidopteran species among which Spoladea recurvalis (F.) (Lepidoptera: Crambidae) is the most devastating (Chang and Ramasamy, Reference Chang and Ramasamy2016; Othim et al., Reference Othim, Agbodzavu, Kahuthia-Gathu, Akutse, Muchemi, Ekesi and Fiaboe2017; Agbodzavu et al., Reference Agbodzavu, Lagat, Gikungu, Akutse, Ekesi and Fiaboeunpublished data). Up to 100% yield losses due to S. recurvalis were reported by Kahuthia-Gathu (Reference Kahuthia-Gathu2011). The larvae wrap and roll amaranth leaves into shelters from which they feed skeletonizing the foliage and leaving frass on leaves often leading to entire foliage loss (Bhattacherjee and Ramdas Menon, Reference Bhattacherjee and Ramdas Menon1964; Pande, Reference Pande1972; James et al., Reference James, Atcha-Ahowé, Godonou, Baimey, Goergen, Sikirou and Toko2010). Chemical control with the use of pyrethroid is inefficient (Clarke-Harris and Fleischer, Reference Clarke-Harris and Fleischer2003) and spinosad application gave only relatively low protection against the lepidopteran complex that feeds on amaranth (Clarke-Harris et al., Reference Clarke-Harris, Fleischer, Fuller and Bolton2004). In addition to their inefficiency, the use of insecticides is considered as non-environment friendly and costly for small-scale farmers. In a study carried by Macharia (Reference Macharia2015) in Kenya, it was reported an increase of pesticide-related acute illness by over 70%. Therefore, the economic value of pesticide-related health cost is non-negligible (Maumbe and Swinton, Reference Maumbe and Swinton2003; Houndekon et al., Reference Houndekon, De Groote and Lomer2006) and is creating negative consumer sentiment around the use of insecticides which puts pressure on growers to use alternative control measures, such as biological control (Wohlfarter and Addison, Reference Wohlfarter and Addison2014).
Biological control programs, whether using the introduction, conservation or augmentation approaches, are facilitated when the climatic responses of biocontrol agents are known, especially temperature (Roy et al., Reference Roy, Brodeur and Cloutier2002). Poor ecological adaptability of a parasitoid to the environment in the field is reported to be one of the factors explaining the failure of biological programs. Stiling (Reference Stiling1993) meta-analyses on natural enemy failures, estimated that ~35% of biocontrol introductions or programs might have been unsuccessful because of climate-related factors (Hoddle et al., Reference Hoddle, Warner, Steggall and Jetter2014). Failure of Trichogramma chilonis (Ishii) (Hymenoptera: Trichogrammatidae) in controlling Chilo infuscatellus (Snellen) (Lepidoptera: Crambidae) in Uttar Pradesh, India, was due to the high temperature and low humidity prevailing during April–July (Tiwari and Tanwar, Reference Tiwari, Tanwar, Upadhyay, Mukerji and Chamola2001) whereas in Punjab, India, it was effective in suppressing Chilo auricilius (Dudgeon) (Lepidoptera: Crambidae) on sugarcane by reducing its incidence from 61 to 12.6% through inundative releases (Varma et al., Reference Varma, Rataul, Shenhmar, Singh and Jalali1991). In Thailand, Quadrastichus citrella (Reina and La Salle) (Hymenoptera: Eulophidae) is considered one of the key factors in regulating Phyllocnistis citrella (Stainton) (Lepidoptera: Gracillariidae) field populations (Kernasa et al., Reference Kernasa, Suasa-ard and Charernsom2008) and also in Israel where up to 84% parasitization of the leafminer's larvae was recorded (Argov and Rossler, Reference Argov and Rossler1996). However, in a study carried out by Llacer et al. (Reference Llacer, Urbaneja, Garrido and Jacas2006), it was reported that overwintering of Q. citrella in Spain may present a barrier, especially in areas where average winter temperatures are around 11 °C and could account for the low recovery rates observed on the citrus leafminer P. citrella. It is thus important to establish how a parasitoid will respond to a range of temperatures to predict its survival, establishment and performance in a given area as well as identify the best timing of releases.
Apanteles hemara (Nixon) (Hymenoptera: Braconidae) is a solitary koinobiont endoparasitoid reported in Africa and Asia, but detailed information on its biology is scarce. The only available literature is just based on reports of its presence in different regions. In Cape Verde Islands, the parasitoid was recovered from its host S. recurvalis on Achyranthes aspera L. (Amaranthaceae) (Papp, Reference Papp1996). It was also cited in India, where the field parasitism rate reached 11% on the same host (Peter and Balasubramanian, Reference Peter and Balasubramanian1984) and Iran, Qazvin province (Ghahari et al., Reference Ghahari, Fischer and Tobias2012). Work done by Othim et al. (Reference Othim, Agbodzavu, Kahuthia-Gathu, Akutse, Muchemi, Ekesi and Fiaboe2017) showed high parasitism rates of the parasitoid in laboratory conditions. Agbodzavu et al. (Reference Agbodzavu, Lagat, Gikungu, Akutse, Ekesi and Fiaboeunpublished data) found that A. hemara represented the most commonly found parasitoid attacking S. recurvalis in the field. It is, therefore, a good potential candidate for an augmentative or classical biological control for other areas where S. recurvalis represents a serious threat to vegetable production such as aubergines, bean, beetroot and cucurbits (James et al., Reference James, Atcha-Ahowé, Godonou, Baimey, Goergen, Sikirou and Toko2010). The objective of this research was to assess the effect of temperature on the development and performance of the parasitoid A. hemara on its preferred host S. recurvalis.
