Introduction
The abundance and diversity of many farmland invertebrates are now recognized to be threatened by intensive farming practices (Carcamo et al., Reference Cárcamo, Niemala and Spence1995). Species of ground beetle (Coleoptera: Carabidae) and rove beetle (Coleoptera: Staphylinidae) feed on crop pests and are themselves food to members of higher trophic groups such as birds and small mammals. Their conservation is therefore important for the ecosystem services they provide and the contribution they make to overall biodiversity. In recent decades considerable effort has been spent evaluating various aspects of their ecology including their effectiveness as predators (reviewed by Symondson et al., Reference Symondson, Sunderland and Greenstone2002); the adverse effects of agricultural inputs (reviewed by Kromp, Reference Kromp1999; Holland & Luff, Reference Holland and Luff2000) and habitat loss (Driscoll & Weir, Reference Driscoll and Weir2005); and the importance of population spatial structure and dynamics on the sustainability of their populations (Thomas et al., Reference Thomas, Parkinson and Marshall1998, Reference Thomas, Parkinson, Griffiths and Fernández-García2001; Holland et al., Reference Holland, Perry and Winder1999, Reference Holland, Begbie, Birkett, Southway, Thomas, Alexander and Thomas2004, Reference Holland, Thomas, Birkett, Southway and Oaten2005a).
Attempts to counteract the declining abundance and diversity of beetle populations in farmland have centred on strategies that involve reducing pesticide inputs and increasing habitat diversity, for example conservation headlands (Chiverton & Sotherton, Reference Chiverton and Sotherton1991), strip management (Lys et al., Reference Lys, Zimmermann and Nentwig1994), beetle banks (Thomas et al., Reference Thomas, Wratten and Sotherton1991) and field margins (Thomas & Marshall, Reference Thomas and Marshall1999). These approaches depend primarily on reducing mortality by providing refugia from pesticides, cultivations and extremes of winter weather. Although relatively easily implemented, they are passive methods inasmuch as they are usually introduced at locations convenient for the farmer, without knowledge of the local natural distribution or requirements of beetle populations. The success of implementing an agri-environmental practice may, therefore, be hit-or-miss and such deployments are rarely followed up with routine monitoring. These methods are at present, however, the best available.
Alternative approaches to managing beetle populations in the field necessarily depend on more detailed knowledge of the key ecological requirements of various species. Much of this detail is still wanting and remains difficult to obtain. Moreover, some factors may not be considered at all as their management is thought beyond the realms of practical intervention. An exception may be soil moisture since it is both managed by farmers and critical to soil-dwelling invertebrates.
Soil moisture is a dynamic quantity dependent on a large range of factors including weather patterns, soil type, geology and topography. Nevertheless, it can be measured qualitatively and quantitatively and is managed to some extent by farmers. The importance of soil moisture to the health, growth and cultivation of crops means that farmers are acutely aware of its variation on their land and so is sometimes managed by drainage to prevent waterlogging. The adoption of conservation tillage to prevent water loss is widespread in more arid areas, but the addition of manures and other organic or inorganic matter to enhance water retention is less common.
Soil moisture is also one of the most important factors affecting habitat selection among carabids, different species prefer habitats lying within specific but fairly narrow ranges (Thiele, Reference Thiele1977). It influences females in their selection of sites for oviposition and affects the subsequent survival of eggs and soil-dwelling larval stages (Huk & Kühne, Reference Huk and Kühne1999). It is therefore probably a key factor in the population dynamics of many species. Soil moisture has been shown to be key to larval survival in some species with extremes of dryness and wetness most detrimental (Van Dijk & Den Boer, Reference Van Dijk and Den Boer1992). High winter rainfall is strongly correlated with synchronous high mortality among many sub-populations of an autumn breeding carabid (Van Dijk & Den Boer, Reference Van Dijk and Den Boer1992).
