Introduction
Despite considerable effort, weed invasion is a major threat to both the crop production and natural ecosystems. Kanatas et al. (Reference Kanatas, Travlos, Gazoulis, Tataridas, Tsekoura and Antonopoulos2020) estimated that weeds generally cause 5, 10 and 25% yield loss in developed, less developed and least developed countries, respectively. The genus Polygonum (Polygonaceae) consists of ca. 250 species, which is commonly known as ‘knotweed’ or ‘smartweed’, while the genus Rumex is commonly designated as ‘dock’ or ‘sorrel’ and is comprised of ca. 200 species (Muller, Reference Muller1981; Sambamurty, Reference Sambamurty2005). Rumex dentatus L., is a major weed of wheat in India and Pakistan, while Polygonum glabrum Willd., is considered as a significant weed of rice in India (Anjum and Bajwa, Reference Anjum and Bajwa2010; Singh et al., Reference Singh, Gupta, Singh and Raghubanshi2015; Koner et al., Reference Koner, Debnath and Barik2019). In India, P. glabrum is abundant throughout the year, while the emergence and development of R. dentatus is analogous to that of wheat and mustard in India and Pakistan. Rumex dentatus is abundant in Europe, Asia, Mediterranean region and Egypt, while P. glabrum is present in India, Sri Lanka, Nepal, Afghanistan, South Japan, Malaysia, South China and Africa (Hadjichambis et al., Reference Hadjichambis, Paraskeva-Hadjichambi, Della, Giusti, De Pasquale, Lenzarini, Censorii, Gonzales-Tejero, Sanchez-Rojas, Ramiro-Gutierrez, Skoula, Johnson, Sarpaki, Hmamouchi, Jorhi, El-Demerdash, El-Zayat and Pieroni2008; Elzaawely and Tawata, Reference Elzaawely and Tawata2012; Al-Sherif et al., Reference Al-Sherif, Ismael, Karam and Elfayoumi2018). Wheat is one of the major cereal crops in India and is cultivated ca. 29.8 million ha area in India (Gupta et al., Reference Gupta, Verma, Chhokar, Sheoran, Sendhil and Sharma2013), while rice is grown ca. 44 million ha area in India. Both weeds, P. glabrum and R. dentatus impede production of rice and wheat, respectively. Herbicides are applied by growers to manage these weeds (Abbas et al., Reference Abbas, Ali, Abbas, Aslam and Akram2009; Naseer-ud-Din et al., Reference Naseer-ud-Din, Shehzad and Nasrullah2011). Application of herbicides to control weeds in crop-fields may harm and reduce the biocontrol agents consuming the weeds, and at the same time, weeds may be resistant to herbicides. So, the use of native biocontrol agents to control weeds will be cost effective and will not leave any chemical residues in environment (Culliney, Reference Culliney2005).
The biological control of weeds through insect herbivores is one of the methods to control weeds without disturbing the crop. Larvae and adults of Lema praeusta (Fab.) gregariously consume leaves of two Commelinaceae rice-field weeds, Commelina benghalensis L. and Murdannia nudiflora (L.) Brenan in India (Das et al., Reference Das, Koner and Barik2019a). The chrysomelid, Altica cyanea Weber is considered as a biocontrol agent of Onagraceae weeds – Ludwigia adscendens (L.) Hara, L. parviflora Roxb. and L. octovalvis (Jacq.) Raven in India (Mitra et al., Reference Mitra, Mobarak and Barik2021). Both larvae and adults of Galerucella placida Baly (Coleoptera: Chrysomelidae) feed on leaves of P. glabrum and R. dentatus (Koner et al., Reference Koner, Debnath and Barik2019). Larvae of G. placida voraciously consume leaves for 10.55 ± 0.08 and 12.28 ± 0.09 days through three instars to complete their larval development on R. dentatus and P. glabrum, respectively (Koner et al., Reference Koner, Debnath and Barik2019). After pupation (3–4 days), newly emerged females feed for 44.77 ± 0.16 and 41.12 ± 0.57 days on leaves of R. dentatus and P. glabrum, respectively (Koner et al., Reference Koner, Debnath and Barik2019). This insect is abundant in Bangladesh, Nepal, Sri Lanka, Myanmar (Burma), Thailand, Laos, Vietnam, Sumatra and Java (Kimoto, Reference Kimoto1989; Hnatiuk, Reference Hnatiuk1990; Beenen, Reference Beenen1998). Galerucella placida has been recorded to feed on Persicaria hydropiper (synonym Polygonum hydropiper) in Australia (Reid, Reference Reid2001), while the insect has been reported to feed on Polygonum perfoliatum L., P. lapathifolium L. and P. hydropiper L. (Polygonaceae) in China (Ding et al., Reference Ding, Fu, Reardon, Wu and Zhang2004). In addition, the insect feeds on P. orientale L. in India (Malik et al., Reference Malik, Das and Barik2018). The insect, G. placida is perfectly harmless on crop plants such as rice, wheat, mustard and potato as R. dentatus and P. glabrum grow in association with these crop plants. An intense competition between R. dentatus and wheat occurs when weed density is exceeded ca. 20 plants m−2, and in extreme condition, i.e., 30 R. dentatus plants m−2 result 80% yield loss of wheat (Waheed et al., Reference Waheed, Usman and Ali2017). Rice faces maximum competition with P. glabrum weeds in its early growth stage and when the crop growth is 2–3% in the early establishment stage, the weeds already attain 20–30% growth (Deka and Barua, Reference Deka and Barua2015). The aqueous extracts and residues of Polygonum species inhibit germination and seedling growth of rice (Abbas et al., Reference Abbas, Tanveer, Khaliq and Safdar2016).
The leaf surface is the site where the first physical contact with the biocontrol agent, G. placida occurs, and if the leaf surface is suitable then females lay eggs on the abaxial surface of the leaves, implicating that leaf surface wax compounds might act as semiochemicals to stimulate the females to lay eggs. Therefore, an understanding of the insect egg laying behavior, and identification and quantification of the leaf surface wax compounds accountable for such a behavior could help to monitor the biocontrol agent in biocontrol program (Padovan et al., Reference Padovan, Keszei, Köllner, Degenhardt and Foley2010; Smith and Beck, Reference Smith and Beck2013; Wheeler and Schaffner, Reference Wheeler and Schaffner2013). The leaf surface wax profile such as long-chain alkanes, free fatty acids, alcohols, aldehydes, esters and acetates differ among plant species (Jetter et al., Reference Jetter, Schäffer and Riederer2000; Mitra et al., Reference Mitra, Sarkar and Barik2017). After reaching close range to the host plant, long-chain alkanes and free fatty acids, which are major components of leaf surface wax, can serve as short-range attractant and oviposition stimulant of an insect herbivore (Müller and Hilker, Reference Müller and Hilker2001; Müller, Reference Müller, Riederer and Müller2006; Manosalva et al., Reference Manosalva, Pardo, Perich, Mutis, Parra, Ortega, Isaacs and Quiroz2011; Mitra et al., Reference Mitra, Das and Barik2020). Five n-alkanes, hexacosane, heptacosane, octacosane, nonacosane and tritriacontane present in the surface wax of corn leaves serve as ovipositional stimulant in Ostrinia nubilalis (Hübner) (Udayagiri and Mason, Reference Udayagiri and Mason1997). Different blends consisting of n-alkanes and fatty acids present in the surface waxes of young, mature and senescent leaves of P. orientale served as short-range attractant in G. placida (Malik and Barik, Reference Malik and Barik2015; Malik et al., Reference Malik, Mitra and Barik2017). Therefore, it is of considerable interest to study whether leaf surface waxes of both weeds, R. dentatus and P. glabrum could serve as short-range attractant and oviposition stimulant of their biocontrol agent, G. placida.