Materials and methods
Rearing of Spoladea recurvalis and Apanteles hemara
The insects used in this experiment were reared at the insectaries of the International Centre of Insect Physiology and Ecology in Nairobi. Larvae of S. recurvalis were collected from a field survey of amaranth lepidopteran pests in Narok county, Transmara (0° 35′ 32.892″ N, 3° 0′ 49.14″ E) and Yatta, Machakos County (01° 08.295′ S, 037° 25.892′ E) in May and June 2014. A colony of A. hemara was established from pupal samples collected during a survey conducted in Yatta, Machakos County (01° 07.878′ S, 037° 33.274′ E) in June 2014. Field insects' materials were later collected from Yatta to infuse the laboratory colonies. Both S. recurvalis and A. hemara were reared according to the methods described by Othim et al. (Reference Othim, Agbodzavu, Kahuthia-Gathu, Akutse, Muchemi, Ekesi and Fiaboe2017).
Effect of different constant temperatures on the developmental time of Apanteles hemara
Newly emerged adults of A. hemara were isolated from the stock culture in separate cages (20 cm × 20 cm × 25 cm) and allowed to mate for 2 to 3 days. The cages were made up of Perspex materials with fine netting materials (150 μm × 150 μm mesh) fitted on the backside to allow efficient air circulation inside the cages. A sex ratio of one male to two females was used. They were fed with a drop of honey smeared on paper and hung with masking tape on one inner face of the cage and also with 10% of honey solution soaked in cotton and kept in an opened Petri dish (90 mm × 12 mm).
A single 2 to 3 days old mated naïve female wasp (with no oviposition experience) was introduced in a cage as indicated above. The cage contained 20 larvae (3–4 days old on their second instar) of S. recurvalis kept in an opened Petri dish for parasitization. Parasitization was observed visually. One female parasitoid was allowed to parasitized ten host larvae out of the 20; it was after that replaced by another female supplied with another batch of 20 larvae. Once the female parasitoid was observed introducing its ovipositor in a host, this host larva was removed using a fine camel brush, isolated in a vial (20 ml) plugged with cotton wool, and introduced in the incubator (Environmental chambers, SANYO MIR-553 and MIR-554, Sanyo Electrical Ltd., Tokyo, Japan), with known temperatures (i.e., 10, 15, 20, 25, 30 and 35 °C) under a relative humidity ranging from 60–70%. To maintain this range of relative humidity, two plastic boxes containing water were placed on the lower shelf of the incubator and refilled as needed. Every 2 days, fresh amaranth leaves were provided to the larvae until parasitoid cocoon formation, host pupation and subsequent emergence of parasitoid or host adults or death of larvae. A total of 200 exposed larvae for parasitization were used for each temperature. For those with effective parasitization, the developmental time of each stage of the parasitoid, the number of cocoons formed (which is termed successful parasitism rate or successful oviposition), the number of cocoons from which adult wasps emerged, the sex ratio of the emerged adults and the parasitoid pupal mortality were determined. Due to the deteriorated nature of the dead exposed host larvae, it was not possible to dissect them and assess the parasitoid egg-larval mortality.
Longevity of adults
Before adult emergence, a drop of honey was put on the internal wall of the vial to allow them to start feeding once they emerged from the cocoons. Vials were kept in an incubator set at the same conditions as mentioned above. They were followed daily at the same time. Mortality was recorded until all adult parasitoids died.
Fecundity
Due to host larvae limitations (difficulties of having continuous and sustainable colonies in laboratory conditions), fecundity was studied only at 20 and 25 °C. Newly emerged females were coupled with males of the same age in individual containers (12 cm in diameter, 6.5 cm in height) which had their top covered in the middle with fine mesh for ventilation. A drop of honey was put on the wall of the container to allow the adults to feed. Each couple was provided with 20 S. recurvalis larvae (3–4 days old) daily until the female died. The number of larvae offered daily was based on results of Othim et al. (Reference Othim, Agbodzavu, Kahuthia-Gathu, Akutse, Muchemi, Ekesi and Fiaboe2017), who found that the highest number of cocoon of A. hemara recovered after 24 h exposure on 50 larvae was 14. A fresh amaranth leaf was hung on the top of the container as a food source for the exposed larvae. A new container was used for daily exposure of the larvae. A total of 10 and 15 pairs (replicates) of wasps were used for 20 and 25 °C respectively. Daily exposed larvae were isolated individually in vials plugged with cotton wool and reared on amaranth leaves until the emergence of either a parasitoid or a moth as described above. The parasitoid lifespan was recorded as well as the pre-oviposition period, oviposition period and a post-oviposition period. The post-oviposition period refers to the time when a parasitoid ceased to parasitize hosts until the death of the parasitoid. Two fecundity parameters were calculated, realized fecundity as the number of parasitized larvae that developed in a cocoon (whether it developed into an adult parasitoid or not) over the life-span of the parasitoid, and fertility as the number of adult parasitoids that emerged from cocoons (Murillo et al., Reference Murillo, Hunt and VanLaerhoven2012).
Data analyses
Data were subjected to Shapiro–Wilk and Bartlett tests to test for normality and homogeneity of variance respectively. The developmental durations for each life stage and adult longevity were compared between temperatures with a Dunnett test using dunn.test package and between sex with a Wilcoxon test because data were not normally distributed. Where data were normally distributed between sex (adult longevity), an independent samples t test was used. Larval and pupal mortality, as well as the parasitism rate and the emergence rate of the parasitoid, were compared between temperatures with a proportion test. The sex ratio was examined at each temperature using a χ2 test.