Thiele (Reference Thiele1977) reports studies on the relationship between beetles and soil moisture that include some farmland species, and importance of soil moisture to carabid assemblages in cereals was highlighted by Luff (Reference Luff1996). Soil characteristics that directly or indirectly influence moisture retention and levels were also highly ranked by Holopainen et al. (Reference Holopainen, Bergman, Hautala and Oksanen1995). However, most studies investigating environmental preferences have been conducted on carabid species of heathland and moorland (Van Dijk & Den Boer, Reference Van Dijk and Den Boer1992; McCracken, Reference McCraken1994; Sanderson et al., Reference Sanderson, Rushton, Cherrill and Byrne1995), permanently or seasonally flooded wetlands (Huk & Kühne, Reference Huk and Kühne1999; Ni Bhriain et al., Reference Ni Bhriain, Skeffington and Gormally2002), grasslands (Rushton et al., Reference Rushton, Luff and Eyre1991) and forest (Antvogel & Bonn, Reference Antvogel and Bonn2001). The relationship between the field-scale distributions of beetles and soil moisture has rarely been investigated and not within arable fields, partly due to difficulties in measuring soil moisture and because of the logistics of adequately sampling beetles across large areas. Where this was achieved in hay meadow, the distribution of one species, Pterostichus versicolor Sturm, was concentrated in the wettest corner of a field where reproduction was considered to occur, followed by a period of redistribution across the field (Hengeveld, Reference Hengeveld1987). Furthermore, beetle distribution is most frequently measured using pitfall traps that are activity dependent but the extent to which these indicate oviposition site preferences is unknown.
We hypothesize, therefore, that the spatial distributions of female beetles seeking oviposition sites are likely to be related to the distribution of soil moisture since this is important, to some extent, for egg survival and, to a greater extent, for larval survival (Van Dijk & Den Boer, Reference Van Dijk and Den Boer1992). We also hypothesize that adults would lay eggs in these preferred sites where larval survival is maximized. Since it is not currently feasible to measure egg density and larval development in the soil on such a large spatial scale, we assume that since the mobility of beetle larvae is limited, that the emergence of new generation adults from the soil will be correlated with the distribution of egg laying adults in the previous season, and that both will be correlated with soil moisture.
This paper describes the distribution and density of nine carabid species and one staphylinid measured using emergence trapping across two arable fields in southern England, UK. Their distribution and abundance in relation to soil moisture during the previous winter and adult distribution the previous summer was examined. The results are discussed with respect to implications for the management of these populations at the farm scale.
Materials and methods
Study site and beetle sampling
Two adjacent arable fields were used near Cranborne, Dorset, UK during 2001–2002. A grid of sampling locations with 40×40 m spacing was established in each field (fig. 1). The grid was established across the whole of a 12 ha field of winter barley (field A) with 86 sampling locations. In the larger 32 ha field of winter wheat (field B) where there was a 24 m wide cover strip sown with a plant mix designed to encourage wild birds, a grid with 114 sampling locations was established across 18 ha. Each sample location was surveyed and located using a differential Global Positioning System (Geoexplorer 3, Trimble, California, USA). To measure the distribution of invertebrates during the peak breeding period a pair of pitfall traps (6 cm diameter, positioned 2 m apart) were set up at each sampling location and opened for two periods (4–11 June 2001; 9–16 July 2001). The population density of invertebrates emerging from the soil in the following year was measured using emergence boxes, similar to those described by Purvis & Fadl (Reference Purvis and Fadl1996). These were placed at each sampling location in early April 2002 before emergence began. Each consisted of a 1 m2×0.2 m high wooden box covered with an insectproof mesh. The sides of each box were buried 5 cm deep into the soil. Within each box, a 10 cm high guidance plate was placed diagonally, at the end of which was placed a pitfall trap (6 cm diameter, partly filled with 50% ethylene glycol and detergent). The pitfall traps within each box were opened on 3 May and emptied on 21 May, 30 May, 10 June, 17 June, 4 July and 11 July 2002. After collection all arthropods were removed and stored in 70% alcohol. The majority of the catch comprised carabid beetles and rove beetles which were identified to species.

Fig. 1. Location of sampling points within fields A and B (––, field boundaries; 
Soil moisture
A variety of techniques exist for measuring soil moisture. Sampling for wet-weight–dry-weight comparisons are straightforward but require extensive resources if many samples need collecting and analysing at one time. Soil probes are also available. However, wide fluctuations in saturation to field capacity and rates of drainage mean its variation over a wide area is best measured rapidly as a relative quantity in situ. This was achieved using the Magnascan system (ASE Solutech Ltd., Biggleswade, Bedfordshire, UK) by which the fields were scanned for electrical conductivity (deciSiemens m−1) to identify relative differences in soil moisture on 11 December 2001. Soil samples were also taken across the study area to facilitate calibration of the Magnascan. A mean electrical conductivity value was calculated for each sampling location using GIS. Delaunay triangulation was used to create natural neighbourhoods (Boots et al., Reference Boots, Okabe, Sugihara and Nok Chiu2000) around each trap location and a mean value calculated for each emergence trap using the electrical conductivity values that fell inside each natural neighbourhood (Vertical Mapper, version 3.1, Mapinfo Corporation, 2004).
