Introduction
Various biotic and abiotic factors affect the behaviour of pests and the efficiency of their biocontrol agents. Temperature is one prominent abiotic factor influencing development (Visser & Both, Reference Visser and Both2005) and reproductive potential of insects (Mironidis & Savopoulou-Soultani, Reference Mironidis and Savopoulou-Soultani2008) and subsequently their population dynamics (Logan et al., Reference Logan, Wolesensky and Joern2006). An increase in temperature may contract or expand the distribution of a given pest (Powell & Logan, Reference Powell and Logan2005) and its natural enemies (Furlong et al., Reference Furlong, Zalucki, Shabir and Adamson2016).
The likely increase in temperature due to changing climate will have a great bearing on the effectiveness of natural enemies for pest management, which in turn will affect insect host-natural enemy associations, crop production and food security (Dhillon & Sharma, Reference Dhillon and Sharma2009) because both host and parasitoid may have different thermal curves (Hance et al., Reference Hance, van Baaren, Vernon and Boivin2007; Furlong & Zalucki, Reference Furlong and Zalucki2017). Our understanding of biological control and ability to manipulate the interaction is facilitated when the relationship between climate, predominately temperature and biocontrol agents and their hosts is known (Golizadeh et al., Reference Golizadeh, Kamali, Fathipour and Abbasipour2008). Thermal requirements of insects have both ecological and practical utility (Damos & Savopoulou-Soultani, Reference Damos and Savopoulou-Soultani2011). Thermal constants and developmental thresholds influence the success of biocontrol agents by affecting their activity and abundance (Furlong & Zalucki, Reference Furlong and Zalucki2017). Differences in lower developmental thresholds of insect predator and prey affect predator–prey dynamics (Dixon, Reference Dixon2006). In addition, time of appearance of natural enemies can be predicted using thermal constants (Malina & Praslicka, Reference Malina and Praslicka2008) and thermal thresholds help to predict occurrence and abundance of biocontrol agents (Dhillon & Sharma, Reference Dhillon and Sharma2009). Developmental thresholds and thermal requirements can be used to select biocontrol agents that are adapted to conditions, which are suitable for their prey (Obrycki & Kring, Reference Obrycki and Kring1998; Perdikis & Lykouressis, Reference Perdikis and Lykouressis2002) and to select suitable temperatures for mass rearing of insects (Torres et al., Reference Torres, Musolin and Zanuncio2002). Knowing the thermal requirements of an insect can aid interpretation of its present geographical distribution and in predicting its future distribution (Hance et al., Reference Hance, van Baaren, Vernon and Boivin2007).
Helicoverpa armigera, also known as the cotton bollworm, is one of the most important pests of cotton. H. armigera is found almost throughout the world (Europe, Africa, Asia and Australia), recently invaded South America and is likely to spread to North America (Kriticos et al., Reference Kriticos, Ota, Hutchison, Beddow, Walsh, Tay, Paula-Moreas, Czepak and Zalucki2015; Downes et al., Reference Downes, Kriticos, Parry, Paull, Schellhorn and Zalucki2016). Habrobracon hebetor (Say) is an idiobiont, gregarious ecto-parasitoid that targets pyralid species (Brower & Press, Reference Brower and Press1990) as well as various other lepidopteran larvae (Chen et al., Reference Chen, Zhang, Zhu and James2013). It has potential as a biological control agent against H. armigera (Nikam & Pawar, Reference Nikam and Pawar1993) in different parts of world (Imam et al., Reference Imam, Uwais, Namat, Akbar and Ahmat2007; Ashfaq et al., Reference Ashfaq, Khan and Farooq2011). Females first paralyse the host larvae and then lay 2–3 eggs on the larval surface. After 3–5 days, the eggs hatch and larvae start to feed on the paralysed host larva for approximately 10 days after which pupation occurs. Adults emerge from pupae after 8–9 days and start searching for new host larvae (Ode et al., Reference Ode, Antolin and Storand1996).
Thermal effects on development of H. armgiera have been studied in various parts of world, including Australia (Foley, Reference Foley1981; Kay, Reference Kay1981; Room, Reference Room1983), Japan (Jallow & Matsumura, Reference Jallow and Matsumura2001) and Greece (Mironidis & Savopoulou-Soultani, Reference Mironidis and Savopoulou-Soultani2008). Forouzan et al. (Reference Forouzan, Amirmaafi and Sahragard2008) and Ahmad et al. (Reference Ahmad, Al-Maliky, Al-Taweel, Jabo and Al-Hakkak1985) studied development of H. hebetor in relation to temperature with Galleria mellonella L. and Ephestia cautella (Walker), respectively, as the hosts but did not cover a wider range of temperatures suitable for development of H. hebetor. Development and reproductive biology of insects may differ with geographical regions (Tsoukanas et al., Reference Tsoukanas, Papadopoulos, Fantinou and Papadoulis2006). Therefore species thermal requirements should be tested with local populations. There are no reports on the effect of temperature on H. hebetor with H. armigera as a host. Vingradova & Reznik (Reference Vingradova and Reznik2015) concluded that using a wide range of constant temperatures is comparable with natural thermorhythms in relation to rate of development. Therefore, we establish the influence of temperature on H. armigera and its parasitoid, H. hebetor across a wide range of constant temperatures. Both host and parasitoid were sourced from Pakistan. We were interested in the thermal optima and high-temperature effects, as well as establishing the developmental threshold and degree days for each species.
Materials and Methods
Culturing of H. armigera and H. hebetor
Adult H. armigera collected from cotton fields near Faisalabad (Latitude, 31.4181N; Longitude, 73.0776E), (Punjab), Pakistan in light traps were placed in plastic jars (45 cm diameter and 30 cm depth) covered with muslin cloth. A 10% solution of honey in water was provided, on soaked cotton pads, to feed the adults. A piece of nappy liner was hung inside the jar in order to collect eggs. Larvae were reared on a semi-synthetic diet (Ahmad et al., Reference Ahmad, Arif and Ahmad1995) for up to four generations.
