Introduction
The limiting effect of seed predation on population growth of herbaceous plants was recognized decades ago (Firbank & Watkinson, Reference Firbank and Watkinson1986), but its potential contribution to weed control in agriculture has not been appreciated until recently. Now that there is increased awareness that seed predation may be a crucially important component in weed management systems (e.g. Westerman et al., Reference Westerman, Liebman, Menalled, Heggenstaller, Hartzler and Dixon2005), there is a growing need to understand which factors shape it. Modelling studies have indicated that 25–50% seed mortality is generally sufficient to stop or slow down the population growth of annual weeds (Westerman et al., Reference Westerman, Liebman, Menalled, Heggenstaller, Hartzler and Dixon2005). Field studies have demonstrated predation levels exceeding this intensity (Hulme, Reference Hulme1994; Harrison et al., Reference Harrison, Regnier and Schmoll2003; Westerman et al., Reference Westerman, Wes, Kropff and van der Werf2003b; Honek et al., Reference Honek, Martinkova and Saska2005), but seed predation levels are highly variable.
One of the main factors responsible for variability in predation rates between studies is the type of predator. Each climatic region has typical groups of seed predators (Hulme & Kollmann, Reference Hulme, Kollmann, Forget, Lambert, Hulme and Vander Wall2005). In temperate agro-ecosystems, rodents and carabid beetles are the most important (Cromar et al., Reference Cromar, Murphy and Swanton1999; Honek et al., Reference Honek, Martinkova and Jarosik2003, Reference Honek, Martinkova and Saska2005; Westerman et al., Reference Westerman, Hofman, Vet and van der Werf2003a; Marino et al., Reference Marino, Westerman, Pinkert and Van der Werf2005), although locally other groups, such as slugs, crickets and birds may be involved (Brust, Reference Brust1994; Kollmann & Bassin, Reference Kollmann and Bassin2001; Holmes & Froud-Williams, Reference Holmes and Froud-Williams2005; Holland et al., Reference Holland, Hutchison, Smith and Aebischer2006). Rodents prefer larger seeds while carabid beetles prefer smaller seeds (Mittelbach & Gross, Reference Mittelbach and Gross1984; Brust, Reference Brust1994).
Carabid beetles are present and active when weed seeds are being shed (Honek et al., Reference Honek, Martinkova and Saska2005) and can predate on seeds before these get buried in the soil. Moreover, carabids consume seeds that come to the soil surface after burial (Martinkova et al., Reference Martinkova, Saska and Honek2006). Because seed is an important part of the food of many species of field carabid and their larvae, including those that are predominantly carnivorous (Skuhravý, Reference Skuhravý1959; Hengeveld, Reference Hengeveld1980; Jørgensen & Toft, Reference Jørgensen and Toft1997; Saska & Jarošík, Reference Saska and Jarošík2001), carabids may reduce seed density on the soil substantially.
It is widely assumed that higher predation rates may be expected when predators are more abundant (Cromar et al., Reference Cromar, Murphy and Swanton1999; Kromp, Reference Kromp1999; Tooley & Brust, Reference Tooley, Brust and Holland2002). However, data that study a relationship between the changes in density of granivorous carabids (or other seed predators) across agricultural landscapes and over the season and intensity of seed predation are limited.
The density of carabid beetles within fields is often higher near the field edge than in the interior (Kromp & Steinberger, Reference Kromp and Steinberger1992; Holland et al., Reference Holland, Perry and Winder1999; Kromp, Reference Kromp1999; Holland, Reference Holland2002). In northern temperate agro-ecosystems, a few studies have compared invertebrate seed predation between sites at different distances from the field edge and observed no significant difference between locations (Marino et al., Reference Marino, Gross and Landis1997; Tooley et al., Reference Tooley, Froud-Williams, Boatman and Holland1999b; Westerman et al., Reference Westerman, Hofman, Vet and van der Werf2003a). In the available studies, spatial variability in predator abundance was not measured. Whether there is a link between spatial patterns of activity-density of carabids and levels of seed predation within fields is, therefore, unknown. Available information suggests that there may be an incongruity; carabid density varies spatially over the field but seed predation does not. There is a need for studying spatial variability in seed predation and predator abundance in the same experiment.