Data analysis
To determine whether the distributions of insects or the environmental parameters were spatially aggregated into patches of higher than average numbers, or gaps of lower than average numbers, their distributions were analysed using spatial analysis by distance indices (SADIE) (Perry et al., Reference Perry, Winder, Holland and Alston1999), termed ‘red/blue’ analysis. This calculates the degree of clustering in the form of: (i) ‘patches’ of large counts, using the overall index 

To test whether two sets of count data were spatially correlated, the correlation coefficient, Χ, between the clustering indices of each set was calculated according to the method described by Perry & Dixon (Reference Perry and Dixon2002). Hence, if the indices of set one are denoted z i1, with mean q 1 and those of set two z i2, with mean q 2, then a measure of local spatial association for position i is given by:
The overall spatial association is the mean of these local values, Χ=Σiχi/n. The significance of Χ was tested against values Χrand from a randomization test that included a Dutilleul adjustment procedure (Dutilleul, Reference Dutilleul1993) to provide a probability value PD.
We used SADIE association to test the following three hypotheses: (i) that any patchiness in the distributions of adult beetles, revealed by SADIE analysis of aggregation, remains stable throughout a season; (ii) that the spatial distributions of beetles and soil moisture are related; and (iii) that new generation adults emerge from sites preferred by adults in the previous year. These hypotheses are tested by analysing spatial association between: (i) distributions of adult beetles in consecutive pitfall trap samples; (ii) the population density of emerging adults in 2002 and soil moisture levels measured in the autumn of 2001; and (iii) the population density of emerging beetles in 2002 with (egg-laying) adult densities in 2001.
To identify whether there was a relationship between soil moisture and the emergence of each species, the data from each field were analysed separately using the method of residual maximum likelihood (REML) and a spatial model with an irregular grid with soil moisture as a fixed effect (Genstat version 8.2, Lawes Agricultural Trust, Rothamsted Experimental Station).
Results
Emergence trap captures showed the mean density of invertebrates over-wintering in the soil to be 157 and 86 m−2 for fields A and B respectively (table 1). A crude statistic of the emerging adult populations of carabid and staphylinid species can be estimated from the product of mean density per square metre (table 1) and total area of the fields. This gives a figure of over 18 million beetles in the smaller field A and over 27 million in the larger field B. The species composition was dominated by the larger Carabidae (e.g. Pterostichus spp. and Poecilus cupreus (Linnaeus)), Amara species and Staphylinidae (Philonthus cognatus Stephens) (table 1). The time of peak emergence varied between species with Nebria brevicollis (Fabricius) and P. cognatus occurring early in the year, Harpalus affinis (Schrank), Notiophilus biguttatus (Fabricius), P. cupreus and Pterostichus melanarius (Illiger) mid-season and Calathus fuscipes Goeze, Harpalus rufipes De Geer, Loricera pilicornis (Fabricius) and Pterostichus madidus (Fabricius) latest (fig. 2).
Table 1. Larval periodFootnote * and total number of invertebrates captured within emergence boxes within the small and large arable fields in Dorset, UK during 2001–2002.

* According to Den Boer & Den Boer-Daanje, Reference Den Boer, Den Boer-Daanje and Stork1990; Fadl & Purvis, Reference Fadl and Purvis1998; Holland, Reference Holland and Holland2002.

Fig. 2. Period of emergence pooling data from the two fields for (a) the four most abundant species (- - -◆- - -, Nebria brevicollis; –◆–, Philonthus cognatus; – –▲– –, Poecilus cupreus; –·×·–, Pterostichus madidus; 
All species exhibited heterogeneous emergence patterns with patches and gaps with higher or lower than average density occurring across the fields on most sampling occasions (table 2). Where there was less evidence of spatial pattern this was frequently a consequence of low numbers, as found with C. fuscipes, H. affinis, H. rufipes and N. biguttatus on some sampling occasions. Nebria brevicollis and L. pilicornis showed little evidence of spatial pattern and emerged throughout field A, however, clustering was found on some sample dates in field B (table 2). When total number captured was considered there was no evidence of spatial pattern in field A for H. affinis, H. rufipes, N. brevicollis and N. biguttatus and in field B for C. fuscipes, H. affinis and N. biguttatus; all other species emerged in patches.