All developmental stages of the Greater Wax Moth, G. mellonella were collected from infested bee hives. Adults were kept in plastic jars 25 cm diameter and 30 cm deep, for mating and egg collection and larvae were reared on artificial diet (Khan et al., Reference Khan, Ashfaq, Ahmed and Sahi2011). Adults and larvae were incubated in a growth chamber, at optimum conditions of 30 ± 1 °C and 75 ± 5% RH (Khan et al., Reference Khan, Ashfaq, Ahmed and Sahi2011). Adults of H. hebetor were obtained from the parasitized larvae of H. armigera from cotton and tomato field, from Faisalabad, (Punjab), Pakistan. The adults were then reared, in the laboratory up to four generations, on larvae of G. mellonella to establish a colony, at 29 ± 1 °C and 65 ± 1% RH, with 16:8 L:D hours, in glass vials of 5 cm diameter and 10 cm depth. Each female in a vial was offered a 4th–5th instar larva of the host along with 20% honey solution in water soaked in cotton swab. After 24 h, the female H. hebetor were moved to another vial containing a larva of G. mellonella and the parasitized larvae incubated as described above.
Temperature-dependent development and survival of immature stages of H. armigera and H. hebetor
Eggs of H. armigera (<24 h old) were placed in Petri dishes (6 cm in diameter) containing artificial diet and subjected to a range of constant temperatures, i.e. 10, 15, 17.5, 20, 25, 27.5, 30, 35, 37.5, 40 °C (all ± ½°C) with 65 ± 1% RH, with 16:8 L:D hours in controlled environmental chamber (GC-1000 DD, Usman Technical Services, Faisalabad, Punjab, Pakistan). A batch of 150–350 eggs of H. armigera was placed at 10–30 °C, while 400–550 eggs were used at 35, 37.5 and 40 °C. Hatching of eggs (and later larval development) was recorded daily at 10, 15, 20 and 25 °C and at twice a day for the higher temperatures (30, 35, 37.5 and 40 °C). Larvae were placed in individual Petri dishes (6 cm in diameter) to avoid cannibalism. Larval development was recorded until pupation and the duration of pupal development was observed.
Four-day-old mated female H. hebetor were transferred individually into glass vials (10 × 5 cm2) each containing a 5th instar larva of H. armigera with its artificial diet. Fifth instar larvae of H. armigera are a suitable stage for parasitism by H. hebetor (Saxena et al., Reference Saxena, Ponnusamy and Iquebal2012). Wasp females were provided with 20% honey solution via cotton swabs. Egg laying was checked at 4 h intervals when larvae with eggs were transferred to a Petri dish (6 cm in diameter), and then subjected to constant temperatures range (10–40 °C). The duration of development of eggs of H. hebetor was recorded at 4 h intervals, while larval and pupal development was observed at 24 h intervals.
Temperature-dependent parasitism of H. hebetor
Pairs of 4 d old H. hebetor adults (n = 30 pairs per replicate) were each provided with ten 5th instar larvae at each constant temperatures from 10 to 40 °C. Larvae of H. armigera, along with artificial diet, were provided individually to each pair to avoid cannibalism in a glass vial (10 × 5 cm2) for a period of 48 h. Parasitoids were provided with 20% honey solution supplied by cotton swab, until all 10 larvae had been proffered (each pair took 20 days). The number of parasitized larvae was scored based on parasitoid pupal formation. This procedure was replicated three times, i.e. 90 pairs of parasitoids in total were trialled at each tested temperature to estimate parasitism.
Model fitting and analysis
The effect of different temperatures on developmental stages was analysed by One-way analysis of variance (ANOVA) using SPSS software and means were compared by Tukey's Honest Significant Difference (HSD) test at P ≤ 0.05. The survival rate and parasitism were subjected to quadratic regression in order to show the relationship with temperature. The effect of temperature on insect development was established using a linear regression; viz. Y = a + bT, where Y is the developmental rate (1/time in stage) at each temperature T, a is the intercept, and b is the slope of the fitted line. The lower developmental threshold (T 0) and thermal constant (k) were calculated as: T 0 = −a/b and k = 1/b (Campbell et al., Reference Campbell, Frazer, Gilbert, Gutierrez and Mackauer1974). The Ikemoto & Takai (Reference Ikemoto and Takai2000) regression method, used to calculate DD and T 0, fits DT = k + T 0 × D, where D is the duration of development (in days), Biological parameters (T 0 and k) for both linear models were compared by the paired t-test.
Various non-linear models available in the literature to describe insect development with temperature; Pradhan–Taylor (Gaussian), Davidsons logistic, Logan-6, Logan-6/Lactin-2, Logan-6/Lactin 1, Logan-10, Hilbert and Logan, Analytis-1/Allahyari, Analytis-1, Analytis-3/Kontodimas, Analytis-3/Briere-1, Analytis-3/Briere-2, Janisch/Analytis, Janisch/Rochat, Polynomial (cubic), Shi-1, Shi-2 and Wang model; were applied to development data across the range of temperatures used for both H. armigera and H. hebetor. These models are frequently used to depict the relationship between temperature and arthropod developmental rates (e.g. Zahiri et al., Reference Zahiri, Fathipour, Khanjani, Moharramipour and Zalucki2010; Shi et al., Reference Shi, Ge, Sun and Chen2011; Bahar et al., Reference Bahar, Soroka, Grenkow and Dosdall2014). Calculations were made using MATLAB R 2016b.