The aims of the present study are (i) to identify the composition, and quantify the amounts of n-alkanes and free fatty acids present in the leaf surface waxes of R. dentatus and P. glabrum, (ii) to observe whether the leaf surface waxes of both R. dentatus and P. glabrum serve as short-range attractant and ovipositional stimulant in G. placida, (iii) to find the role of individual synthetic compounds, and blends of synthetic alkanes and fatty acids resembling the leaf surface waxes of both weeds in G. placida through a short Y-tube olfactometer, and (iv) to observe whether the most attractive synthetic blends comparable to the leaf surface waxes of both weeds could serve as ovipositional stimulant in G. placida. If the long-chain n-alkanes and free fatty acids present in the leaf surface waxes of both weeds are used by the insect in short-range host finding and oviposition, then this approach will contribute to the evaluation of host plant specificity of the prospective biological control agent.
Materials and methods
Insects
Light traps were used to collect adults of G. placida from P. orientale weeds growing in fields neighboring to the University of Burdwan (23°16′ N & 87°54′ E), Burdwan, West Bengal, India and reared on the same leaves at 21°C, 65 ± 5% relative humidity (RH) and 12L : 12D photoperiod in a biological oxygen demand incubator. A moist piece of cotton was wrapped around the petiole of P. orientale leaves followed by encasing an aluminum foil to prevent moisture loss from leaves, and new leaves were supplied daily by replacing the preceding one. Newly emerged F2 males and females started to mate after 1–2 days. Mated females of 4–6 days old (3–4 days after initial mating) were used for olfactometer and ovipositional bioassays.
Plant materials
Two to three weeks old mature leaves of R. dentatus and P. glabrum were collected from wheat- and rice-fields, respectively, adjoining to this university during December, 2018–January, 2019. Collected leaves were immediately washed with distilled water and dried by paper toweling.
Extraction of leaf surface waxes
Seventy-five grams leaves each of R. dentatus and P. glabrum were separately collected for five times in the morning at 8 a.m., and the gum arabic method of Jetter and Schäffer (Reference Jetter and Schäffer2001) was employed to isolate leaf surface waxes from an intact leaf. Before use of gum arabic (Sigma Aldrich, Germany), the contaminants of gum arabic were removed by soxhlet extraction with hot chloroform. An aqueous solution of ca. 0.1 ml [50% (w/w)] gum arabic was put on per cm2 of leaf surface on the adaxial and abaxial surfaces of each leaf by a small paintbrush. After drying for 1 h, a thin whitish adhesive layer was removed from each leaf by forceps, keeping the leaves as undamaged and intact (without an injury in the epidermal and mesophyll tissue). The gum arabic fractions of adaxial and abaxial leaf surfaces were separately collected for each leaf followed by put together to get the crude surface waxes and extracted with water and chloroform (v/v). After vigorous agitation and phase separation, the organic solution was removed, and the solvent was evaporated under reduced pressure. The isolation of leaf surface waxes using gum arabic method was repeated five times from five different batches of 75 g leaves of either R. dentatus or P. glabrum. Each dried crude extract obtained from a batch of 75 g leaves of either R. dentatus or P. glabrum was then dissolved in 30 ml chloroform and equally divided into three crude fractions [each 10 ml fraction was ca. 25 g leaves; number of leaves for each 25 g of R. dentatus and P. glabrum were 15 ± 1 and 35 ± 1 (mean ± standard error; five replicates of each type of leaf), respectively]. The first, second and third fractions (each fraction was corresponding to ca. 25 g leaves) of each crude extract obtained from 75 g leaves of either R. dentatus or P. glabrum were utilized for (i) identification and quantification of alkanes, (ii) identification and quantification of free fatty acids and (iii) bioassays, respectively.
Identification and quantification of alkanes
Alkanes were identified and quantified according the protocol of Mobarak et al. (Reference Mobarak, Koner, Mitra, Mitra and Barik2020). The first fraction of each crude extract (equivalent to ca. 25 g of leaves) was fractioned by thin layer chromatography (TLC) on silica gel G (Sigma St. Louis, MO, USA) layers (thickness 0.5 mm) with carbon tetrachloride as the mobile phase. A faint yellowish band appeared on the TLC plate, and the plate was air-dried under laboratory conditions. The single hydrocarbon band appeared in each TLC plate was scrapped from the plate and then extracted with chloroform. Five separate crude extracts obtained from five different batches of leaves of either R. dentatus or P. glabrum were used to isolate five purified alkane samples for either R. dentatus or P. glabrum. Each crude extract obtained from a batch of leaves was used to isolate a purified alkane sample, and five separate alkane samples for either R. dentatus or P. glabrum leaves were prepared for gas chromatography-mass spectrometry (GC-MS) and GC- flame ionization detector (GC-FID) for identification and quantification, respectively. Half portion of each sample was used for identification by GC-MS and the remainder for quantification of alkane compounds by GC-FID.