Table 2. Degree of clustering into ‘patches’ using overall index 


(***=Pi or Pj<0.001, **=Pi or Pj P<0.01, **=Pi or Pj <0.05; *=5%, PD<0.025 or >0.975; **=1%, PD<0.005 or >0.995, ***=0.1%, PD<0.0005 or >0.9995).
When the pattern of emergence between successive sampling dates was compared, all species showed spatial association between some dates with the exception of H. affinis (table 2). Associations were almost exclusively positive indicating that emergence was occurring from the same locations within the fields. The strength and number of associations differed between species and fields, although this would be expected given the variation in the spatial pattern that occurred. Loricera pilicornis, N. bigutattus, N. brevicollis, Philonthus cognatus, Poecilus cupreus and the two Pterostichus species all showed significant associations between most sampling occasions in one or both fields. Significant associations were also detected for L. pilicornis and N. brevicollis for which spatial pattern was only evident, if at all, on the early and late sampling occasions. It was, however, their low abundance that prohibited the detection of spatial pattern during the mid-season.
The range of soil moisture levels differed between the two fields: field A had a narrower range and was generally drier than field B (fig. 3). The SADIE analysis identified a patch along the south-western edge of field A where it was wettest (−775 to −825 deciSiemens m−1) as contributing to a patch where emergence was highest for eight species showing a positive association with soil moisture (table 3). By contrast, in field B the wettest area (−800 to −950 deciSiemens m−1) was also identified as a patch by SADIE but in this case there was dissociation with emergence densities of eight species (table 3). Although these results initially appeared contradictory, it was noted that the difference in overall wetness of the two fields meant that the wettest area of field A and the driest area of field B had similar moisture content and the beetles selected the appropriate areas within each, as shown for P. cupreus in fig. 3. The REML analysis showed a significant relationship between soil moisture and emergence density (log x+1) in both fields for C. fuscipes, N. brevicollis and P. cognatus (fig. 4a, d, h). Significant relationships were also found in one of the fields for H. affinis, H. rufipes, P. madidus and P. melanarius (fig. 4b, c, f, g). Other species showed non-linear relationships with soil moisture and were found within a restricted soil moisture range (e.g. P. cupreus, fig. 4e).

Fig. 3. Soil moisture levels within (a) field A and (b) field B and the location of patches of Poecilus cupreus (+ and − indicate where the clustering value exceeds the 90th centile for patches and gaps respectively, from randomization distributions).

Fig. 4. Relationship between soil moisture and density of nine beetle species (log x+1 m−2) emerging during spring to summer. a, Calathus fuscipes (+, Field A, Wald statistic/d.f.=1.36 chi p<0.05; ■, Field B, Wald statistic/d.f.=2.57 chi p<0.001); b, Harpalus affinis (+, Field A, Wald statistic/d.f.=1.1 NS; ■, Field B, Wald statistic/d.f.=1.45 chi p<0.01); c, H. rufipes (+, Field A, Wald statistic/d.f.=1.47 chi p<0.05; ■, Field B, Wald statistic/d.f.=1.25 NS); d, Nebria brevicollis (+, Field A, Wald statistic/d.f.=1.51 chi p<0.01; ■, Field B, Wald statistic/d.f.=3.3 chi p<0.001); e, Poecilus cupreus (+, Field A, Wald statistic/d.f.=1.16 NS; ■, Field B, Wald statistic/d.f.=1.0 NS); f, Pterostichus madidus (+, Field A, Wald statistic/d.f.=1.45 chi p<0.01; ■, Field B, Wald statistic/d.f.=1.2 NS); g, P. melanarius (+, Field A, Wald statistic/d.f.=1.0 NS; ■, Field B, Wald statistic/d.f.=1.48 chi p<0.01); h, Philonthus cognatus (+, Field A, Wald statistic/d.f.=2.1 chi p<0.001; ■, Field B, Wald statistic/d.f.=1.48 chi p<0.01).
Table 3. Spatial association χi with probability level (Dutilleul adjusted) PD between beetles and soil moisture.