Model evaluation
The best-fit model was assessed based on the coefficient of nonlinear regression (for non-linear models; R 2), sum of squares error (SSE) and biological criteria. High R 2 and lower SSE are the usual criteria for best-fit models (Walgama & Zalucki, Reference Walgama and Zalucki2006). Because non-linear models differ in the number of parameters that need to be estimated an addition criteria is often applied to discriminate amongst the models. We employed Akaike information criterion (AIC) to appraise goodness of fit of non-linear models (Akaike, Reference Akaike1974). AIC is defined as: AIC = 2k − 2ln(L), where k is the number of estimated parameters for the model, L is the maximum value of the likelihood function for the model and ln is the natural log. The best-fit model would have lower value of AIC.
Lastly the best-fitting non-linear models were assessed on biological grounds as parameters by comparing estimates of T o, T opt and T max: the low-temperature threshold, optimal temperature and high-temperature threshold, respectively with experimental data. Non-linear models can give nonsensical values for these due to the form of the function at temperature extremes and extrapolation errors; such model could not consider as best fit model, even though they may have high R 2 values.
Results
Temperature influence on H. armigera and H. hebetor
Development
No stage of H. armigera could complete development at 40 °C. Below 40 °C temperature had a significant effect on the developmental period of eggs (F = 3389, DF = 1, 5 and P < 0.001), larvae (F = 2671, DF = 1, 4 and P < 0.001) and pupae (F = 556, DF = 1, 3 and P < 0.001) (fig. 1). Eggs of H. armigera completed their development 2.2 days at 35 °C and took longer at temperatures below and above 35 °C. Larvae of H. armigera were not able to complete their development at 10 °C but, as with the eggs, the development period decreased with increasing temperatures; 70 days at 15 °C to 11 days at 35 °C. Pupae of H. armigera could not complete development at 10 and 15 °C, but the developmental period decreased from 22 days at 20 °C to 9 days at 35 °C. Developmental period of H. armigera increased for all immature stages at 37.5 °C (table 1). Temperature significantly (P < 0.001) affected the developmental period of eggs (F = 387, DF = 1, 6), larvae (F = 1076, DF = 1, 5) and pupae (F = 439, DF = 1, 4), of H. hebetor (fig. 1). As expected the developmental period of each immature stage of H. hebetor decreased with an increase in temperature except at 37.5 and 40 °C. Unlike H. armigera, eggs of the parasitoid completed their development at 40 °C and pupae developed at 15 °C; as with H. armigera, larvae of H. hebetor could not survive at 10 °C.
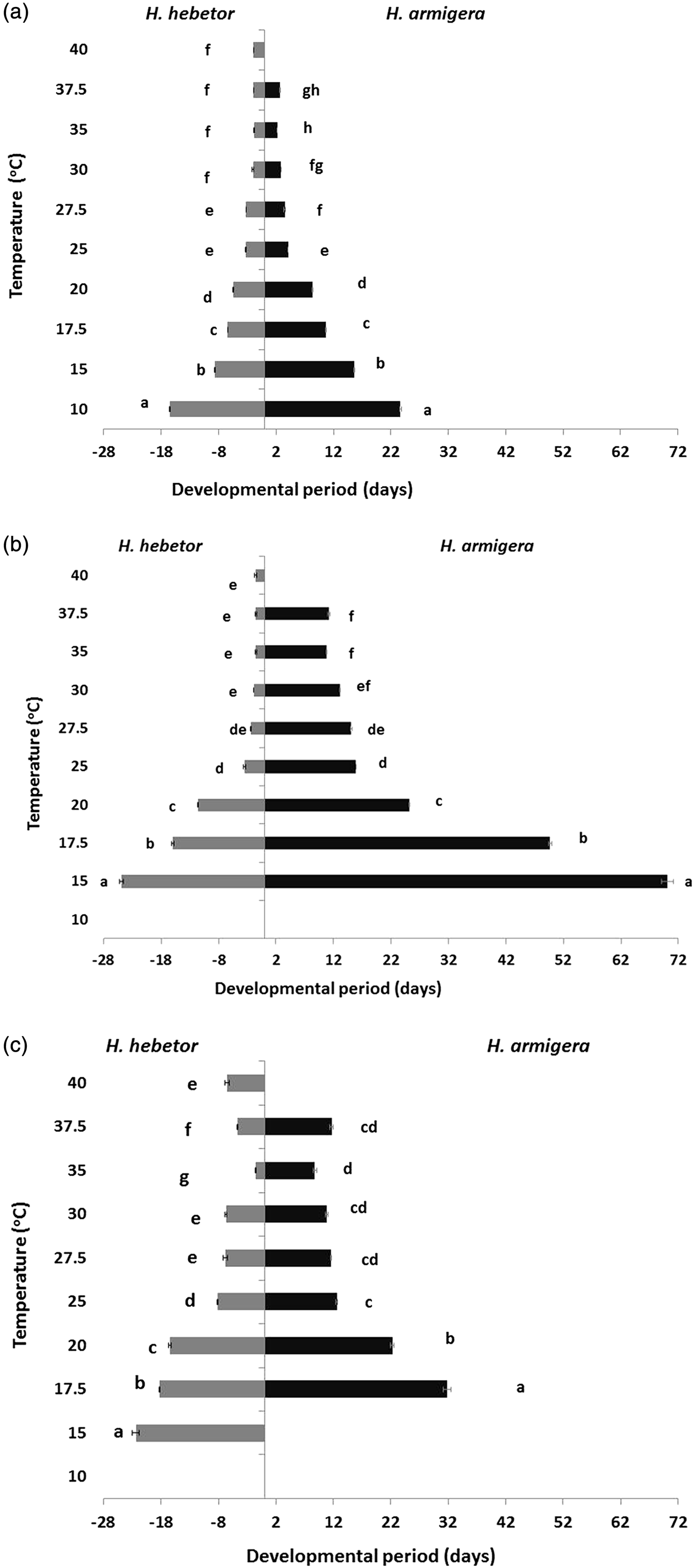
Fig. 1. Development time (days ± SE) of immature stages of H. hebetor and H. armigera (eggs, larvae and pupae) at ten constant temperatures from 10 to 40 °C (see the text for details).