The development rate was calculated as the inverse median development time (development rate = 1/median development time) (Régnière, Reference Régnière1984), for each immature stage (egg-larval and pupal) and plotted against temperature. The degree-day model states that the relationship between development rate r (T) (1/development time in days) vs. temperature can be described by a linear equation: r (T) = a + bT, where T is the rearing temperature, a is the intercept and b is the slope of the linear function. The lower threshold temperature T min (T min = −a/b) and the thermal constant K (i.e., the number of degree-days above the lower threshold required to complete development, DD) (K = 1/b) were calculated based on the linear equation (Mathieu et al., Reference Mathieu, Dumont, Chiroleu, Duyck, Flores, Lebreton, Reynaud and Quilici2014). The data points for extreme temperatures (nonlinear points) were excluded. Linear functions cannot correctly capture the development rate at extreme temperatures. For that reason, three non-linear models, Taylor, Lactin-1 and Ratkowsky were used to describe the relationship between temperature and development rates. Taylor function is defined as follows rT = R m × exp(−1/2 × ((T−T m)/T o)2), where rT is the development rate, T the temperature, R m the maximum development rate, T m the optimum temperature and T o the rate at which development rate falls away from T m (Taylor, Reference Taylor1981). The Lactin-1 model is defined as rT = exp(aa × T) − exp(aa × T max − (T max − T)/ΔT) where rT is the development rate, T the temperature and aa, T max and ΔT fitted parameters. The Ratkowsky model is formulated as rT = (cc × (T − T 1) × (1 − exp(k × (T − T 2))))2 where rT is the development rate, T the temperature, T 1 and T 2 the minimum and maximum temperatures at which the rate of growth is zero, cc the slope of the regression and k a constant (Ratkowsky_83: Ratkowsky Equation Of Development Rate. Retrieved from https://rdrr.io/cran/devRate/man/ratkowsky_83.html). The devRate package for R (Rebaudo et al., Reference Rebaudo, Struelens and Dangles2017) was used to quantify the relationship between development rate and temperature. All statistical analyses were done in R statistical software version 3.4.1 (R Core Team, 2017).
Results
Developmental time of Apanteles hemara at different temperatures
A. hemara larvae reared at constant 10 and 35 °C failed to complete development and died before emergence from the host, thus these data were only analysed from individuals that successfully emerged from S. recurvalis. At 15 °C, the egg-larval developmental time of male parasitoids was not statistically different from females (W = 11.5, df = 1, P = 0.794). The same trend was observed at 20 °C (W = 435.5, df = 1, P = 0.059) and 30 °C (W = 744, df = 1, P = 0.786). It was, however, significantly different at 25 °C (W = 619, df = 1, P = 0.024). The pupal developmental time was not significantly different between sex at 15 °C (t = 0.099, df = 7, P = 0.924) and 30 °C (W = 827, df = 1, P = 0.385). The difference was, however, significant at 20 °C (W = 952.5, df = 1, P < 0.0001) as well as at 25 °C (W = 763.5, df = 1, P < 0.0001). The total developmental time was not significantly different between males and females at 15 °C (t = 0.355, df = 4.35, P = 0.739) and at 30 °C (W = 812, df = 1, P = 0.476) but was significantly different at 20 °C (W = 1003.5, df = 1, P < 0.0001) and 25 °C (W = 811.5, df = 1, P < 0.0001) (Table 1).
Table 1. Developmental time (mean ± SE in days) of immature stages of A. hemara reared on S. recurvalis
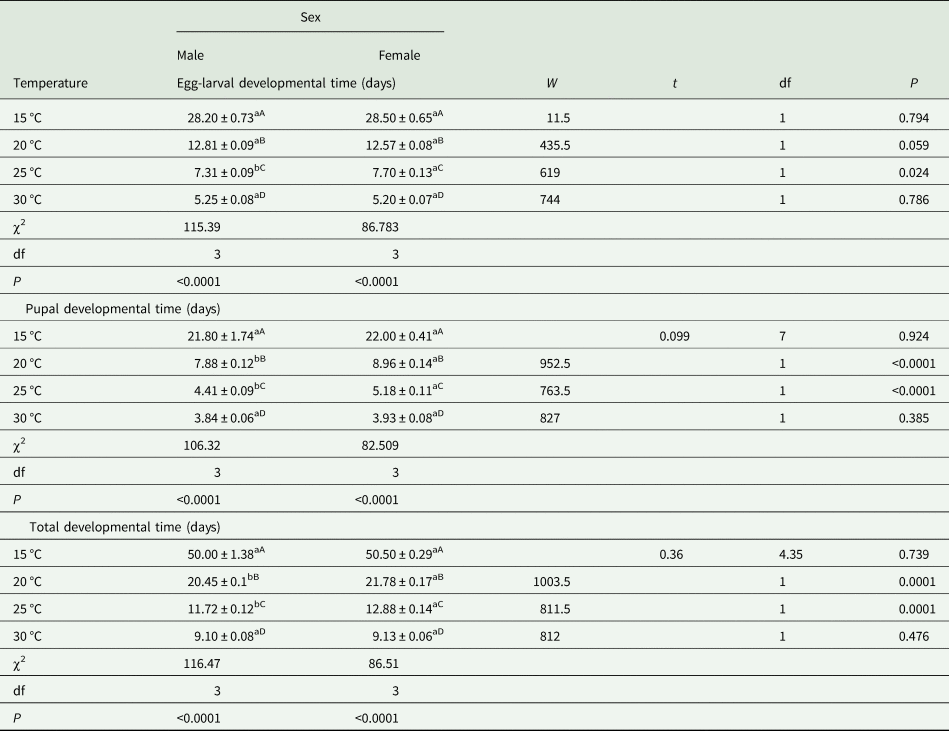
Means followed by the same lower (upper) case letters in the same row (column for the same parameter) are not significantly different at P < 0.05 by the Wilcoxon rank sum test, two-sample t-test or Welch two-sample t-test (Dunnett test).
