Introduction
Insects inhabit all kinds of aquatic habitats, and often dominate the fauna in wetlands, including temporary wetlands (Leeper & Taylor, Reference Leeper and Taylor1998a). Insects play an important role in wetland ecosystems (Cummins, Reference Cummins1973; Berg, Reference Berg, Armitage, Cranston and Pinder1995; Batzer & Wissinger, Reference Batzer and Wissinger1996) and are important food sources for many birds (King & Wrubleski, Reference King and Wrubleski1999), bats (Fukui et al., Reference Fukui, Murakami, Nakano and Aoi2006), frogs (Vignes, Reference Vignes1995) and other arthropods (Paetzold & Tockner, Reference Paetzold and Tockner2005). Temporary wetlands, especially floodplains, are also the main larval habitats for flood-water mosquitoes (Becker et al., Reference Becker, Zgomba, Petrić, Dahl, Boase, Lane and Kaiser2003).
Used at the appropriate dosage for mosquito control, Bacillus thuringiensis var. israelensis (Bti) has no significant impact on most other animals or plants in the short term (Boisvert & Boisvert, Reference Boisvert and Boisvert2000). At higher dosages, negative effects on non-target organisms have been reported for Chironomidae (Diptera: Nematocera) (Charbonneau et al., Reference Charbonneau, Drobney and Rabeni1994; Fillinger, Reference Fillinger1998; Hershey et al., Reference Hershey, Lima, Niemi and Regal1998; Pont et al., Reference Pont, Franquet and Tourenq1999). However, neither Chironomidae production nor species richness was negatively affected by the Bti-based mosquito control in the River Dalälven floodplains (Lundström et al., Reference Lundström, Brodin, Schäfer, Persson Vinnersten and Östman2009a,Reference Lundström, Schäfer, Petersson, Persson Vinnersten, Landin and Brodinb).
Most impact studies had a duration of a few weeks to a year and showed no negative effects of Bti on non-target organisms (Boisvert & Boisvert, Reference Boisvert and Boisvert2000). However, a three-year study of non-target organisms in Minnesota wetlands showed increasing negative impact on order Diptera, especially sub-order Nematocera and family Chironomidae and aquatic predatory Coleoptera. The negative effects were small the first year, larger the second year, and an approximately 60–80% reduction in insect abundance the third year (Hershey et al., Reference Hershey, Lima, Niemi and Regal1998; Niemi et al., Reference Niemi, Hershey, Shannon, Hanowski, Lima, Axler and Regal1999). These unique results were questioned by other researchers, which motivated the Minnesota team to continue the study for two more years using the same study design. However, the initial results were not confirmed in the continued study, and the only negative effect observed was a slightly reduced abundance of Chironomini, a tribus within the subfamily of Chironominae (Diptera: Chironomidae) (Read et al., Reference Read, Balcer, Schmude and Lima1999). Thus, there is a need for more long-term studies of non-target organisms in relation to Bti-based mosquito control covering several years and including insects in general and Nematocera in particular.
Recurrent floods in the River Dalälven floodplains during April to September produce massive numbers of Aedes sticticus and other flood-water mosquitoes (Schäfer et al., Reference Schäfer, Lundström and Petersson2008). These mosquitoes are a huge nuisance for humans and livestock in the near and far surroundings of the temporary flooded wetlands. Since 2002, aerial application of VectoBac G©, with Bti as the active ingredient, is used against larvae of flood-water mosquitoes. With a dosage of 13–15 kg VectoBac G© per ha, we achieve close to 100% reduction in larvae abundance and about 95% reduction in adult female mosquito abundance (J.O. Lundström & M.L. Schäfer, unpublished data).
Our aim was to investigate the effect of Bti used for mosquito larval control on the non-target insect fauna. This was performed by (i) studying the emergence of insects by taxonomic order and sub-order, (ii) studying the emergence of Nematocera by family, and (iii) thoroughly investigating the effect of Bti-based mosquito control on the non-target insect fauna composition and abundance in temporary wetlands. Because the initial evaluation gave indication of a treatment effect on order Coleoptera, we decided (iv) to perform a more detailed study of the Coleoptera with identification to family level.