There is some evidence for correspondence in the temporal trends of predator density and seed predation within a single field (Honek et al., Reference Honek, Martinkova and Jarosik2003; Mauchline et al., Reference Mauchline, Watson, Brown and Froud-Williams2005; O'Rourke et al., Reference O'Rourke, Heggenstaller, Liebman and Rice2006). However, a high density of predators does not guarantee a high seed predation rate. For example, Honek et al. (Reference Honek, Martinkova and Jarosik2003) found, in only one out of nine fields, a positive relationship between activity-density of carabids and seed predation. Here again, there is a need for studies that quantify seed predation and predator density in the same trial.
Studies that included several species of seeds usually obtained different levels of predation for each of the seeds (Tooley et al., Reference Tooley, Froud-Williams, Boatman and Holland1999b; Westerman et al., Reference Westerman, Hofman, Vet and van der Werf2003a,Reference Westerman, Wes, Kropff and van der Werfb). This suggests that particular seed species differ in attractiveness for carabids. Laboratory studies provide clear evidence that carabids select for specific seeds (Tooley et al., Reference Tooley, Froud-Williams, Boatman and Holland1999a; Honek et al., Reference Honek, Martinkova and Jarosik2003, Reference Honek, Martinkova, Saska and Pekar2007) and that these preferences are innate and do not change during the year (Honek et al., Reference Honek, Saska and Martinkova2006). Selection for seeds is driven both by taxonomic and morphological constraints (Honek et al., Reference Honek, Martinkova, Saska and Pekar2007). How the preferences, established under artificial laboratory conditions, reflect the situation in the field has so far been addressed in one study (Honek et al., Reference Honek, Martinkova and Jarosik2003). Although Honek et al. (Reference Honek, Martinkova and Jarosik2003) found a correlation between field seed losses and laboratory preferences for seeds by granivorous carabids, more data is necessary.
In this study, we first ask whether there is spatial variation in seed predation with distance from the field edge and whether this variation can be explained by spatial variation in abundance of seed-eating carabids. Second, we ask whether temporal variation in seed predation in the field can be explained by the temporal variation in carabid activity-density. As a null model, we expect that the highest impact of seed predators on weed seed survival occurs when their activity-density, as measured in pitfall traps, is highest. The most abundant predator species occurring in the field sites were brought to the laboratory to determine their consumption of the weed seeds that were used in the field studies.
Material and methods
Experimental fields
Experiments were located at the research farm ‘Droevendaal’ of Wageningen University and the Research Centre in Wageningen, the Netherlands (51°58′N, 05°40′E, 20 m of altitude). This farm is located on sandy soil and has been managed organically since 2003. Measurements were made in two fields of winter wheat (Triticum aestivum L. var. Cordos). Both wheat crops were sown in late October 2003 and harvested on 4 August 2004. The fields are about 500 m apart and are designated here field 1 and field 2 (equals parcels 10 and 8 according to the farm plan). Field 1 measures 100×150 m and was surrounded by 2–3 m wide boundary strips of rye-grass (Lolium perenne L.) on all sides. Field 2 measures 100×170 m and was surrounded by rye-grass strips on three sides and by a strip with weedy vegetation on the south side. In both fields, an observation plot of 50×50 m was selected, adjacent to the grassy boundary strip. The wheat crop extended for at least another 50 m past the edges of the remaining three sides. Outside the study plots, composted manure was applied on 16 April, and mechanical weed control was carried out on 19 April 2004 using a harrow. Further details with respect to the farm, soils, crop fields and management can be found at http://www.droevendaal.wur.nl/uk/.
Background weed population
Weed density was virtually zero at the beginning of experiment in April, but increased during the growing season. In field 1, the dominant weeds were Stellaria media (L.) Vill., Poa annua L., Taraxacum officinale Wigg., Capsella bursa-pastoris (L.) Medik. and Viola arvensis Murray. Most of the weed plants were located within 1 m of the field edge, where the crop had developed poorly. In field 2, weed densities were higher than in field 1. Dominant weeds were Apera spica-venti (L.) P.B., Tripleurospermum inodorum (L.) Schultz-Bip., Persicaria maculosa S.F. Gray, V. arvensis, T. officinale, S. media and Cirsium arvense (L.) Scop. The latter three weeds only occurred close to the field edge.