The developmental time varied greatly among tested temperatures. For the males (χ2 = 115.39, df = 3, P < 0.0001) and females (χ2 = 86.783, df = 3, P < 0.0001), egg-larval developmental times were significantly different between temperatures. Similarly, there was a highly significant decrease in A. hemara's pupal developmental time with increasing temperature in males (χ2 = 106.32, df = 3, P < 0.0001) and females (χ2 = 82.509, df = 3, P < 0.0001). The total developmental time was also significantly affected by the temperatures in males (χ2 = 116.47, df = 3, P < 0.0001) and females (χ2 = 116.47, df = 3, P < 0.0001) (Table 1).
Development time models and estimated values
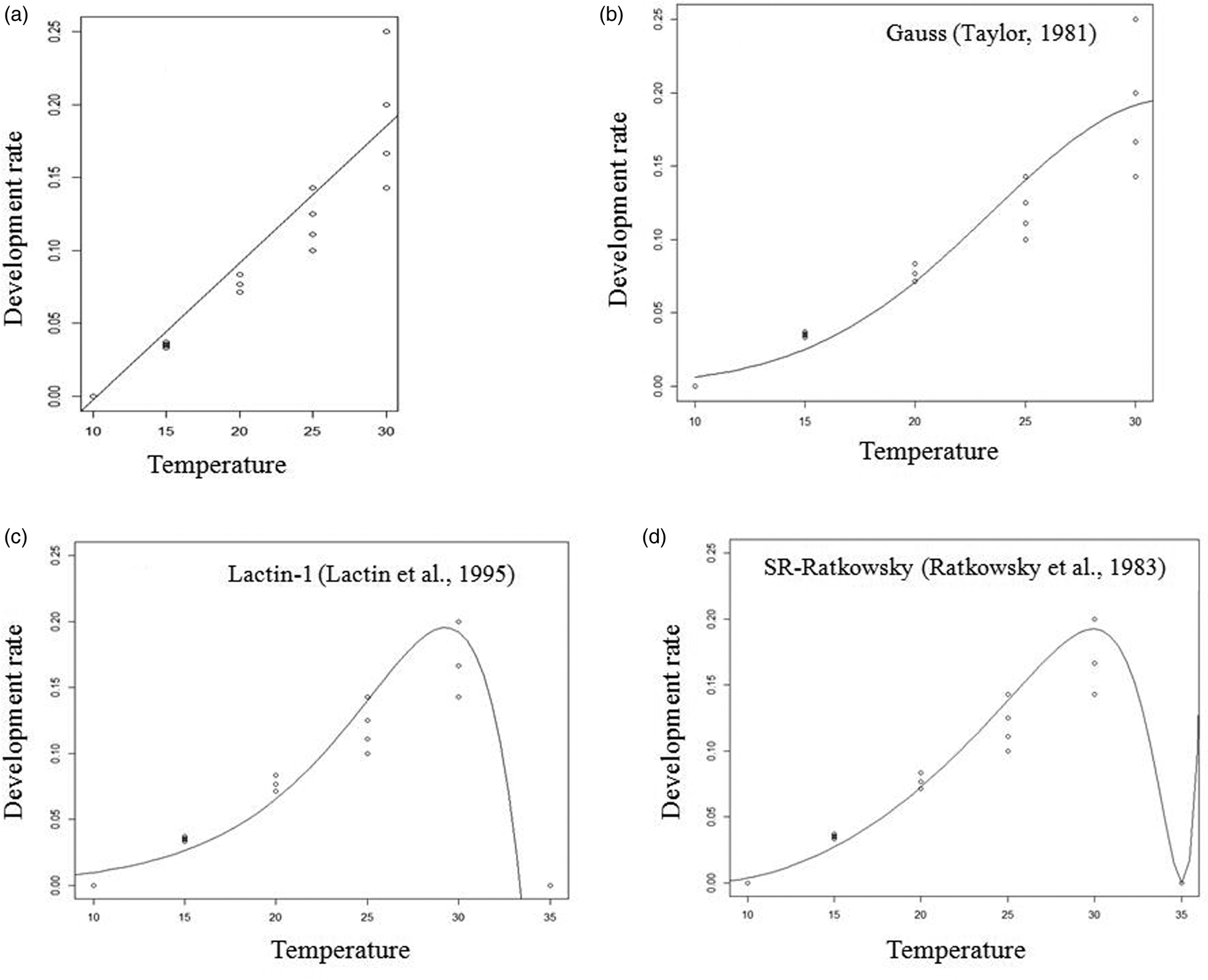
Figures 1–3 represent estimation of linear regression, Taylor, Lactin-1 and Ratkowsky models for different stages of development. Table 2 shows the effect of temperature on the development rate of A. hemara.