Material and methods
Study areas
The study was performed in six temporary flooded wetlands, four wet meadows (Fågle, Lusmyren, Nordmyra and Laggarbo) and two alder swamps (Koversta and Valmbäcken), in the River Dalälven floodplains in central Sweden. Floods with varying duration from a few days to several months occur irregularly between April and December. Wet meadows are characterized by low vegetation (mainly grasses and herbs), with few or no woody plants, and are subject to relatively frequent floods of relatively long duration. Alder swamps are characterized by woody plants and trees forming a canopy and are subject to less frequent floods and shorter flood duration. All study areas are located in the Lake Färnebofjärden area (WGS 84: Lat 60.2°N, Long 16.8°E); a detailed map of the six study areas is provided in Lundström et al. (Reference Lundström, Schäfer, Petersson, Persson Vinnersten, Landin and Brodin2009b). A brief description of the six wetlands, including treatment frequency, is provided in Persson Vinnersten et al. (Reference Persson Vinnersten, Lundström, Petersson and Landin2009), and a more detailed description in Lundström et al. (Reference Lundström, Schäfer, Petersson, Persson Vinnersten, Landin and Brodin2009b). Flood-water mosquitoes are prevalent in the floodplains, and a massive emergence of mainly Aedes sticticus occurs after floods in May to September (Schäfer et al., Reference Schäfer, Lundström and Petersson2008). Three experimental wetlands, Nordmyra, Laggarbo and Valmbäcken, were treated with VectoBac G© against mosquito larvae, while three wetlands, Lusmyren, Fågle and Koversta, remained untreated reference wetlands.
Insect sampling
Emerging insects were collected using modified Mundie's emergence traps (Silver, Reference Silver2008; Lundström et al., Reference Lundström, Schäfer, Petersson, Persson Vinnersten, Landin and Brodin2009b). The trap height was 0.6 m with a 0.31 m2 bottom sampling area. A floating device attached to the base of the trap allowed for catching emerging insects during floods (trap floating on the water surface) as well as during dry periods (trap standing on the ground). Insects emerging under the traps were collected in a jar with ethylene glycol at the top of the trap. Four emergence traps were positioned in each of the six wetlands in May and were visited once a week until September to collect the trapped insects and to maintain the traps (addition of ethylene glycol, removal of spiders' webs, removal of vegetation that grow too high inside traps). Insect emergence was monitored every year from 2002 to 2007, i.e. six consecutive years. The continuous annual five-month sampling period probably covers most of the insect emergence period, judging from studies on insect production in similar climatic conditions (Neckles et al., Reference Neckles, Murkin and Cooper1990; Salmela et al., Reference Salmela, Autio and Ilmonen2007).
Insect sorting and identification
All insects were preserved in 70% ethanol, while snails (Gastropoda), spiders (Arachnida) and other non-insects were discarded. Insects were initially counted and sorted by taxonomic order and for Diptera and Hemiptera also to sub-orders (appendix 1). Nematocera and Coleoptera were identified to family (appendix 2 and 3).
Statistical analyses
The numbers of insects per trap and week were not normally distributed, Shapiro-Wilk's test (Shapiro & Wilk, Reference Shapiro and Wilk1965), and thus all abundance data were log-transformed before analysis. Analyses of covariance (ANCOVA) was used to evaluate the effects of Bti treatment on the number of emerging insects by taxonomic order and sub-order, and by Nematocera family and Coleoptera family from 2002 to 2007 in the short term (two weeks before and two weeks after treatment) and in the long term (one to several years after treatment); water depth and time of year and were included as the covariates. ANCOVA is a general linear model with one continuous outcome variable (quantitative) and one or more factor variables (qualitative). ANCOVA is a merger of ANOVA and regression for continuous variables. ANCOVA tests whether certain factors have an effect on the outcome variable after removing the variance for which quantitative predictors (covariates) account. Least-square means and insect production adjusted for time of the year and water levels were used to derive t-values for emergence data by taxonomic order and sub-order, Nematocera families and Coleoptera families in the short term and long term. The in-depth analysis on Nematocera families and Coleoptera families were performed because species within some Nematocera families are known to be sensitive to Bti (Boisvert & Boisvert, Reference Boisvert and Boisvert2000) and because our initial analysis indicated a treatment effect on Coleoptera.