Sample sites
In each field, sample sites where seed predation and activity-density of carabids was measured were arranged on seven transects in the observation plot. Transects were 3 or 6 m apart and perpendicular to the field edge. Per transect, there were six sample sites: field boundary (1 m from the edge), field edge, and within the crop at 4, 11, 24 or 49 m from the edge. Five control cages were placed at random locations within each of the two observation plots (see below). Measurements lasted from 23 March to 27 July 2004, so the whole period of activity-density of granivorous ground beetles was covered from spring emergence of the carabids until harvest of the wheat. The dates given in the results and figures represent collection dates.
Quantification of seed predation
Seed predation was quantified using ‘seed cards’ (Westerman et al., Reference Westerman, Hofman, Vet and van der Werf2003a) that consisted of firm, high quality sand paper (3×10 cm, KWB ‘waved’ grain size 60 or 80) and contained 50 seeds. The weed species were selected on the basis of: (i) presence in the experimental fields (see above); (ii) early maturation and seed shed; and (iii) differences in level of attractiveness to carabid beetles. We selected three species of seed that are preferred by an array of spring breeding carabids (P. annua, S. media, C. bursa-pastoris) and one that is less preferred (Lamium amplexicaule L.) (Tooley et al., Reference Tooley, Froud-Williams, Boatman and Holland1999a; Honek et al., Reference Honek, Martinkova and Jarosik2003). Seeds were purchased from Herbiseed®, Twyford, Great Britain. Individual weight per seed, determined by weighing five batches of 100 seeds per species, was 0.09 mg for C. bursa-pastoris, 0.37 mg for S. media, 0.61 mg for L. amplexicaule and 0.43 mg for P. annua. Seed cards of the four species were nailed to the ground and covered in a wire cage (width×length×height: 25×25×12.5 cm; mesh size: 1 cm) to give access only to small invertebrates. The cages were secured to the ground with two steel tent pegs. Background losses of seed due to wind, rain and loss of adhesive power of the glue were assessed using sets of four seed cards glued to the bottom of a fine meshed sieve (20 cm diameter, 3 cm high rims), which was completely wrapped in a 1×1 mm mesh synthetic fabric to exclude both invertebrate and vertebrate predators. After exposure to seed predators for seven days, cards were collected, put into envelopes and transferred to the laboratory. The number of seeds remaining on each treatment card, N cage, and on the control cards, N control, was counted. If seeds fell off the cards during transport, they were retrieved from the envelope and counted. The percentage seed predation was calculated as the Abbott corrected seed loss per seven days (Abbott, Reference Abbott1925):
In the rare situation that N cage exceeded N control, seed predation was set to 0%.
Insect sampling
On either side of each cage (ca. 20 cm apart), two 0.5 litre pitfall traps (covered with metal roof and half-filled with a saturated saline solution as fixative) were placed with the rims at ground level. Pitfall traps were emptied weekly. Carabid adults were identified to species (Hurka, Reference Hurka1996; Boeken et al., Reference Boeken, Desender, Drost, van Gijzen, Koese, Turin and Vermeulen2002) and larvae to genus (Luff, Reference Luff1993). Carabid species were classified as granivorous if seeds constitute at least a part of their diets (Tooley et al., Reference Tooley, Froud-Williams, Boatman and Holland1999a; Honek et al., Reference Honek, Martinkova and Jarosik2003, Reference Honek, Martinkova, Saska and Pekar2007). Data from each pair of traps were summed before analysis. In this paper, we present only general trends in activity-density and structure of the carabid assemblage; more detailed analysis of that can be found in Saska et al. (Reference Saska, Vodde, Heijerman, Westerman and van der Werf2007).
Laboratory preference experiment
The thirteen most numerous carabid species in the pitfall traps (see table 5) were tested in the laboratory, in order to estimate the contribution of particular carabid species to the observed seed predation in the field, and to compare the order of preference in the field and in the laboratory. Live carabid specimens were collected in the experimental fields described above but outside the experimental area by means of dry pitfall traps. Carabids were acclimated in the refrigerator (5°C) for five days, after which they were used for the experiment. Each carabid individual (5–10 replications per carabid species) was placed in a glass dish (9 cm in diameter, 5 cm high) with a layer of moist sieved soil, which did not contain any seeds, and offered 15 seeds of each seed species (the same as used in the field trial). Seeds were exposed to carabids on small trays, 28 mm in diameter and 6 mm deep, filled with white plasticine (Honek et al., Reference Honek, Martinkova and Jarosik2003). The seeds were pressed into the plasticine to half their transverse width so they could be easily picked up by the beetles. Separate trays were assembled for particular species. After four days of exposure, the remaining seeds were counted.