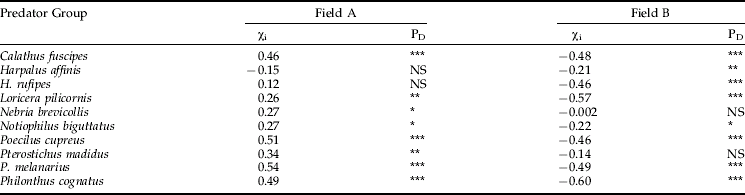
(Two-sided significance thus *=5%, PD<0.025 or >0.975; **=1%, PD<0.005 or >0.995, ***=0.1%, PD<0.0005 or >0.9995).
All species, with the exception of C. fuscipes, and N. biguttatus, showed positive spatial associations between the distributions of their emergence densities in successive years (table 4). These associations were strongest for beetles collected in July of the previous year for all species with the exception of P. cognatus for which there were stronger associations in June.
Table 4. Spatial association χi with probability level (Dutilleul adjusted) PD between arthropods captured in pitfall traps and emergence in the following year. Two-sided significance thus *=5%, PD<0.025 or >0.975; **=1%, PD<0.005 or >0.995; ***=0.1%, PD<0.0005 or >0.9995; NA, insufficient captured in pitfall traps.

† According to Luff, Reference Luff1973; Wallin, Reference Wallin1985; Den Boer & Den Boer-Daanje, Reference Den Boer, Den Boer-Daanje and Stork1990; Fadl & Purvis, Reference Fadl and Purvis1998.
Discussion
Emergence traps provide good estimates of population densities. They are less prone to error than pitfall traps because capture is less dependent on activity (Thiele, Reference Thiele1977; Ulber & Wolf-Schwerin, Reference Ulber and Wolf-Schwerin1995) and small species are more readily captured (Purvis & Fadl, Reference Purvis and Fadl1996; Holland & Smith, Reference Holland and Smith1999) giving better estimates of relative abundance (Desender & Maelfait, Reference Desender and Maelfait1986). Emergence traps are sometimes moved at regular intervals (Helenius, Reference Helenius1995; Ulber & Wolf-Schwerin, Reference Ulber and Wolf-Schwerin1995) to provide a short-term estimate of abundance. However, in the present study, traps were left in the same location throughout the season to obtain an estimate of the total overwintering densities or ‘productivity’ (Purvis & Fadl, Reference Purvis and Fadl1996).
Much previous work has emphasized the importance of field margins and other non-crop habitat as refugia for over-wintering invertebrates (reviewed by Lee & Landis, Reference Lee, Landis and Holland2002). The present study clearly shows that the arable soils within the cropped area are also an important over-wintering habitat for beneficial invertebrates given the tens of millions of beetles that can be found within each field. This represents enormous potential for pest predation and, because Carabidae are one of the most highly ranked food taxa for farmland birds (Wilson et al., Reference Wilson, Morris, Arroyo, Clark and Bradbury1999; Holland et al., Reference Holland, Hutchison, Smith and Aebischer2005b), an important resource for taxa higher up the food chain. Management of soils for biocontrol should therefore be considered when developing integrated pest control programmes and managing conservation of farmland birds.
Typical of arable land (Luff, Reference Luff and Holland2002) the two Pterostichus species were, overall, the most abundant carabids in the present study. Estimates of population density of total beetles and individual species varied considerably between fields, for example, the density of P. melanarius was 29 m−2 in field A, but only 1.2 m−2 in field B. Other season-long trapping programmes have found P. melanarius densities in the same range: 13.8 m−2 in winter wheat and 2.5 m−2 for spring wheat (Purvis & Fadl, Reference Purvis and Fadl1996); 1–3.9 m−2 (Holland & Reynolds, Reference Holland and Reynolds2003). In a study comparing different methods of soil cultivation, the densities of emerging adult Carabidae and Staphylindae were 21.9–84.9 m−2 and 0.6–10.7 m−2 respectively (Holland & Reynolds, Reference Holland and Reynolds2003). For some species (e.g. N. brevicollis) population density may have been underestimated because the rate of emergence was increasing when trapping had to stop to enable harvest.