We estimated the relative importance of year, week, water level at trap (range 0–50 cm), locality and Bti treatment on weekly abundance of emerging insects, by taxonomic order and sub-order, in the short-term and the long-term view. The variance components were estimated in PROC VARCOMP using maximum likelihood (SAS Institute Inc., 2004). For the estimation of variance components water depth was not used as a covariate, instead water depth was grouped into five categories. And in the variance component analysis all factor were treated as random factors.
Wetland similarities based on insect abundances by taxonomic order, and by family for Nematocera and Coleoptera, were evaluated by McQuitty Similarity analysis (i.e. weighted average linkage and weighted pair-group method using arithmetic averages) for individual years and all years combined, and the clusters were visualized in dendrograms (SAS Institute Inc., 2004). McQuitty's method is agglomerative clustering algorithms that differ primarily in how they compute the distance between clusters. All such algorithms begin by placing each observation in a unique cluster, i.e. a cluster of one. The two closest clusters are merged to form a new cluster that replaces the two merged clusters. Merging of the two closest clusters continues until only some specified number of clusters remains. This cluster analysis ranges from zero and upwards depending on the similarities in the groups defined.
SAS Statistical Software, version 9.1 (SAS Institute Inc., 2004) was used for all statistical calculations.
Results
Faunal composition and abundance
A total of 137,153 insect specimens, including 13 taxonomic orders, were collected in the 24 emergence traps over the six years (appendix 1). Diptera dominated the collections (74.3% of all insects), followed by Hymenoptera (18.1%) and Coleoptera (4.2%). Plecoptera and Trichoptera were uncommon, and no Odonata or Ephemeroptera were collected. Thus, insect taxa with terrestrial or semi-terrestrial larvae, and adults were more common than taxa with strictly aquatic larvae and/or adults.
The annual number of insects collected varied between years, with the highest numbers recorded in dry or intermediate dry years (32,153 insects in 2004, 26,228 in 2005 and 26,769 in 2007), and the lowest number recorded in the wettest year (8650 insects in 2002). The remaining years were intermediate wet and had intermediate catches, 19,125 insects in 2003 and 24,228 in 2006. The annual insect emergence in experimental and reference wetlands combined over the six years ranged between 1157 and 4302 insects per m2 (fig. 1). The average annual insect emergence was 1652 insects per m2 for the experimental wetlands and 1406 insects per m2 for the reference wetlands.

Fig. 1. Numbers of emerging insects per m2 by wetland and year in six temporary flooded wetlands in the River Dalälven floodplains, central Sweden. For each group of bars, the first three illustrate annual production in experimental wetlands (treated with Bti against mosquito larvae), and the next three bars illustrates production in three reference wetlands (without treatment) (□, Nordmyra Exp; ![]() , Valmbäcken Exp; ▪, Laggarbo Exp;
, Valmbäcken Exp; ▪, Laggarbo Exp; ![]() , Lusmyren Ref;
, Lusmyren Ref; ![]() , Koversta Ref;
, Koversta Ref; ![]() , Fågle Ref).
, Fågle Ref).
The Diptera sub-order Nematocera dominated with 72,814 individuals (53.1% of all insects), and in total 18 Nematocera families were recorded (appendix 2). The most abundant Nematocera families were Sciaridae (42.7% of all Nematocera), Chironomidae (28.9%), Cecidomyiidae (11.8%), Ceratopogonidae (7.3%), Mycetophilidae (4.1%) and Culicidae (2.1%).