Statistical analysis
Model analysis focuses on the formulated research objectives: to obtain better understanding of (i) the effect of space on seed predation; (ii) the effect of time on seed predation; (iii) the effect of spatially varying carabid abundance on seed predation; (iv) the effect of temporally varying carabid abundance on seed predation; and (v) the effect of weed species on seed predation. Preliminary analysis indicated that climatic effects might have an effect on seed predation; therefore, the weekly sum of precipitation (mm) and average maximum temperature (°C) were also included in the model as predictors. The meteorology data were obtained from the meteorological station of Wageningen-UR (www.met.wau.nl), located ca. 3 km from the experimental fields.
As an initial step, a simple correlation analysis on time courses of the spatially integrated total predation of the four seeds and total carabid activity-density was conducted for each of the fields, using Spearman's distribution free coefficient of correlation.
Then, a comprehensive linear mixed regression model was built. This model described the percentage predation of weed seeds as a function of the experimental factors: (i) field (field 1 or 2); (ii) weed species (S. media, C. bursa-pastoris, P. annua and L. amplexicaule); (iii) environment (crop or boundary); (iv) distance from edge (0, 4, 11, 24 or 49 m); and (v) sampling time (18 weeks). A logit-link function is used to account for a sigmoid relationship between the response variable and the predictors. The statistical approach takes account of non-normal errors and heteroscedasticity by using a binomial variance function that allows for overdispersion. The model was fitted to the data using Iteratively Reweighted Restricted Maximum Likelihood (IRREML) (Keen & Engel, Reference Keen, Engel, Goedhart and Thissen2005) in Genstat (version 8).
As the first model indicated significant differences between the two fields and between weed species within each field (results not shown), further analyses with IRREML were conducted for the two fields separately (second analysis; table 2) and for each weed species in each field (third analysis; tables 3 and 4). Three further covariables were included: temperature, precipitation and the total catch of granivorous carabids at each sampling site. The covariables temperature and precipitation are embedded within the factor time (18 levels); hence, although they are not explicitly accounted for in the initial analyses for all weed species together, their effects are contained in the factor time and thus accounted for. An iterative procedure was used to remove non-significant interactions and factors and arrive at a minimal statistical model that contained only significant effects. Only this final model will be presented.
Analyses of the effects of carabids on seed predation were run both with carabid activity-density as predictor and with carabid activity-mass as predictor. Activity-mass is the sum of activity-densities per species weighed by the average body mass per species. This variable could, in theory, provide better prediction of predation capacity, because voracity increases with body size (Honek et al., Reference Honek, Martinkova and Jarosik2003, Reference Honek, Martinkova, Saska and Pekar2007). However, carabid activity-densities gave a better fit to the data; therefore, these analyses are reported here.
In a fourth set of detailed analyses with IRREML, an attempt was made to relate seed predation on given weed species to activity-densities of carabid species that showed preference for these species in the laboratory tests. These analyses were restricted to data collected in those weeks in which these predators were abundant, and they contained the factors time and distance plus their interaction in addition to the covariable of carabid activity-density.
In the fifth and final set of analyses with IRREML, seed predation on a specific weed in a given field and week was related to distance from the edge and the activity-density of carabids as covariable. In the fourth and fifth set of analysis, the critical significance levels were Bonferroni-corrected to avoid an increased number of type I errors resulting from the multiple comparisons.