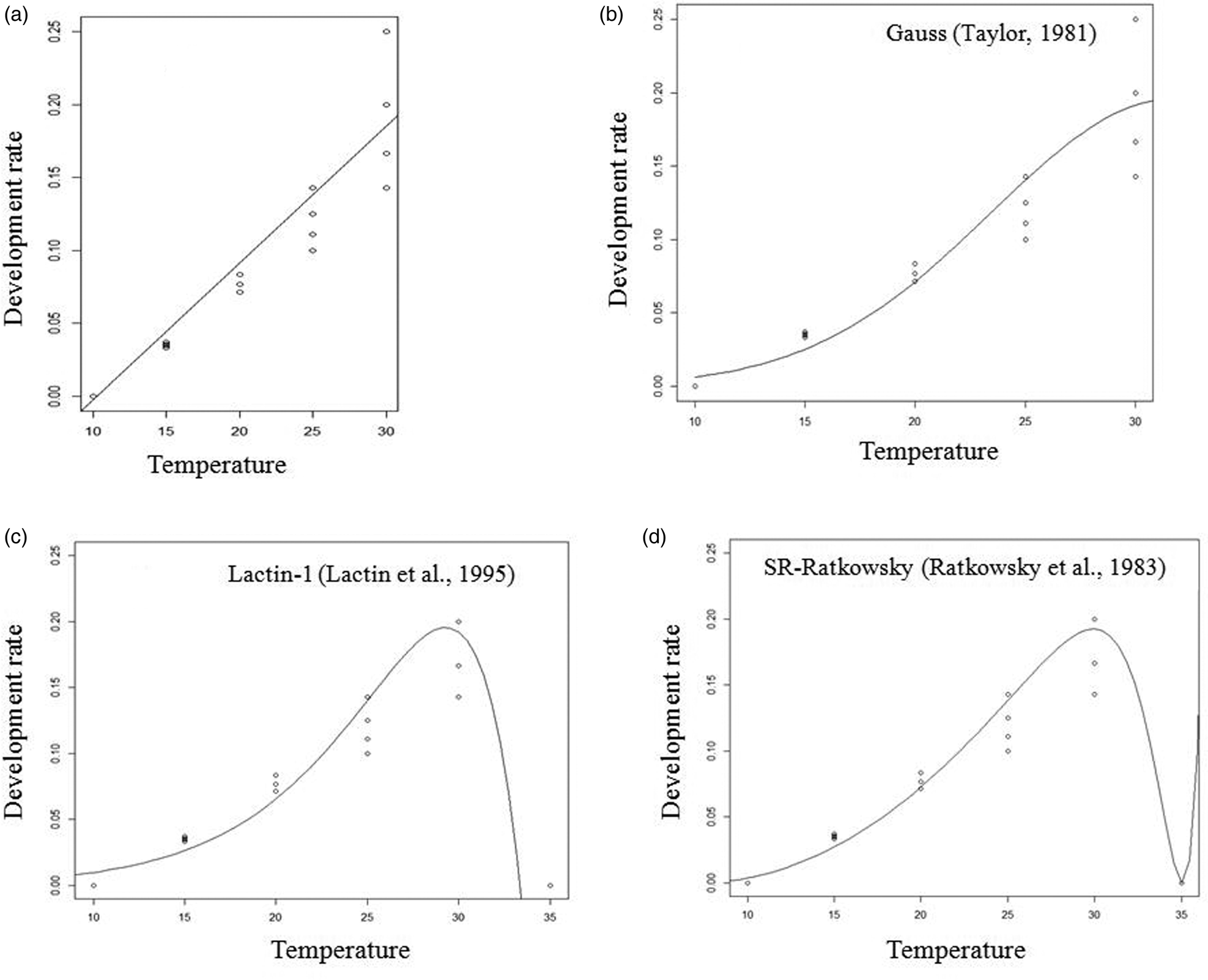
Figure 1. Linear (a), Taylor (b), Lactin-1 (c) and Ratkowsky (d) fitted models of the development rate (1/development duration) for the larval stage of A. hemara.
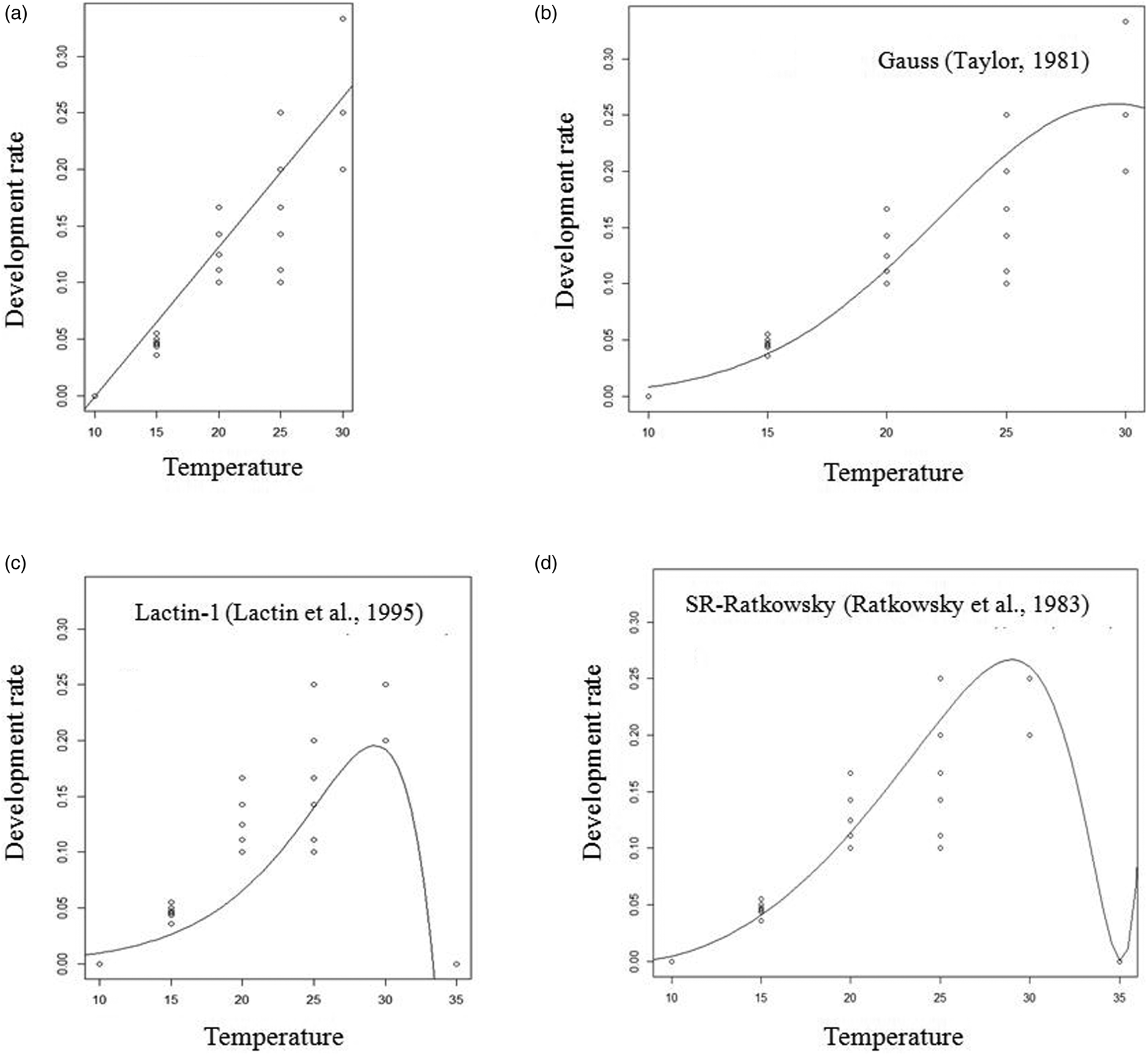
Figure 2. Linear (a), Taylor (b), Lactin-1 (c) and Ratkowsky (d) fitted models of the development rate (1/development duration) for the pupal stage of A. hemara.