Table 1. Linear regression models (ordinary and Ikemoto) parameters and R 2 values for temperature dependent development of immature stages (eggs, larvae, pupae and combined immature stages) of H. armigera and H. hebetor.
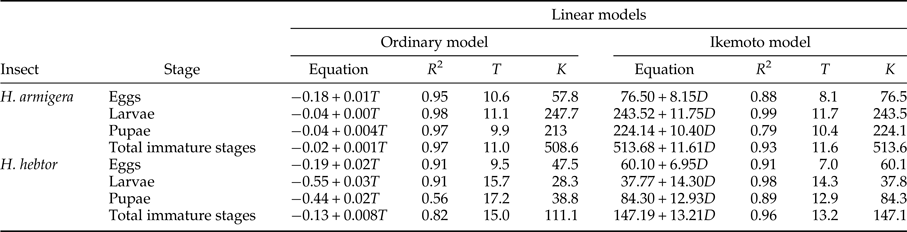
T o, developmental threshold; K (DD), thermal constant (degree-day), *(egg to adult emergence).
Survival
The highest survival of H. armigera was observed at 25 °C (fig. 2). Based on the fitted curves survival was optimal at 23.2 °C (61%), 24.3 °C (87%) and 25.1 °C (77%) for eggs, larvae, pupae of H. armigera, respectively (fig. 2). Larvae of H. armigera showed the highest survival and were less affected by temperature above and below the optimum than eggs or pupae. As was observed with its H. armigera host, survival of all stages of H. hebtor was greatest at 25 °C (fig. 2) and then tailed off at either higher or lower temperatures. Based on the fitted curves survival rate of H. hebetor was 53% at 25.2 °C, 81% at 25.8 °C and 76% at 26 °C for eggs, larvae and pupae, respectively (fig. 2).
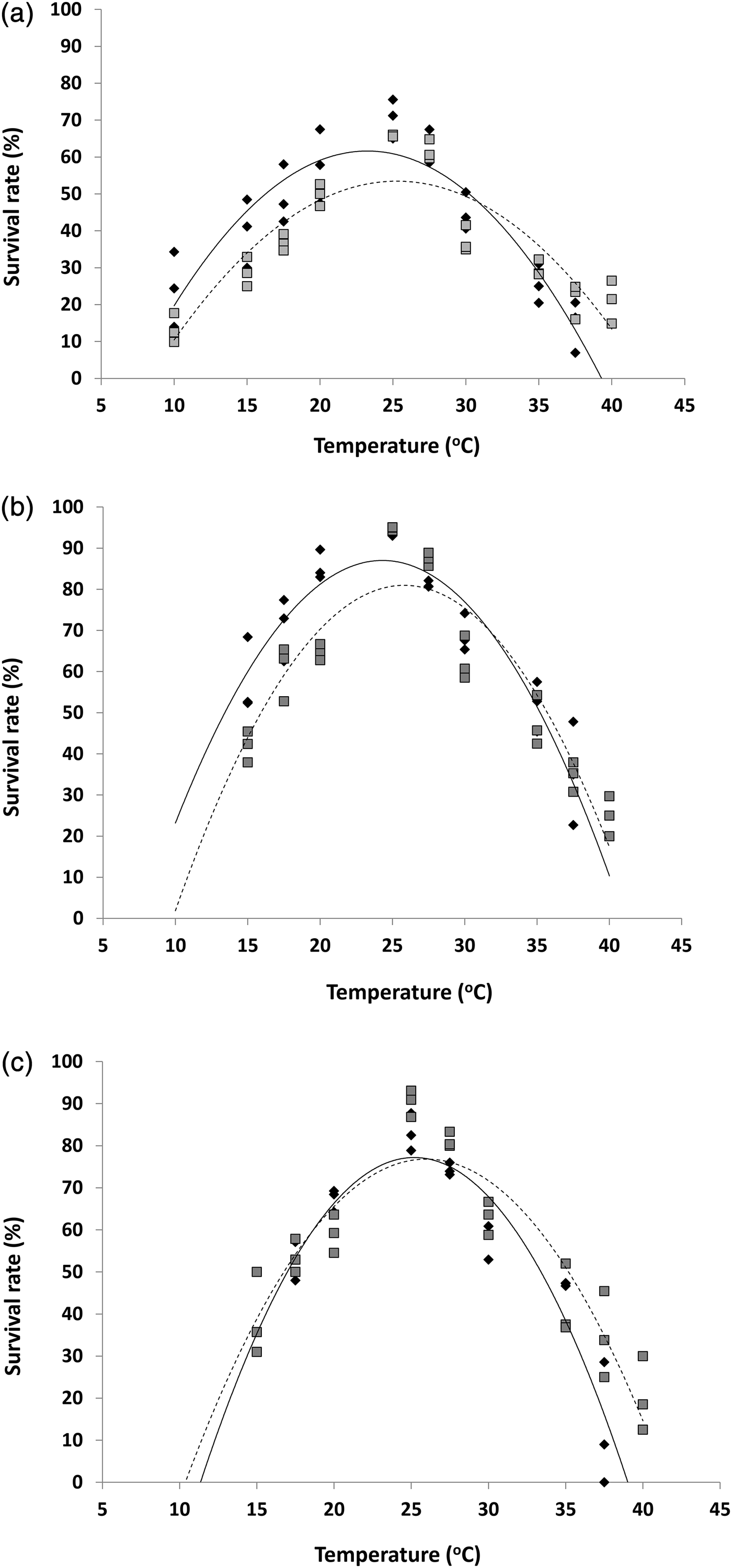
Fig. 2. Survival rate (%) of (a) eggs, (b) larvae, (c) pupae of H. armigera (![]() ) and H. hebetor (
) and H. hebetor (![]() ) at ten constant temperatures from 10 to 40 °C. Quadratic lines for H. armgiera (solid lines) and H. hebetor (dotted lines) (a) Eggs: H. armigera, Y = −0.2389x 2 + 11.107x − 67.456, R 2 = 0.82 and H. hebetor, Y = −0.1845x 2 + 9.3246x − 64.355, R 2 = 0.77 (b) Larvae: H. armigera, Y = −0.3115x 2 + 15.148x − 97.14, R 2 = 0.86 and H. hebetor, Y = −0.3162x 2 + 16.324x − 129.73, R 2 = 0.85, and (c) Pupae: H. armigera: Y = −0.4032x 2 + 20.311x − 178.61, R 2 = 0.88 and H. hebetor: Y = −0.3156x 2 + 16.394x − 136.05, R 2 = 0.83.