Results
Carabid activity-density
A total of 11,148 individual carabid adults were collected (including those that never eat seeds); 6733 individuals, thereof, were collected in field 1 and 4415 in field 2. They belonged to 75 species in total; 65 species were found in field 1 and 61 species in field 2. The most numerous species were Amara spreta (29.6%), Harpalus affinis (10.9%), Bembidion femoratum (8.3%), Clivina fossor (7.3%), Pseudoophonus rufipes (7.2%), Demetrias atricapillus (5.3%), Poecilus versicolor (3.2%), Bembidion lampros (3.2%), Agonum muelleri (2.9%), Amara aenea (2.3%), Harpalus distinguendus (2.2%) and Amara plebeja (1.5%). The other species each constituted 1% or less of the total catch; and, taken together, these other species represented 16.2% of the total catch. Granivorous species constituted 63% of the catch (70% in field 1 and 53% in field 2). Composition of the assemblage varied over the season (fig. 1). Throughout the experimental period, it was dominated by the spring-breeding carabids; but, starting at the end of June, autumn-breeding species, such as P. rufipes, became more active (fig. 1)
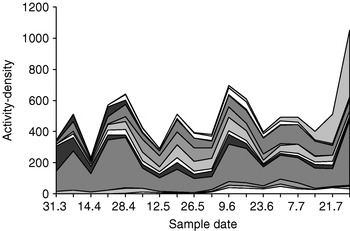
Fig. 1. Temporal variation in activity-density of the 12 most numerous carabids in pitfall traps placed in two fields of organic winter wheat in Wageningen in 2004. The order of appearance (bottom-up) is as follows: Agonum muelleri, Amara aenea, A. plebeja, A. spreta, Bembidion femoratum, B. lampros, Clivina fossor, Demetrias atricapillus, Harpalus affinis, H. distinguendus, Poecilus versicolor, Pseudoophonus rufipes.
Observed activity-density of carabids was high at the edge and lower in the field interior (fig. 2a, b). Activity-density varied irregularly over time and reached a seasonal peak in the last week of July (fig. 3a, b).

Fig. 2. Spatial variability in activity-density of carabids ((a) field 1, (b) field 2) and seed predation ((c) field 1, (d) field 2). Symbols in (a) and (b) indicate (○) total activity-density and (●) activity-density of granivorous beetles. Symbols in (c) and (d) indicate the botanical species of seed: (○) Capsella bursa-pastoris, (●) Lamium amplexicaule, (△) Poa annua and (□) Stellaria media.

Fig. 3. Temporal variation in activity-density of carabids ((a) field 1, (b) field 2) and seed predation ((c, e, g, i) field 1. (d, f, h, j) field 2). Symbols in (a) and (b) indicate (○) total activity-density and (●) activity-density of granivorous beetles. Symbols in (c–j) indicate the botanical species of seed: (○) Capsella bursa-pastoris, (●) Lamium amplexicaule, (△) Poa annua and (□) Stellaria media.
Seed predation
Seed predation was 14.3% in field 1 and 16.8% in field 2. Seed predation differed among the four species of weed (fig. 4). The percentage of seed removal per week (averaged over the two fields) was 23.2% in C. bursa-pastoris, 9.2% in L. amplexicaule, 19.8% in P. annua and 13.6% in S. media. Spatial patterns in seed removal differed among the four species of weed seed. For all species, removal was least in the field boundary. Within the field, seed removal increased with distance from the edge for C. bursa pastoris and P. annua, whereas it did not change with distance to the edge in L. amplexicaule and S. media (fig. 2c, d). Accumulated over time, the patterns of seed predation were similar among the three species eaten most: C. bursa-pastoris, P. annua and S. media (table 1). In these three species, removal varied substantially from week to week; and it increased towards the end of the observation period (fig. 3c, d). In contrast, removal of the seed of L. amplexicaule varied relatively little from week to week; and an increasing trend towards the end of the observation period, as observed in the other species, was not found.

Fig. 4. Average removal of seeds (%) of four species of weed, exposed during one-week periods in one of two winter wheat fields, from April through July 2003 on an organic farm near Wageningen (□, Field 1; ![]() , Field 2).
, Field 2).
Table 1. Correlations between weekly seed removal (averaged over all sample sites) in four species of weed and activity-density of carabid beetles (summed over all sample sites) in two organic cereal winter wheat fields near Wageningen in 2004.
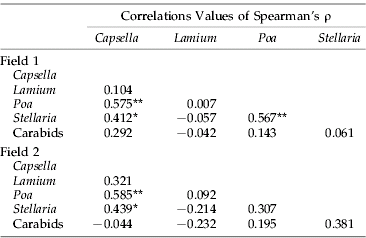
** P<0.05.
* P<0.10.