Culicidae, the target organism for the Bti treatments, had an extremely patchy distribution. Thus, it was not possible to perform a statistical evaluation with a reasonable accuracy.
Wetland fauna similarity
We measured wetland similarity based on abundance by taxonomic order, by Nematocera family and by Coleoptera family for all individual years and for all years combined.
The wetland similarity cluster analysis based on the insect abundance by taxonomic order for all six years combined revealed one cluster and one solitaire at a linkage distance of 1.0 (fig. 2a). The cluster contained both experimental and reference meadows and one swamp, while the solitaire was the reference swamp Koversta. In the more detailed cluster analysis, for the six years the samples from the reference swamp Koversta in 2002 and 2003 formed a cluster at the linkage distance of 0.7. All other samples formed one large cluster at this linkage distance.

Fig. 2. Cluster analysis of six temporary flooded wetlands based on six-year insect abundances sampled in 24 emergence traps during May–September 2002–2007, in the River Dalälven floodplains central Sweden. (a) by taxonomic order, (b) by Nematocera families, and (c) by Coleoptera families. Mosquito larval control with Bti was performed in experimental (Exp) while reference (Ref) wetlands were untreated. The grey line marks the clusters formed at a linking distance of 1.0.
The wetland similarity cluster analysis based on the Nematocera abundance by family for all six years combined revealed two clusters and one solitaire at a linkage distance of 1.0 (fig. 2b). The two alder swamps, Koversta (reference) and Valmbäcken (experimental), formed one cluster. The other cluster included the reference wetlands Fågle and Lusmyren, and the experimental wetland Laggarbo, while the experimental wetland Nordmyra was a solitaire. In the more detailed analysis using the annual samples of Nematocera abundance by family, two clusters and two solitaires at a linkage distance of 1.0 were formed. Again, Nematocera family communities from experimental and reference wetlands clustered together.
The wetland similarity cluster analysis, based on the Coleoptera abundance by family for all six years combined, revealed one cluster and one solitaire at the linkage distance of 1.0. Again, the reference swamp Koversta was the solitaire (fig. 2c). In the more detailed analysis of the annual samples, Koversta 2003 and Koversta 2004 were solitaires at the linkage distance of 1.0 while all the other annual samples formed one large cluster.
The general and the more detailed cluster analyses showed that experimental and reference wetlands clustered together, indicating that Bti treatment did not affect the structuring of insect communities in the temporary flooded wetlands.
Emergence period
The general emergence period was extensive in both experimental and reference wetlands, ranging from the start of sampling in May (week 19) until the end of sampling in September (week 37), and the peak emergence commonly occurred in June to August (fig. 3). However, high or very high insect emergence occurred already at the start of sampling in May 2004 and 2005.

Fig. 3. Temporal patterns of insect emergence in relation to mosquito larval control with Bti in six temporary flooded wetlands in the River Dalälven floodplains, central Sweden. The annual collections of emerging insects included weekly samples from May (week 19) to September (week 37). Each weekly figure is based on total catch in 12 emergence traps operated in three temporary flooded experimental wetlands (treated with Bti against mosquito larvae) and 12 traps in three reference wetlands (without treatment). M, May; J (after M), June; J (before A), July; A, August; and S, September (![]() , experimental; —, reference).
, experimental; —, reference).
The temporal emergence of the three most abundant taxonomic orders varied within and between years. Diptera emergence peaked in June–August in all years except 2005, when the peak was during May–July. The temporal emergence of Hymenoptera peaked in June to August in 2002, 2003, 2005, 2006–2007 and in June to July and September in 2004. The Coleoptera had peak emergence in June to August in 2002 and 2005, and in May to July in 2003, 2004, 2006 and 2007. Thus, the general insect emergence period in the temporary flooded wetlands of the River Dalälven floodplains is extensive, and the timing of peak insect emergence is highly variable.