) at ten constant temperatures from 10 to 40 °C. Quadratic lines for H. armgiera (solid lines) and H. hebetor (dotted lines) (a) Eggs: H. armigera, Y = −0.2389x 2 + 11.107x − 67.456, R 2 = 0.82 and H. hebetor, Y = −0.1845x 2 + 9.3246x − 64.355, R 2 = 0.77 (b) Larvae: H. armigera, Y = −0.3115x 2 + 15.148x − 97.14, R 2 = 0.86 and H. hebetor, Y = −0.3162x 2 + 16.324x − 129.73, R 2 = 0.85, and (c) Pupae: H. armigera: Y = −0.4032x 2 + 20.311x − 178.61, R 2 = 0.88 and H. hebetor: Y = −0.3156x 2 + 16.394x − 136.05, R 2 = 0.83.
Relationship between development rate and temperature
Linear regression models for each developmental stage were used to calculate threshold temperatures and degree days to complete development (table 1). The Ikemoto and Takai model and ordinary regression model were not statistically different for developmental threshold (t = 0.261, P > 0.05) and k (t = −1.581, P > 0.05) in case of H. armigera. Using the Ikemoto and Takai model the pattern of T o for H. armigera was larva (11.7 °C) > pupa (10.4 °C) > Egg (8.1 °C).
In case of H. hebetor the two linear models differed in their estimate of T o (t = 4.029, P < 0.05). Larvae of H. hebetor showed a higher threshold (14.3 °C) than pupae (12.9 °C) or eggs (7 °C) based on the Ikemoto and Takai model (table 1). Most non-linear models could be fitted to all developmental stages except Shi-2 in case of H. armigera. This model could only be fitted to eggs along with larval period as parameter estimates failed to converge in these cases. The selected models for the entire immature period differed in their ability to estimate pivotal temperatures (T min, T opt, and T max) for H. armigera (table 2, fig. 3). Analytis-3/Briere-2 model was adjudged the best model for entire developmental period of H. armigera due to its biological significance (see the Discussion ). The estimated values for T o, T opt and T max are 8.5, 34.8 and 38.7 °C, respectively. Using the best-fit models of H. hebetor (table 2, fig. 3) the calculated values of T o, T opt and T max are 13.3, 37.2 and 40.5 °C, respectively, based on the biological significance (see the section Discussion).
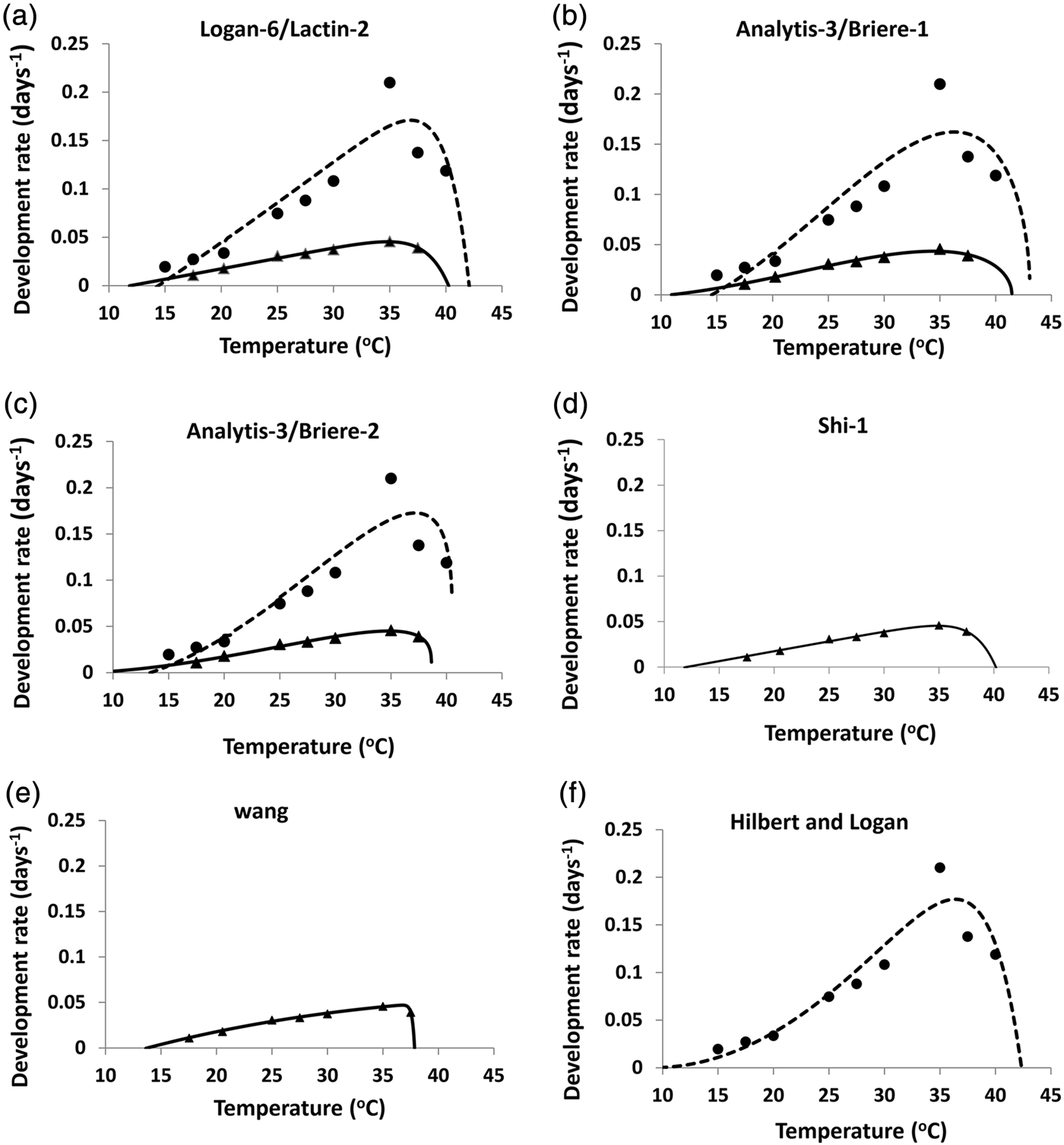
Fig. 3. Development rate for whole immature stages and best fit models for H. armigera and H. hebetor. Observed data (triangles) and fitted non-linear models (solid lines) of H. armigera and Observed data (dots) and fitted non-linear models (dotted lines) of H. hebetor (see table 2 for detail).