Figure 3. Linear (a), Taylor (b), Lactin-1 (c) and Ratkowsky (d) fitted models of the development rate (1/development duration) for the total developmental time of A. hemara.
Table 2. Model parameters of linear regressions, Taylor, Lactin-1 and Ratkowsky models for the temperature effect on A. hemara immature stages' development rate

Significance code: ‘***’P<0.0001. rT is the development rate, a: y intercept, b: slope, K: thermal constant (DD), T min: lower development temperature threshold, T the temperature, R m the maximum development rate, T m the optimum temperature, T o the rate at which the development rate falls away from T m, T the temperature, aa, T max and ΔT are fitted parameters, T 1 and T 2 the minimum and maximum temperatures at which the rate of growth is zero, cc the slope of the regression, k a constant and R 2 coefficient of determination.
The fit of all tested non-linear models for the dependence of development rates of A. hemara on temperature was significant for egg-larval and total developmental time. For egg-larval development, F = 25.33 and P < 0.0001 for the Taylor model, F = 6.57 and P = 0.010 for Ratkowsky and F = 229.5 and P < 0.0001 for Lactin-1. For total developmental time, F = 55.24 and P < 0.0001 for the Taylor model, F = 22.62 and P < 0.0001 for Ratkowsky and F = 602.8 and P < 0.0001 for Lactin-1. For the pupal development time, only Lactin-1 fit was significant (F = 118.1, P < 0.0001). Base on R 2, Lactin-1 was retained as it best fits the data for larval, pupal and total development rates (Table 2). The Lactin-1 model estimated the upper threshold temperature for development at 35.0 °C for larval, pupal and total development stages. Since, the Lactin-1 model does not estimate the optimum temperature, it was derived from the Taylor-81 model and was estimated at 30.8 °C for the total development stage.
Using the linear model, the lower temperature (T min) and the sum of effective temperatures (K) for development were 10.1 °C and 106.38 DD for the egg-larval stage. They were 10.1 °C and 75.76 DD for the pupal stage, and 10.3 °C and 185.18 DD for the total developmental time (Table 2).
Pupal mortality, parasitism and emergence rates
Pupal stage mortality of A. hemara is presented in Table 3. There was a highly significant difference in the mortality recorded at different temperatures (χ2 = 106.50, df = 3, P < 0.0001). The highest pupal mortality was recorded at 15 °C, but the lowest was recorded at 25 °C.
Table 3. Effect of constant temperatures on A. hemara pupal morality when reared on S. recurvalis

Means followed by the same letter in the same column were not significantly different by the proportion test, P < 0.05.
The successful parasitism rate (successful oviposition or realized fecundity) (χ2 = 37.28, df = 3, P < 0.0001) and adult emergence rate (fertility) (χ2 = 106.50, df = 3, P < 0.0001) were significantly affected by temperatures. The highest successful parasitism rate was obtained at 30 °C and the lowest at 15 °C. The highest adult emergence rate occurred at 25 °C and the lowest at 15 °C (Table 4).
Table 4. Effect of constant temperatures on A. hemara parasitism and emergence rates when reared on S. recurvalis

* n represents the number of collected cocoons and emerged adults.
Means followed by the same letter in the same column were not significantly different by the proportion test, P < 0.05.
Adult longevity and sex ratio of Apanteles hemara at different temperatures
Adult longevity of A. hemara was significantly influenced by temperature. Adult longevity decreased with increasing temperatures from 15 to 25 °C. An increase was, however, noted at 30 °C. There were no significant differences in the longevity of females and males at the four tested temperatures (Table 5).
Table 5. Effect of constant temperatures on adult longevity (mean ± SE in days) of A. hemara when reared on S. recurvalis

Means followed by the same lower (upper) case letters in the same row (column) are not significantly different at P < 0.05 by the Wilcoxon rank sum test or two-sample t test (Dunnett test).
There was no significant difference in the sex ratio at 15 °C (χ2 = 0.09, df = 1, P = 0.757) and at 25 °C (χ2 = 0.29, df = 1, P = 0.59). However, this was statistically different at 20 °C (χ2 = 5.68, df = 1, P = 0.017) and 30 °C (χ2 = 9.8765, df = 1, P = 0.001) where it was male biased (Table 6).
Table 6. Effect of constant temperatures on the A. hemara sex ratio when reared on S. recurvalis

Means followed by the same letter in the same row were not significantly different by the χ2 test, P < 0.05.
Female realized fecundity and survival
The realized fecundity of A. hemara was influenced by temperature and showed significant differences between the two tested temperatures (W = 117, df = 1, P = 0.001). The mean daily realized fecundity in terms of number of produced cocoons was 8.15 ± 1.03 at 20 °C and 2.41 ± 0.2 at 25 °C per female. No pre-oviposition period was observed. At 20 °C, the mean oviposition period was 12.20 ± 5.89, and at 25 °C, it was 11.61 ± 5.41 (figs 4 and 5).