Relationships between seed predation and activity-density of carabids
At the highest hierarchical level, the field, there was a negative relationship between seed removal and the catch of granivorous carabids; the level of seed removal in field 1 was 14.3% with a total catch of 4732 individual granivorous beetles, while the level of seed removal in field 2 was 16.8% with only half the total catch of granivorous carabids – 2339 individuals.
Simple correlation analyses of temporal trends, using parameter-free Spearman's ρ, suggest temporal incongruence between seed removal and carabid abundance. The levels of predation of the three most heavily predated species of weed (C. bursa-pastoris, P. annua and S. media) were correlated over time (table 1), indicating that temporal changes in intensity of seed predation in the three species result from the same process. On the other hand, no significant correlations were found with carabid abundance. A temporal incongruence between the pattern of seed removal and the pattern of abundance of granivorous carabid beetles demonstrated in table 1 is further substantiated by statistical analyses (see below).
After a first analysis indicated differences in statistical structure of the data in the two fields (differences in the significance of higher order interactions; results not shown), results of two GLMs (separate for each of the fields) indicate that all explanatory variables plus several interactions have significant effects on seed predation (table 2). The data set was then analysed separately for each species of weed in each field and the minimal models were constructed (tables 3 and 4). In these models, the weather variables were included. The most important factors explaining seed predation in field 1 were time, average daily maximum temperature and precipitation (P<0.001 for all three variables in all species of weed) (table 3). These factors accounted for a minimal complete statistical explanation of the results in two species (L. amplexicaule and S. media) and they usually exceeded the level of significance of other significant terms for the models for C. bursa-pastoris and P. annua, with only one exception in the case of C. bursa-pastoris (table 3). The overall interpretation for the results in field 1 is that differences in seed predation were mostly related to the explanatory variables time, temperature and precipitation. The explanatory variables of distance to the edge and carabid activity-density had, by comparison, smaller effects.
Table 2. Linear regression analysis (GLMM), of seed predation of the four species of weed seed (species), exposed on seed cards placed in the field margin or within the field (margin) at five different distances from the field edge (distance) for 18 weeks of exposure (time).

Table 3. Minimum adequate models, of the linear regression analyses (GLMM) of seed predation separate for the four species of weed seed from field 1, exposed on seed cards placed within the field at five different distances from the field edge (distance) for 18 weeks of exposure (time). Average maximum temperature (temperature), sum of precipitation (precipitation) and activity-density of granivorous carabid beetles (carab_counts) are used as variates.

Significant terms having negative slopes are in bold.
Table 4. Minimum adequate models, of the linear regression analyses (GLMM) of seed predation separate for the four species of weed seed from field 2, exposed on seed cards placed within the field at five different distances from the field edge (distance) for 18 weeks of exposure (time). Average maximum temperature (temperature), sum of precipitation (precipitation) and activity-density of granivorous carabid beetles (carab_counts) are used as variates.

Significant terms (P<0.05) having negative slopes are in bold.
Results of the same analyses for field 2 were very similar. Time was highly significant in all four species (P<0.001), temperature was significant at P<0.001 in all species except C. bursa-pastoris and precipitation was significant at P<0.001 in all species except P. annua where it was significant at P<0.005 (table 4). The factor distance was a significant predictor for the removal of the seed of C. bursa-pastoris and for those of P. annua but not for the other two species.
Taken together over the two fields, these analyses demonstrated the overriding importance of time-depending explanatory variables in the seed removal of all weed species. There were significant effects of distance on the predation of seed of C. bursa-pastoris in field 1 and of P. annua in fields 1 and 2. Significant effects of carabid activity density were only found in field 1 and applied to predation on the seed of C. bursa-pastoris and of P. annua. It should be noted that significance does not imply positive relationship. For instance, the effect of temperature on the removal of the seed of L. amplexicaule is negative in both fields (tables 3 and 4).
Similar results were obtained when relationships between the activity-density of single carabid species (varying over space and time) and removal of seeds of single weed species were analysed for both fields (results not shown). None of the 30 analyses performed showed positive significance of carabid abundance on seed predation.