Effect of Bti treatments
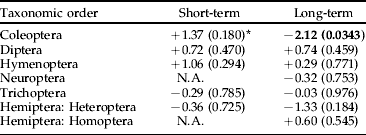
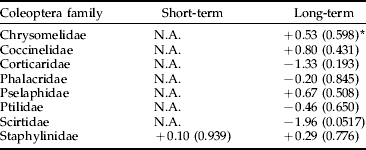
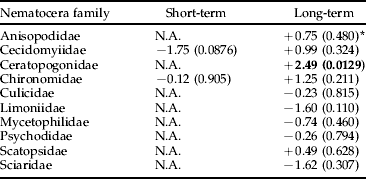
We found no significant negative effects of Bti treatments on the production of insects by taxonomic order, with the exception of Coleoptera in the long term (table 1). However, no significant negative effects were found for the Coleoptera families, neither in the short term nor in the long term (table 2). Scirtidae showed a near significant trend (P<0.06) of higher abundance in reference than in experimental wetlands in the long term (table 2). There was no significant negative treatment effect on Nematocera production, neither when analyzed for the whole sub-order nor when analyzed by family (table 3). However, abundance of Ceratopogonidae was significantly higher in experimental than in reference wetlands.
Table 1. Analysis using ANCOVA of the effects of Bti-based mosquito larval control on the abundance of insects by taxonomic order in wetlands of the River Dalälven floodplains, central Sweden. The short-term analysis was based on weekly samples two weeks before and two weeks after Bti treatment. The long-term analysis was based on weekly samples over six consecutive years (2002 up to and including 2007).
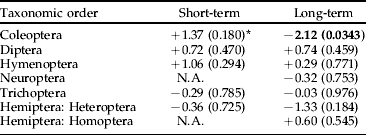
* The figures are t-values based on least-square means and production adjusted for time of the year and water levels, values within parentheses are level of significance, and N.A. (=not applicable) denotes that either temporal or spatial abundance variation made data unsuitable for statistical analysis. Bold=significant change, −=decrease, +=increase.
Table 2. Analysis using ANCOVA of the effects of Bti-based mosquito larval control on the abundance of Coleoptera by family in wetlands of the River Dalälven floodplains, central Sweden. The short-term analysis was based on weekly samples two weeks before and two weeks after Bti treatment. The long-term analysis was based on weekly samples over six consecutive years (2002 up to and including 2007).
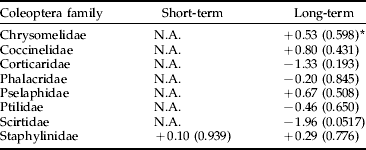
* The figures are t-values based on least-square means and production adjusted for time of the year and water levels, values within parentheses are level of significance, and N.A. (=not applicable) denotes that either temporal or spatial abundance variation made data unsuitable for statistical analysis. −=decrease, +=increase.
Table 3. Analysis using ANCOVA of the effects of Bti-based mosquito larval control on the abundance of Nematocera by family in wetlands of the River Dalälven floodplains, central Sweden. The short-term analysis was based on weekly samples two weeks before and two weeks after Bti treatment. The long-term analysis was based on weekly samples over six consecutive years (2002 up to and including 2007).
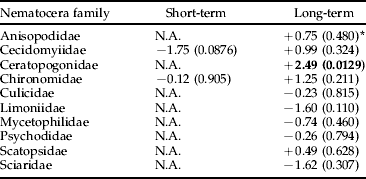
* The figures are t-values based on least-square means and production adjusted for time of the year and water levels, values within parentheses are level of significance, and N.A. (=not applicable) denotes that either temporal or spatial abundance variation made data unsuitable for statistical analysis. Bold=significant change, −=decrease, +=increase.