Figure 4. Age-specific reproduction and survival of adult females of A. hemara reared on S. recurvalis at 20 °C, 60–70% relative humidity and 12:12 h (light:dark) photoperiod.

Figure 5. Age-specific reproduction and survival of adult females of A. hemara reared on S. recurvalis at 25 °C, 60–70% relative humidity and 12:12 h (light:dark) photoperiod.
Discussion
In biological control programmes, detailed information concerning thermal requirements and thresholds is useful in selecting natural enemies that are best adapted to conditions favouring target pests (Jervis and Copland, Reference Jervis and Copland1996). To the best of our knowledge, no data on the development of A. hemara on S. recurvalis at different temperatures are available. Results in the current study clearly showed that the development and the survival of A. hemara on S. recurvalis are highly significantly affected by temperatures. Previous studies have shown the effect of temperatures on the development of various Braconid parasitoids (Cardona and Oatman, Reference Cardona and Oatman1975; Zamani et al., Reference Zamani, Talebi, Fathipour and Baniameri2007; Htwe et al., Reference Htwe, Takagi and Takasu2008). Insects, being poikilothermic, are particularly sensitive to their environmental temperature. A. hemara completed its development within range temperatures of 15–30 °C but not at 10 and 35 °C. Similar results were obtained by Cardona and Oatman (Reference Cardona and Oatman1975) who reported that Apanteles subandinus (Blanchard) (Hymenoptera: Braconidae) completed its development from egg to adult at temperatures of 15.5–32 °C but not at low temperatures of 11.2 °C. Similar developmental time trends were also obtained for Apanteles taragamae (Viereck) (Hymenoptera: Braconidae) for which a total developmental time of 12.3 days was obtained at 24 °C and 9.4 days at 30 °C (Dannon et al., Reference Dannon, Tamò, van Huis and Dicke2010). Our results on the developmental time are also comparable to the development of Cotesia vestalis (Haliday) (Hymenoptera: Braconidae) another Microgastrine parasitoid species at 20, 25, 30 and 35 °C which lasted for 19.6, 12.5 and 9.5 days respectively and no parasitoid pupae were recovered at 35 °C. However, at 15 °C, C. vestalis took 10 days less to complete its immature development as compared to A. hemara (Htwe et al., Reference Htwe, Takagi and Takasu2008). The effect of temperature on an insect is explained by its interference with the metabolism, respiration, nervous system, endocrine system and heat shock protein capacity (Neven, Reference Neven2000). At the lower extreme temperature, a delay in development occurs due to suboptimal feeding; but as the temperature increases, it is accompanied by an increase in the developmental rate up to a lethal limit, where the rate of metabolism decreases (Van Steenis, Reference Van Steenis1994).
In the current study, our findings showed that the developmental time of A. hemara was similar for females and males at only two of the four tested temperatures in the assay. These results are similar to previous studies on Microgastrinae. For instance, Esmat et al. (Reference Esmat, Wedad and Neama2017) reported that the differences between the duration of the immature developmental period of males and females of Apanteles galleriae (Wilkinson) (Hymenoptera: Braconidae) were not significant at 25, 27 and 30 °C but significant at 20 °C at a photoperiod of 0:24 (L:D). The developmental time of A. taragamae males and females on 2 days old larvae of Maruca vitrata (Fabricius) (Lepidoptera: Crambidae) at 25.3 ± 0.5 °C and 78.9 ± 5.6% relative humidity was not significantly different (Dannon et al., Reference Dannon, Tamò, van Huis and Dicke2010).
The selection of mathematical models that describe the relationship between temperatures and the developmental rate is essential. Linear functions fitted well the effect of temperature on the development rate for all A. hemara immature stages (egg-larval and pupal) and the total developmental time, especially for temperature between 10 and 30 °C. However, for higher temperatures, the Lactin-1 model gave good results and allowed the calculation of the maximum temperature threshold. Although the coefficient of determination of the Taylor model was low, the parameters estimates were highly significant. The estimated values of optimum development temperature for the egg-larval and pupal stage were in accordance with the observed successful parasitism and adult emergence rates at 30 °C, though the parasitoid pupal mortality obtained at that temperature was a bit higher than at 25 °C. The lower developmental threshold of the immature stage of S. recurvalis reported in the literature is 10.4 °C (Lee et al., Reference Lee, Kim, Cheong, Kim, Lee and Hwang2013). The one of A. hemara obtained during this study (10.3 °C) is relatively close to the pests' lower threshold, and the parasitoid is therefore expected to keep in check the pest at lower temperature limits. However, the upper lethal temperature for S. recurvalis is 48.8 °C (Lee et al., Reference Lee, Kim, Cheong, Kim, Lee and Hwang2013), far above the upper thermal threshold of A. hemara (35.0 °C) leaving a huge gap where the pest will grow unchecked with temperatures above 35.0 °C. Such risk is fortunately reduced by the fact that the S. recurvalis optimal temperature range for growth is 25.0–30.0 °C (Lee et al., Reference Lee, Kim, Cheong, Kim, Lee and Hwang2013). However, global warming might favour the pest than the parasitoid.