In another study using these two same and four other adjacent fields during 2000, the densities of P. madidus and P. melanarius estimated using mark–release–recapture (MRR) were 2.3–5.8 m−2 and 0.2–0.3 m−2 respectively (Holland et al., Reference Holland, Begbie, Birkett, Southway, Thomas, Alexander and Thomas2004). Other density estimates of P. melanarius have also been reported in the range 0.05–5 m−2 (Ericson, Reference Ericson1978; Hance et al., Reference Hance, Gregoire-Wibo and Lebrun1990; Thomas et al., Reference Thomas, Parkinson and Marshall1998). The relative accuracy of emergence traps and MRR to estimate population density has never been assessed. A large number of traps are required to accurately estimate the density of patchy populations. However, the technique is recommended since the effort involved with emergence traps is no more than that in MRR studies, and they provide population density data of more species, including those with individuals or populations too small to estimate by MRR.
The emergence periods of each species corroborates previous work (Den Boer & Den Boer-Daanje, Reference Den Boer, Den Boer-Daanje and Stork1990; Fadl & Purvis, Reference Fadl and Purvis1998; Holland & Reynolds, Reference Holland and Reynolds2003) with the exception of P. cupreus where, in the present study, the peak was a month later than that found by Holland & Reynolds (Reference Holland and Reynolds2003) and P. melanarius, which was a month earlier than that found by Fadl & Purvis (Reference Fadl and Purvis1998). Philonthus cognatus emergence was highest in June as found in sugarbeet, although a second generation may emerge in September (Purvis & Curry, Reference Purvis and Curry1984). Emergence of most species peaked in June when pests such as cereal aphids and orange wheat blossom midge, on which they feed, infest cereal crops (Edwards et al., Reference Edwards, Sunderland and George1979; Holland & Thomas, Reference Holland and Thomas2000; Winder et al., Reference Winder, Alexander, Holland, Woolley and Perry2001).
In common with previous studies, all species exhibited some degree of patchiness in their distributions but was often not found for occasions when abundances were low. In addition, strong spatial associations between successive distributions were found for most of the species and showed the extent to which emergence was concentrated within particular areas of the fields. Where no associations were detected this was almost exclusively a consequence of low abundances and an associated absence of spatial pattern. For six species the location of stable patches was related to soil moisture levels and for three of these there were significant linear relationships between their emergence densities and soil moisture levels. In the fields where no soil moisture relationship was detected the density of beetles was often low. Likewise, the distribution of three carabid species differed in the period shortly after emergence, with two occurring in patches that provided specific soil moisture levels (Hengeveld, Reference Hengeveld, Cormack and Ord1979). For Carabidae the importance of soil conditions in comparison to vegetation and spatial separation was highlighted in a study conducted on moorland in the UK (Sanderson et al., Reference Sanderson, Rushton, Cherrill and Byrne1995). More specifically, the distribution of nine Pterostichus species inhabiting grassland was related to soil moisture, soil bulk density and altitude although the type and extent of the relationship differed between species (Rushton et al., Reference Rushton, Luff and Eyre1991). The two Pterostichus species studied here exhibited gaussian response curves to all three environmental variables and the optimum soil moisture level was the same, unlike in this study. Sanderson et al. (Reference Sanderson, Rushton, Cherrill and Byrne1995) and Rushton et al. (Reference Rushton, Luff and Eyre1991) used ordination and generalized linear modelling, respectively, to test for relationships with environmental variables. Such approaches may be more appropriate because a wider range of environmental conditions can be sampled as there are no spatial limitations imposed by the grid size that can be logistically sampled. Other abiotic and biotic factors, that may be interacting may have also been influencing beetle distributions and thereby oviposition. Those proven to have an effect on distributions within fields include weed cover (Purvis & Curry, Reference Purvis and Curry1984; Powell et al., Reference Powell, Dean and Dewar1985; Pavuk et al., Reference Pavuk, Purrington, Williams and Stinner1997), crop cover (Honek, Reference Honek1988) and prey abundance (Winder et al., Reference Winder, Alexander, Holland, Woolley and Perry2001, Reference Winder, Griffiths, Perry, Alexander, Holland, Kennedy and Birt2005), although the impact of these may all be mitigated by soil moisture. In reality, a combination of positive and negative mechanisms will drive the spatial dynamics (Thomas et al. Reference Thomas, Holland, Brown and Holland2002) although the apparent drivers may change according to the spatial resolution of the study.