As a final step in the analysis, the spatial variation in seed predation was analysed for each week, seed and field separately. However, none of the tests were significant at Bonferroni-adjusted probability level. Thus, there was no evidence that the level of seed predation was related to the activity-density of carabids.
Three dimensional plots of the data that illustrate the spatio-temporal variation in seed predation for the four weed species and the simultaneous variation in activity-density of carabid beetles are shown in figs 5 and 6. These figures clearly illustrate the extent of incongruence between the two spatio-temporal patterns, as evidenced by the statistical analysis.

Fig. 5. Activity-density (a) and spatio-temporal variation (b–e) in seed predation in field 1. (a) Activity-density of granivorous carabids, (b) Capsella, (c) Lamium, (d) Poa and (e) Stellaria.

Fig. 6. Activity-density (a) and spatio-temporal variation (b–e) in seed predation in field 2. (a) Activity-density of granivorous carabids, (b) Capsella, (c) Lamium, (d) Poa and (e) Stellaria.
Preference in the laboratory
Five species of carabids did not eat seeds (table 5). The remaining eight species accepted at least one species of seed (table 5). The order of preference, calculated from the preferences of individual carabid species, was similar to that observed in the field; P. annua and C. bursa-pastoris were the most preferred, S. media was intermediate and L. amplexicaule was the least preferred species of seed (cf. table 5 and fig. 4).
Table 5. Consumption of seeds of the four species of weeds (mean±s.e.) by eight carabid species over four days. Five other species, Bembidion lampros (5 replicates), B. femoratum (5), Clivina fossor (5), Demetrias atricapillus (5) and Poecilus versicolor (8), did not eat any seeds.

1 calculated only from the species that ate seeds.
N, number of replicates.
Discussion
The work reported in this paper shows that, while carabid activity-density in two fields was higher at the edge than in the field interior, seed predation by carabids was lower near the edge than in the field. Over the course of the season, seed predation by carabids varied from week to week, but these fluctuations were not mirrored by parallel changes in activity-density of carabids. Hence, the results point to spatial, as well as temporal, incongruence between patterns of seed predation by carabids and patterns of their activity-density. There was good correspondence, however, between field and laboratory studies on preference for different species of weed seed.
We think that the reasons for spatial incongruence and temporal incongruence are different. In space, incongruence may be the result of varying densities of seeds. Corollary observations in field 1 suggest that seed density was higher near the edge. A greater supply of seeds (as well as alternative prey) near the edge could have decreased the predation chance per seed per unit time per unit of predator density as a result of a ‘dilution’ of predator impact over a greater number of seeds. Such a dilution effect was demonstrated for seed predation by rodents by Marino et al. (Reference Marino, Westerman, Pinkert and Van der Werf2005). Honek et al. (Reference Honek, Martinkova and Jarosik2003) found a negative relationship between weed abundance and seed predation levels by carabids and ascribed this also to a dilution effect. The reduction of relative mortality in larger populations of prey (both in temporal and spatial scale) is well documented in ecological literature (e.g. Begon et al., Reference Begon, Townsend and Harper2006). A lower removal rate of weed seed by carabid beetles at higher densities of the seeds is yet another example of this universal mechanism.
In time, there are three confounded factors that are likely to affect seed predation: (i) changes in community composition of predators; (ii) changes in phenological stage of individual species; and (iii) weather effects. Approximately 50 species of granivorous carabids occurred in our study fields (Saska et al., Reference Saska, Vodde, Heijerman, Westerman and van der Werf2007). It is known (e.g. Tooley et al., Reference Tooley, Froud-Williams, Boatman and Holland1999a; Honek et al., Reference Honek, Martinkova and Jarosik2003, Reference Honek, Martinkova, Saska and Pekar2007) that different carabid species have different food demands; hence, a change in community composition must be expected to affect the per capita food demand averaged over the whole community. Furthermore, there is a progression, through phenological stages within each species, e.g. from pre-reproductive to reproductive and finally post-reproductive adults. Major differences in food demand have been reported for carabids according to their reproductive stage (van Dijk, Reference van Dijk, Brandmayr, den Boer and Weber1983; Honek et al., Reference Honek, Saska and Martinkova2006). All these changes affect food demand by the carabid community. Finally, weather has multiple effects, both on the seeds and on the carabids. High soil moisture after rainfall can increase water content of seeds (Bradford, Reference Bradford, Kigel and Galili1995). That imbibed seeds could be more readily eaten by seed predators than unimbibed ones was shown by Cardina et al. (Reference Cardina, Norquay, Stinner and McCartney1996). Insects, in general, are more active (Cloudsley-Thompson, Reference Cloudsley-Thompson1989; Honek, Reference Honek1997) and have higher rates of metabolism at higher temperatures (Neven, Reference Neven2000); hence, their food demand is generally increased. We assume this is also the case for carabids. We have unpublished laboratory data that confirm an increase in food intake at higher temperatures (P. Saska & A. Honek, unpublished data).