The results of these experiments showed that temperature affected adult longevity. A constant decrease in adult parasitoid longevity as per the increase of the temperature (showing, therefore, an inverse relationship between longevity and temperature) is usually reported in other Braconidae species; for instance, in Microplitis manila (Ashmead) (Qiu et al., Reference Qiu, Zhou, Luo and Xu2012), Chelonus inanitus (L.) (Rechav, Reference Rechav1978), Chelonus sp. nr. curvimaculatus (Cameron) (Hentz et al., Reference Hentz, Ellsworth, Naranjo and Watson1998), Bracon vulgaris (Ashmead) (Ramalho et al., Reference Ramalho, Wanderley, Malaquias, Fernandes, Nascimento and Zanuncio2011), Chelonus murakatae (Munakata) (Qureshi et al., Reference Qureshi, Quan, Zhou, Zhu and Wang2017) and Psyttalia cosyrae (Wilkinson) (Mohamed et al., Reference Mohamed, Overholt, Wharton and Lux2006). Our results, however, showed a partial difference at 30.0 °C where the longevity experienced an increase. There are some cases in Microgastrinae matching our findings, where a constant decrease was not always observed as the temperature increases such as in A. galleriae (Uçkan and Erginin, Reference Uçkan and Erginin2003), C. murakatae males (Qureshi et al., Reference Qureshi, Quan, Zhou, Zhu and Wang2017) and B. vulgaris males (Ramalho et al., Reference Ramalho, Wanderley, Malaquias, Fernandes, Nascimento and Zanuncio2011).
The sex ratio was male-biased at 20 and 30 °C whereas it was balanced at 15 and 25 °C. The sex ratio is an important parameter when considering biological control agents. A female-biased sex ratio is sought as females are the ones responsible for attacking the pests through host feeding or oviposition (Berndt and Wratten, Reference Berndt and Wratten2005; Chow and Heinz, Reference Chow and Heinz2005). Female parasitoids also cause the death of their host via nonreproductive effects (Abram et al., Reference Abram, Brodeur, Urbaneja and Tena2019). In a study carried by Othim et al. (Reference Othim, Agbodzavu, Kahuthia-Gathu, Akutse, Muchemi, Ekesi and Fiaboe2017) on A. hemara at 25 ± 2 °C and 60 ± 10% RH, they obtained a female-biased sex ratio in a proportion of 59.09% which is close to 53.23% that was obtained in the current study at 25 °C. Our results on the sex ratio are also similar to the ones obtained in other Braconidae species where variable sex ratios were recorded according to the temperature. Mohamad et al. (Reference Mohamad, Mansour and Ramadan2015) reported in Ascogaster quadridentata (Wesmael) a balanced sex ratio at 20, 25 and 30 °C but male-biased at 35 °C. The Cotesia flavipes (Cameron) sex ratio was female-biased at 30 °C, whereas more males emerged at lower temperatures (22 and 26 °C) (Jiang et al., Reference Jiang, Sétamou, Ngi-Song and Omwega2004). Hentz et al. (Reference Hentz, Ellsworth, Naranjo and Watson1998) found that the progeny of Bracon hebetor (Say) was female-biased at 20 °C but male-biased at 30 and 40°C. It was, however, unclear why in the present study, no clear gradient of temperature was obtained for male-biased and balanced sex ratio, and further studies are needed to elucidate this finding.
There was a significant difference in the daily realized fecundity registered at 20 and 25 °C, with 20 °C recording the highest fecundity. This result can be explained by the higher mortality registered at the larval stage at 25 °C as compared to 20 °C and the higher number of eggs deposited at 20 °C. Daily fecundity recorded in A. taragamae varied from 5.5 at 20 °C to 1.2 at 30 °C (Dannon et al., Reference Dannon, Tamò, van Huis and Dicke2010), results which are not far from what we observed in the current study in A. hemara, and support the idea that the daily fecundity is affected by temperature. The extended longevity of the female at 20 °C is translated into longer post-oviposition period, meaning that A. hemara lays most of their eggs in the first 2 weeks following their emergence. Şengonca and Peters (Reference Şengonca and Peters1993) showed that the Cotesia rubecula (Marsh.) (Hymenoptera: Braconidae) oviposition period lasts about 17 days which is similar to the results we obtained in this study. No pre-oviposition was observed during this experiment on A. hemara. The absence of pre-oviposition period was reported in other Microgastrinae species such as Pseudapanteles dignus (Muesebeck), a primary parasitoid of the tomato pinworm Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) (Cardona and Oatman, Reference Cardona and Oatman1971), Apanteles machaeralis (Wilkinson) a parasitoid of Diaphania indica (Saunders) (Lepidoptera: Pyralidae) (Peter and David, Reference Peter and David1990), Apanteles s. (Blanchard) (Cardona and Oatman, Reference Cardona and Oatman1975), C. rubecula (Şengonca and Peters, Reference Şengonca and Peters1993) and Glyptapanteles thompsoni (Lyle) (Hymenoptera: Braconidae) (Vance, Reference Vance1931).
Conclusion
This study is the first to report the effect of temperature on A. hemara, an important parasitoid of amaranth lepidopteran defoliator, S. recurvalis. The study showed that temperature has a strong effect on the development rate, mortality, sex ratio, fecundity and longevity of A. hemara. This parasitoid has the estimated optimal total development at 30.8 °C, which gives an indication of the optimal conditions for mass rearing. It also provides the first data on the temperature range suitable for field releases in augmentative biological control. Moreover, this study has contributed to the deeper understanding of the biology of A. hemara and has formed a basis for future phenology modelling works such as validation of the model under fluctuating temperatures, forecasting phenology of A. hemara with existing climate data especially temperature. This will allow to determine the number of generations of the parasitoid per year in a given agro-ecological zone base on its degree-days requirement, and to know which period of the year the parasitoid will be likely absent. Also, a comparative study of thermal requirements between the pest and the natural enemy is warranted.
Acknowledgements
The present study was funded by the German Federal Ministry for Economic Cooperation and Development (BMZ). We gratefully acknowledge also the financial support for the core research agenda of icipe by UK Aid from the UK Government; Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); and the Kenyan Government. The first author received a scholarship from the German Academic Exchange Service (DAAD) through icipe Capacity Building Program (ARPPIS). The authors express their gratitude to the project team for their technical assistance.