Explanatory value of the measured factors
The variance component analysis on production of insect orders showed that, in the short term, year explained 5.7% of the total variation, followed by week (4.9%), water level (0.42%), locality (0.32%) and Bti treatment (0.04%). About 11% of the total short-term variation in insect order production was explained by the factors we studied. In the long term, year (3.8%) and week (1.8%) provided again the largest explanatory values, while less important factors were locality (0.33%), water level (0.10%) and Bti treatment (0.04%). About 6% of the total long-term variation was explained by these factors.
The variance component analysis on production of Nematocera families showed that, in the short term, week explained 7.2% of the total variation, followed by locality (4.8%), Bti treatment (0.34%), year (0.12%) and water level (0.03%). In the long term, week explained 2.4% of the variation, followed by year (1.4%), locality (0.67%), Bti treatment (0.08%) and water level (0.07%). The studied temporal and environmental factors explained 12.6% of the total Nematocera production variation in the short term and 4.5% in the long term.
The variance component analysis on production of Coleoptera families showed that, in the short term, week explained 16.8% of the total variation, followed by water level (16.4%), Bti treatment (15.2%), locality (9.1%) and year (0%). About 57.4% of the total short-term variation in Coleoptera production was explained by the factors we studied. In the long term, Bti treatment explained 5.7% of the variation, year explained 5.4% while locality (3.8%), week (2.2%) and water level (0%) added little to the explanation of the variation. About 17% of the total long-term variation was explained by these factors.
Both for insect production by taxonomic order, and by Nematocera family level, the residual variances not explained by the temporal and environmental factors were high in the short term and the long term, indicating that other factors were more important. Bti treatment contributed less than 0.5% to the variation in these analyses. In the analyses of Coleoptera family, however, Bti treatment explained a larger proportion of the variation.
Discussion
Our analyses of possible effects of Bti in both short term and long term on insects collected in temporary wetlands during six consecutive years only revealed two effects in the long term. The order Coleoptera was more abundant in reference wetlands than in experimental wetlands, and the family Ceratopogonidae was more abundant in experimental than in reference wetlands.
In the more detailed analysis of Coleoptera by family, Scirtidae showed a near significant trend to be more abundant in reference than in experimental wetlands in the long term. This difference could be explained by the extraordinary high Scirtidae production in the reference swamp Koversta. Koversta had the highest Scirtidae abundance of all wetlands in all years except for 2007. There is no previous indication of Bti susceptibility in Coleoptera (Boisvert & Boisvert, Reference Boisvert and Boisvert2000). Regarding Ceratopogonidae, an effect of Bti leading to higher abundance in treated areas seems unlikely and anyway could not be considered as a negative effect on non-target organisms. Thus, we conclude that Bti treatments had no adverse effects on the production of Ceratopogonidae, neither in the short nor the long term.
Flood-water mosquitoes are the target organisms for the Bti treatments (Schäfer et al., Reference Schäfer, Lundström and Petersson2008). We used the standard white enamel dipper method for measuring flood-water mosquito abundance before and 24 h after Bti treatment. Usually, the mortality rate is close to 100% (J.O. Lundström & M.L. Schäfer, unpublished data). However, the occurrence of adult flood-water mosquitoes in the emergence traps was extremely irregular and patchy, both in experimental and in reference wetlands, which precluded the statistical analysis at the short term. Flood-water mosquito larvae adapt to rapid changes in hydrology by following with the redrawing water. Thus, although the fourth instar larvae and the pupae are abundant and widely distributed during the flood-period, they have a patchy distribution, closely related to the occurrence of ground depressions. Therefore, fixed emergence traps are not suitable for evaluation of adult flood-water mosquito abundance. Adult flood-water mosquitoes monitored with CO2-baited traps (Schäfer et al., Reference Schäfer, Lundström and Petersson2008). Other insect groups that may be underrepresented in emergence trap catches are insects which will not leave the water, e.g. aquatic predatory insects such as dytiscids and notonectids. These can be monitored by other methods, such as activity traps (Persson Vinnersten et al., Reference Persson Vinnersten, Lundström, Petersson and Landin2009). In spite of this, the use of emergence traps is a reliable and practical method for monitoring emerging insects from both aquatic and terrestrial habitats (Hövemeyer & Havelka, Reference Hövemeyer and Havelka1996; Whiles & Goldowitz, Reference Whiles and Goldowitz2001; Lundström et al., Reference Lundström, Brodin, Schäfer, Persson Vinnersten and Östman2009a,Reference Lundström, Schäfer, Petersson, Persson Vinnersten, Landin and Brodinb).