Table 2. Parameter estimates ± SE for selected non-linear models fitted (AIC-based ranking and biological significance) to describe the development rate of entire immature stages of H. armigera and H. hebetor. Estimated T min, T opt and T max are also provided.
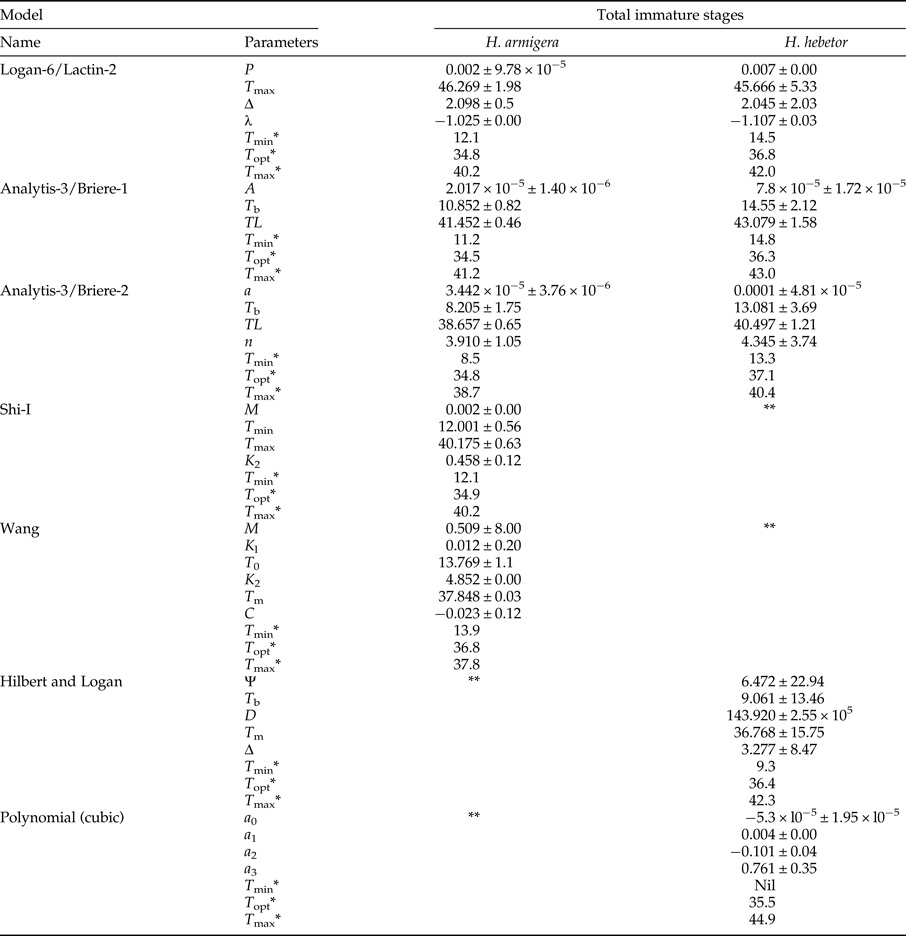
*Values obtained mathematically using Microsoft excel. **Not selected based on AIC and biological significance.
Parasitism
The level of parasitism varied with temperature (fig. 4) with the highest level (99.8%) being observed at 25 °C. This reduced to 52% at 15 °C and 13% at 40 °C.