Because of the influences of changing community composition, phenological stage in beetle species and weather effects, the seed demand per carabid, averaged over the community, changes in time. These changes are greater and more difficult to predict in highly diverse community than in communities that are dominated by a single species or a few species. Our results suggest that the confounding factors have a greater influence on the level of seed predation in the field than the numbers of carabids.
The ranking of weed species in order of preference for their seeds by carabids was the same in the field as in laboratory studies with the 13 carabid species that were most numerous in the field. The correspondence indicated that differences between weed seeds in the level of predation under field conditions were the result of a selection process by the predators and supports the observation of Honek et al. (Reference Honek, Martinkova and Jarosik2003).
Although carabids were by far the most important invertebrate seed predators in our experiments, we showed that the level of seed predation at a particular location in the field is not linked directly to the activity-density of carabid beetles in this place. This result differed from findings at higher levels of scale, for instance in a comparison of different fields (Brust & House, Reference Brust and House1988; Honek et al., Reference Honek, Martinkova and Jarosik2003, Reference Honek, Martinkova and Saska2005); but it agrees with observations of Reed et al. (Reference Reed, Kaufman and Kaufman2005) who studied seed predation by rodents and did not find any relationship between rodent density and intensity of seed predation in burned and unburned prairie. Discrepancies in results may be related to the spatial scale of experimental setups in comparison to the scale of animal movement. We surmise that measurements made at a certain sample location are a more unique and distinguishing representation of the density at that site when the distance between sample sites (vastly) exceeds the range of movement of individual predators than when the reverse is the case. In the latter case, differences in catches between sample sites do not so much represent local differences in density as random variation and local differences in factors that affect activity and movement.
We conclude, from our findings, that predicting seed predation from observations on the activity-density of seed predators within a field is extremely hard, if at all possible. To quantify seed predation, we cannot rely on invertebrate predator counts, but have to measure seed predation instead. Thereby, it is important to realise that measurements on seed cards quantify removal chance per unit time. They quantify seed demand at that time in that location relative to the amount of seeds available to predators. They do not quantify total seed removal from a population of seeds, as that depends on the seed population present, seed burial rates, and the timing of seed shed and seed burial (Westerman et al., Reference Westerman, Liebman, Menalled, Heggenstaller, Hartzler and Dixon2005). Moreover, it needs to be assumed a priori that this chance, as measured with seed cards, is representative of the chance of removal of naturally exposed seeds on the soil (Brust & House, Reference Brust and House1988; Westerman et al., Reference Westerman, Hofman, Vet and van der Werf2003a; Gallandt, Reference Gallandt2005). It is important to know natural seed densities and their spatial pattern in studies of seed predation.
As to the biology of seed predators, much needs to be learnt about their basic biology before their impact on weed population dynamics can be understood and predicted. We especially need to know how food demand by predators changes according to their reproductive stage. Much additional work will also be needed to unravel and explain how rainfall and temperature affect seed predation.
Acknowledgements
The work was funded by the EU Mme M. Curie Training Site program (contract no. HPMT-CT-2000-00199). Completion of the manuscript was supported by a visiting scientist grant from the C.T. de Wit Research School for Production Ecology and Resource Conservation of Wageningen University and by grant no. 522/06/P366 from the Grant Agency of the Czech Republic, both to P.S. The authors acknowledge J. Withagen for statistical advice, A. Honěk, J.M. Holland and two anonymous reviewers for commenting on the manuscript, and staff of Plantkundig Proefcentrum Wageningen for managing the fields and help with the assembly of the seed cards.