Our results stands in sharp contrast to Hershey et al. (Reference Hershey, Lima, Niemi and Regal1998), who found substantial negative effects of Bti (60–80% abundance reduction) on non-target benthic insects in Minnesota wetlands after three years. Their results were, however, neither confirmed nor supported when the study was continued for two more years (Read et al., Reference Read, Balcer, Schmude and Lima1999). In accordance, Lundström et al. (Reference Lundström, Brodin, Schäfer, Persson Vinnersten and Östman2009a,Reference Lundström, Schäfer, Petersson, Persson Vinnersten, Landin and Brodinb) found no negative effect of Bti on the most susceptible Nematocera family, Chironomidae. Thus, we conclude that Bti, at the dosage used for mosquito control in Sweden, does not cause any significant negative effect on the insect emergence in the River Dalälven floodplains.
The production of insects from the temporary flooded wetlands in the River Dalälven floodplains, measured as insect emergence per m2, was modest. Most similar to the insect production from Finnish bogs (Paasivirta et al., Reference Paasivirta, Lahti and Perätie1988) but lower than the production from a small wetland in south eastern USA (Stagliano et al., Reference Stagliano, Benke and Anderson1998) and higher than the production from a temporary pond in South Carolina (Leeper & Taylor, Reference Leeper and Taylor1998b). The order Diptera dominated the insect fauna, and almost all the Diptera belonged to the sub-order Nematocera. The overall structure of the wetland insect community, with high dominance of Diptera, was similar to those found in other temporary wetlands (Whiles & Goldowitz, Reference Whiles and Goldowitz2001; MacKenzie & Kaster, Reference MacKenzie and Kaster2004; Wrubleski, Reference Wrubleski2005; Kratzer & Batzer, Reference Kratzer and Batzer2007) and temporary ponds (Boix et al., Reference Boix, Sala and Moreno-Amich2001).
The emergence period of insects varied among orders and covered the complete sampling period May–September, with peak emergence most often occurring during June–August. This emergence pattern is in accordance with previous studies on wetland insects in general and Chironomidae in particular (Leeper & Taylor, Reference Leeper and Taylor1998b; Whiles & Goldowitz, Reference Whiles and Goldowitz2001; MacKenzie & Kaster, Reference MacKenzie and Kaster2004; Lundström et al., Reference Lundström, Schäfer, Petersson, Persson Vinnersten, Landin and Brodin2009b).
We observed that the production of insects from the temporary flooded wetlands was consistently higher during the terrestrial time periods (with no surface water or <1 cm of surface water) than during the aquatic time periods (when the study wetlands had 1 cm to 120 cm of water). Thus, as suggested by Wissinger et al. (Reference Wissinger, Batzer, Rader and Wissinger1999), hydrology structures the insect communities of wetland areas. Our results are in line with several studies that have shown a negative correlation between insect emergence and hydroperiod, with reduced emergence during wet years (Neckles et al., Reference Neckles, Murkin and Cooper1990; Leeper & Taylor, Reference Leeper and Taylor1998a; Whiles & Goldowitz, Reference Whiles and Goldowitz2001). Alternation between terrestrial and aquatic conditions is a main characteristic of the temporary wetlands, and the production of insects is highly influenced by this hydrology.