The clear positive regression of increasing emergence density with decreasing soil moisture found for some species was surprising considering the theoretical and observed importance of adequate moisture for oviposition and subsequent survival of eggs and larvae (Hengeveld, Reference Hengeveld, Cormack and Ord1979; Holopainen et al., Reference Holopainen, Bergman, Hautala and Oksanen1995; Luff, Reference Luff1996; Huk & Kühne, Reference Huk and Kühne1999). Magnascan readings of electrical conductivity only indirectly measure relative levels of soil moisture at the time they are taken. Since soil moisture is a dynamic quantity dependent on, among other factors, quantity of and time since last rainfall, and soil drainage properties, levels are likely to vary only by expansion and contraction around spatially fixed foci. These indirect relative measurements can therefore still provide indicative data provided they are collected within a very narrow time frame.
Although significant linear relations fitted some of these data, the range of ambient soil moisture in the fields was limited and uncontrolled: there were few extremely dry areas. The independent variable in the data should therefore be viewed as a subset of a wider range over which emergence densities are probably distributed as found by Sanderson et al. (Reference Sanderson, Rushton, Cherrill and Byrne1995). Optimum preferred soil moisture levels for different species would be expected since species with winter and summer larvae should respectively avoid areas prone to waterlogging and parching (Huk & Kühne, Reference Huk and Kühne1999). Such an optimum would be represented by the modal value of the distribution and is hinted at in the data presented here, exemplified by the emergence of P. melanarius clustering within a narrow band at soil moisture levels yielding readings of between −725 and −800 deciSiemens m−1. In this study, however, there appeared to be no differentiation between species according to breeding periods as most species emerged predominantly within a similar moisture range. Further experimental work under controlled conditions may confirm the observations presented here and define species-specific soil moisture range preferences more precisely.
Two principal mechanisms may explain the emergence patterns detected here: (i) no selection for specific oviposition sites but spatial variation in survival and mortality over previous seasons; or (ii) preferences shown by ovipositing females in the previous year for particular areas where optimum moisture maximizes survival to adulthood, as was shown to occur, for example, with Carabus clatratus Linnaeus (Huk & Kühne, Reference Huk and Kühne1999). In the present study, for all but two species, there were positive correlations between 2001 distributions and where they emerged in 2002 suggesting the second mechanism, although we were unable to prove that the distribution of ovipositing females was being measured in the previous year. However, many field-inhabiting carabids were found to have stable distributions within years (Thomas et al. Reference Thomas, Parkinson and Marshall1998, Reference Thomas, Parkinson, Griffiths and Fernández-García2001; Holland et al. Reference Holland, Perry and Winder1999, Reference Holland, Thomas, Birkett, Southway and Oaten2005a; Fernández García et al. Reference Fernández-García, Griffiths and Thomas2000; Winder et al., Reference Winder, Griffiths, Perry, Alexander, Holland, Kennedy and Birt2005) indicating that females do not move beyond these patches in search of oviposition sites. Moreover, larvae are considered to be relatively immobile, having to survive where oviposition occurred (Lövei & Sunderland, Reference Lövei and Sunderland1996). Instability between years, as found with some species (Holland et al., Reference Holland, Thomas, Birkett, Southway and Oaten2005a) would suggest that survival plays an important part in some years, although both mechanisms are likely to operate to some degree. Survival of eggs and larvae may also be affected by levels of disease and parasitism, temperature, starvation and by certain farming operations (Luff, Reference Luff1987; Holland & Luff, Reference Holland and Luff2000) and the impact of these may differ between- and within-fields thereby creating heterogeneous distribution patterns. Whatever the mechanism, results from this study indicate that benefits may accrue from managing soil moisture by a combination of drainage and adding organic matter to provide an adequate range in all fields where populations of predatory arthropods are required.
Acknowledgements
The study was conducted as part of the 3D Farming Project which was funded under the Sustainable Arable LINK Programme by the Department of the Environment, Food and Rural Affairs and Scottish Executive Environmental Rural Affairs Department with additional financial support from Dow AgroSciences, Home-Grown Cereals Authority, Horticultural Development Council, Processors and Growers Research Organisation, Tesco, Unilever, The Game Conservancy Trust, The Chadacre Agricultural Trust, The Dulverton Trust, The Manydown Company, The Worshipful Company of Farmers and The Yorkshire Agricultural Society. Sincere thanks to all those that helped with the study. The authors gratefully thank Lord Cranborne for permission to use Cranborne farm and the staff of Cranborne Estates.