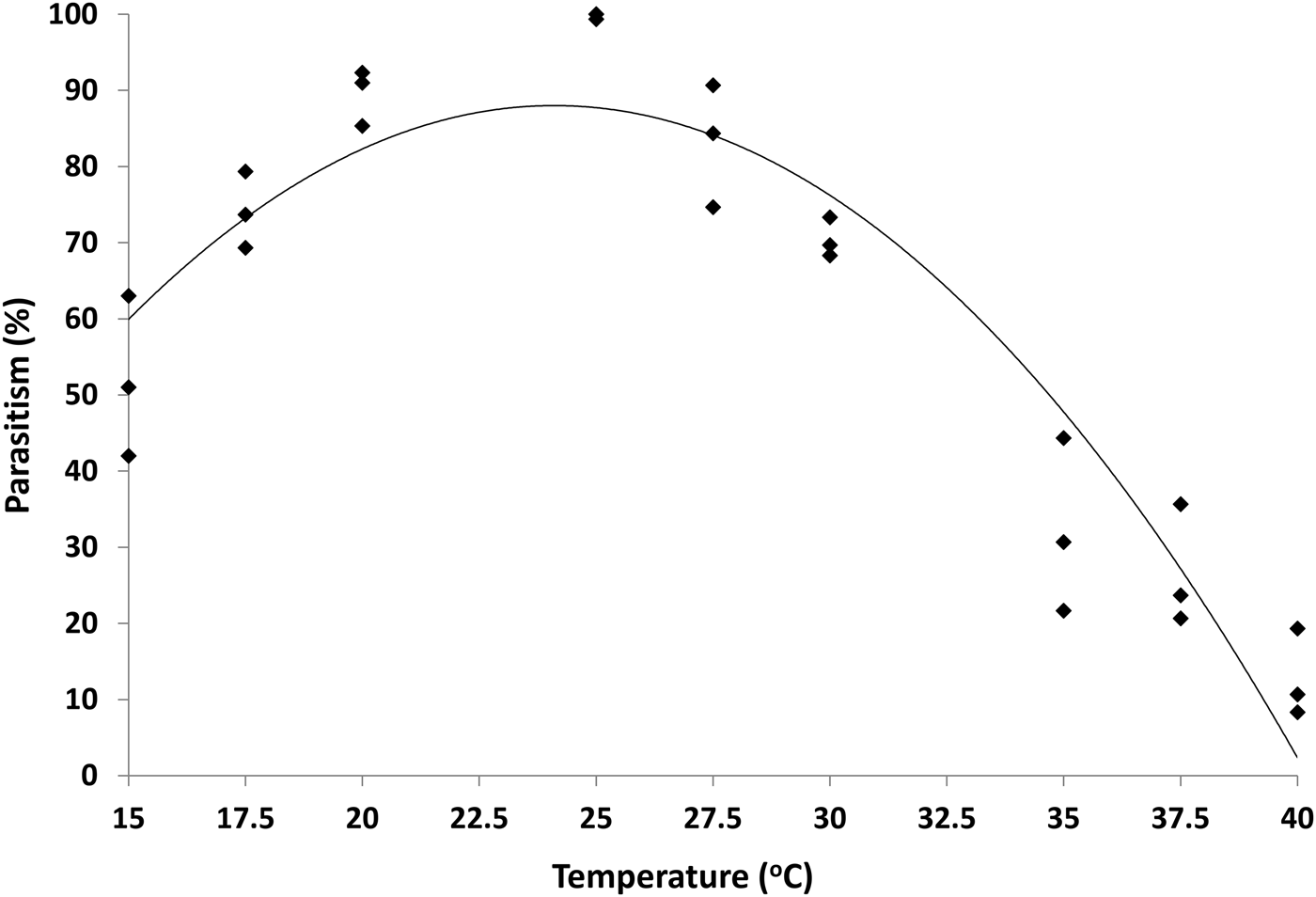
Fig. 4. Percentage of successful parasitism (Y) by H. hebetor of H. armigera late instar larvae at constant temperatures. Fitted line: Y = −0.3388x 2 + 16.334x − 108.84, R 2 = 0.88.
Discussion
As expected immature life stages of both H. armigera and H. hebetor completed their development in shorter times at high temperatures up to an optimum temperature. A similar pattern of developmental period against temperature was previously recorded for H. armigera (Jallow & Matsumura, Reference Jallow and Matsumura2001; Bartekova & Praslicka, Reference Bartekova and Praslicka2006) and H. hebetor (Engroff & Watson, Reference Engroff and Watson1975; Thanavendan & Jeyarani, Reference Thanavendan and Jeyarani2010). However, developmental period in our study is shorter than observed in other studies on H. armigera, which were carried out on a population from Japan when reared on tomato (Jallow & Matsumura, Reference Jallow and Matsumura2001), on one from Slovakia reared on corn seeds (Bartekova & Praslicka, Reference Bartekova and Praslicka2006) but higher values of developmental period than a population from Greece reared on artificial diet (Mironidis & Savopoulou-Soultani, Reference Mironidis and Savopoulou-Soultani2008). Developmental thresholds of H. armigera recorded in our study (11–12 °C) were in agreement with other studies (Jallow & Matsumura, Reference Jallow and Matsumura2001; Mironidis & Savopoulou-Soultani, Reference Mironidis and Savopoulou-Soultani2008). However, Bartekova & Praslicka (Reference Bartekova and Praslicka2006) and Foley (Reference Foley1981) determined a higher developmental threshold for eggs (14.8 °C), and non-diapusing pupae (14.8 °C). The life history traits may differ among the population of different geographical regions (Tsoukanas et al., Reference Tsoukanas, Papadopoulos, Fantinou and Papadoulis2006), rearing techniques and diet.
The developmental period of H. hebetor observed in the present study differed from that of Forouzan et al. (Reference Forouzan, Amirmaafi and Sahragard2008) who observed higher values of developmental period for H. hebetor on G. mellonella. In contrast, Ahmad et al. (Reference Ahmad, Al-Maliky, Al-Taweel, Jabo and Al-Hakkak1985) recorded a slightly lower developmental duration of H. hebetor on E. cautella. In the present study, H. hebetor were unable to survive and complete development only at 10 °C. In contrast, survival rate of H. hebetor was zero at 16 °C for larvae and pupae when reared on G. mellonella in Iran (Forouzan et al., Reference Forouzan, Amirmaafi and Sahragard2008). These differences in aspects of life history traits may be due to different strain of H. hebetor, rearing host and/or geographical origin. The lower developmental threshold (15 °C) for H. hebetor calculated in our study is higher than that calculated (13 °C) by Forouzan et al. (Reference Forouzan, Amirmaafi and Sahragard2008) who used a different host (G. mellonella). Variation in lower developmental threshold of insect predators and prey plays an important role in predator and prey dynamics (Dixon, Reference Dixon2006).