The Nematocera of the temporary wetlands in the River Dalälven floodplains include at least 18 families. The most abundant families are Sciaridae and Chironomidae, followed by Cecidomyiidae, Ceratopogonidae, Mycetophilidae and Culicidae. Of these families, only Culicidae include species with strictly aquatic larvae, while Chironomidae and Ceratopogonidae include both species with aquatic larvae and species with terrestrial larvae. Sciaridae have larvae in humid soil, Mycetophilidae utilize mushrooms as larval habitats, and Cecidomyiidae form galls, feed on fungi or predate on small invertebrates. The insect species composition probably changes over time, following the hydrological transitions. Lundström et al. (Reference Lundström, Brodin, Schäfer, Persson Vinnersten and Östman2009a) found that terrestrial Chironomidae species dominated the Chironomidae fauna during dry years, while more aquatic species dominated during wet years. This pattern could be observed in the present study for Nematocera families as well.
The cluster analyses of insect abundances by taxonomic order and by Coleoptera family showed high similarities, with the reference swamp Koversta as the only solitaire. Thus, it seemed that temporary flooded wetlands in the River Dalälven floodplains contain a relatively homogenous insect fauna. In contrast, the cluster analysis of Nematocera abundance by family pointed to a difference between the Nematocera communities of swamps and wet meadows, with the experimental meadow Nordmyra as the only exception. There may be several reasons why the swamps differed from the other wetlands in insect abundance and composition. These include: higher moisture conditions during dry periods due to shading effects of trees, more aqueous nutrients released during floods (Goonan et al., Reference Goonan, Beer, Thompson and Suter1992) and leaf fall from trees that may promote insect production (McArthur et al., Reference McArthur, Aho, Rader and Mills1994). These factors may be beneficial for several Nematocera species that require humid to wet conditions during the larval development (e.g. Sciaridae and Mycetophilidae species), and for Scirtidae that are deposit feeders on leaves and other substrates in small water bodies (Klausnitzer, Reference Klausnitzer and Nilsson1996). In addition, Bti treatment did not seem to have any structuring effect on the production or composition of the studied taxa, since the insect fauna of experimental and reference wetlands were similar.
Our results show no negative effect of Bti treatment on the general insect production emergence, which is in accordance with most previous studies of Bti-based mosquito larval control (Boisvert & Boisvert, Reference Boisvert and Boisvert2000; Davis & Peterson, Reference Davis and Peterson2008; Lundström et al., Reference Lundström, Schäfer, Petersson, Persson Vinnersten, Landin and Brodin2009b). Thus, negative indirect effects on birds, bats, frogs and other generalist predators are not expected. We conclude that Bti treatment effects on insect production may be minute in comparison to other environmental factors structuring the insect fauna of the temporary flooded wetlands.
Acknowledgement
We acknowledge grants from Swedish Environmental Protection Agency to J.O.L, and the invaluable help provided by Björn Forsberg, Anna Hagelin and Andreas Rudh (field sampling and insect identification), Axel Berglund (Nematocera identification), and Anna-Sara Liman and Antti Vähäkari (field sampling).
Appendix 1. Number of insects by taxonomic order and sub-order, collected in 24 emergence traps during 2002 to 2007 in six temporary flooded wetlands in the River Dalälven floodplains. In experimental wetlands (Exp) Bti-based mosquito control was performed in 2002, 2003, 2005 and 2006, while no mosquito control was performed in reference wetlands (Ref).

* Insect taxonomy according to Chinery (Reference Chinery1988).
Appendix 2. Number of insects by Nematocera family, collected in 24 emergence traps during 2002 to 2007 in six temporary flooded wetlands in the River Dalälven floodplains. In experimental wetlands (Exp) Bti-based mosquito control was performed in 2002, 2003, 2005 and 2006, while no mosquito control was performed in reference wetlands (Ref).

Appendix 3. Number of insects by Coleoptera family, collected in 24 emergence traps during 2002 to 2007 in six temporary flooded wetlands in the River Dalälven floodplains. In experimental wetlands (Exp) Bti-based mosquito control was performed in 2002, 2003, 2005 and 2006, while no mosquito control was performed in reference wetlands (Ref).