In this study, H. armgiera completed its development within a narrower range of temperature (17.5–37.5 °C) than its parasitoid (15–40 °C), which contrasts to normal paradigm that parasitoids have generally narrow thermal tolerance than its host (Messenger & Bosch, Reference Messenger, Bosch and Huffaker1971). Parasitoids with higher tolerance than its host may go extinct locally due to absence of its host (Bahar et al., Reference Bahar, Soroka, Grenkow and Dosdall2014). As H. hebetor is a generalist parasitoid, having a broad host range within the Lepidoptera, there is less chance of extinction of H. hebetor with climatic warming. The developmental periods for H. hebetor were shorter than for H. armigera over all temperatures. A shorter developmental period for the natural enemy is helpful for successful biological control programs (Snyder & Ives, Reference Snyder and Ives2003) as there would be more generations compared with the host. Higher temperatures (>35 °C) are lethal to all stages of H. armigera (this study) as has been found by others (Bashi & Tunc, Reference Bashi and Tunc2008; Tran et al., Reference Tran, Worner, Hale and Teulon2012). We found that H. hebetor completed its development at higher temperatures as compared with H. armigera. This finding suggests that H. hebetor can be used to control cotton bollworm even in tropical areas. Low survival rates of H. hebetor at high temperatures (>37 °C) may be due to increased defence reaction such production of prophenoloxidase (PPOs) by the hosts (Spanoudis & Andreadis, Reference Spanoudis and Andreadis2012). Larvae of both H. armigera and H. hebetor were comparatively more heat tolerant than pupae or eggs. A possible explanation of this difference is production of more heat-shock proteins (Hsps) in the larval stage than at other immature stages (Sinha & Sanyal, Reference Sinha and Sanyal2013).
To overcome the pitfall of linear models, we evaluated the appropriateness of non-linear models based on how well they described the data (AIC ranking) and the estimated biological parameters (T o, T opt, T max) (see the section Materials and Methods). Five models were selected based on AIC ranking for H. armigera: Wang, Shi-1, Logan-6/Lactin-2, Analytis-3/Briere-2, and Analytis-3/Briere-1. All provided adequate description of the data set (fig. 3). Estimates of key biological parameters were compared (table 2). Even though the Wang model provided reasonable estimates of T max and T opt; 37.8 and 36.8 °C, respectively; the value of T o (13.93 °C) appears to be too high (see linear models) and the model had more fitted parameters (6). The best fit must meet criteria of parsimony and fewer parameters with goodness of fit and biological significance (Walgama & Zalucki, Reference Walgama and Zalucki2006). The remaining four models (Shi-1, Logan-6/Lactin-2, Analytis-3/Briere-2, and Analytis-3/Briere-1) selected on AIC gave similar estimated values for biological parameters (table 3). We favoured the Analytis-3/Briere-2 model because it gave a T max (38.7 °C) very similar to experimental result and had fewer fitted parameters.
The non-linear models for H. hebetor presented an interesting problem. Based on AIC criteria the top five models were Janisch/Rochat, Janisch/Analytis, Logan-10, Analytis-1/Allahyari and Pradhan-Taylor (Gaussian), but each gave unrealistic values for T max (over 50 °C) or T o (less than zero), which did not accord with experimental observations. We chose to look more closely at the next best fitting models: Polynomial (cubic), Hilbert and Logan, Analytis-3/Briere-2, Logan-6/Lactin-2 and Analytis-3/Briere-1 on the basis of AIC. We rejected the Polynomial (cubic) model as it cannot be used to calculate T o. Hilbert and Logan model calculated appropriate T opt, unrealistic T min and slightly higher T max than the remaining models (table 3). We did not consider this model further due to the higher number of parameters that need to be estimated (5). Failure of Analytis-3/Briere-2 model to be best fit for H. hebetor was due to unrealistic T opt and T max. Of the remaining models Analytis-1/Briere-1 was adjudged best as it required only three fitted parameters and gave appropriate biological parameters (T opt and T max). Using an appropriate biofix the first emergence of H. hebetor adults can be predicted using both linear and non-linear models.
Our data indicate that H. hebetor has the potential to be used as a biological control agent for H. armigera over a wide range of temperatures (fig. 4). The effect of temperature on life history traits for the two insects are very similar e.g. reductions in the development time of the host stages at higher temperatures are mirrored by equivalent reductions in the parasitoid. However, H. hebetor does better than H. armigera at higher temperatures. Higher thermal tolerance of H. hebetor indicates that this parasitoid has features of an effective biocontrol agent both in cooler areas but also in warmer areas. It may coincide with the changing distribution of H. armigera in climate warming scenarios due to its high thermal tolerance. We directly tested the efficiency of parasitism at various temperatures and found that although the level dropped at the higher temperatures H. hebetor was still able to parasitize H. armigera in laboratory assays at 40 °C and complete development. Thermal tolerance and parasitism indicate the potential of H. hebetor as an effective biological control agent to control H. armigera. Further studies are needed to explore the relationship of temperature with H. hebetor and its pest, H. armigea under fluctuating temperatures/natural conditions, as well as the effects of host plant and humidity.
Acknowledgements
The authors are thankful to Higher Education Commission, Pakistan to sponsor this research, Yaghoub Fathipour, Department of Entomology, Faculty of Agriculture, Tarbiat Modares University, Tehran, Iran, Dr M. Ashfaq and Dr Jalal Arif from University of Agriculture Faisalabad, Pakistan for helping out in different capacities and two anonymous referees for comments on early versions of the manuscript.
Disclosure
The authors have no potential conflicts of interests to disclose.