For identification of alkanes, the extracts were analyzed with a Clarus 690 GC coupled to a SQ8C Mass Selective Detector using a SE-30 column (Agilent, USA; length: 30 m × 0.32 mm × 0.25-μm film thickness). The oven temperature program was initially 170°C held for 1 min, then raised at 4°C min−1 to 300°C and finally held for 15 min (Sarkar et al., Reference Sarkar, Malik and Barik2014). Helium was the carrier gas. The flow rate of helium was 1 ml min−1. The MS parameters were 280°C at the interface, ionization energy 70 eV, scan speed ca. 5 scans/sec and scanned over the mass range of 40–600 mass units. The identity of compounds was confirmed by injections of mixture of synthetic n-alkanes (n-C14 to n-C35). Alkanes were verified by comparison of the diagnostic ions and GC retention times with those of respective authentic standards.
For quantification of compounds, five separate extracts obtained from five different batches of leaves of either R. dentatus or P. glabrum were analyzed by a Techcomp Gas Chromatograph (Em Macau, Rua De Pequim, Nos. 202A-246, Centro Financeiro F7, Hong Kong) model 7900 fitted with a SE-30 capillary column (Agilent, USA; length: 30 m × 0.32 mm × 0.25-μm film thickness) and a flame ionization detector which was run under the same temperature conditions as mentioned in GC-MS analysis. The carrier gas was nitrogen with a flow rate of 18.5 ml min−1. The volume of the sample injected was 1 μl with a split ratio of 1:5. The peaks were identified by comparing retention times with those of standard n-alkanes from n-C14 to n-C35, and the area of each peak was converted into quantity of n-alkanes based on internal standard heneicosane (n-C21). All n-alkanes (>99% purity) between n-C14 and n-C35 were purchased from Sigma Aldrich, Steinheim, Germany.
Identification and quantification of free fatty acids
Free fatty acids were identified and quantified according to the protocol of Mitra et al. (Reference Mitra, Das and Barik2020). The second fraction of each crude extract obtained from either R. dentatus or P. glabrum leaves (equivalent to ca. 25 g of leaves) was mixed with diethyl ether and filtered through Whatman No. 41 filter paper. The extract was purified by TLC on silica gel G layers (thickness 0.5 mm) with n-butanol: acetic acid: water (4:1:5; this mixture was shaken and water was separated from this mixture by a separating funnel and discarded) as the mobile phase (Das et al., Reference Das, Koner and Barik2019b). The band was eluted from the silica gel layer with diethyl ether, and diethyl ether was removed under reduced pressure to get purified free fatty acids. The purified free fatty acids were esterified with 3 ml BF3-methanol followed by warming for 5 min in a hot water bath at 50–60°C, and cooled. Hexane (30 ml) was added to this mixture followed by washing with saturated NaCl twice in a separating funnel. The aqueous layer of each sample was discarded and the hexane fraction was passed through 50 g anhydrous Na2SO4 twice. One portion of each esterified sample (hexane fraction) was used for GC-MS and another for GC-FID. Five separate crude extracts obtained from five different batches of leaves of either R. dentatus or P. glabrum leaves were used to separate free fatty acids samples followed by esterification, and in this way, five separate samples for either R. dentatus or P. glabrum leaves were prepared.
One portion of the esterified fatty acids was analyzed with a Clarus 690 GC coupled to a SQ8C Mass Selective Detector with a SE-30 column (Agilent, USA; length: 30 m × 0.32 mm × 0.25-μm film thickness). The oven temperature program was initially held at 160 °C for 2 min, then raised at the rate of 3°C min−1 to 220°C and finally held at 220°C for 18 min (Mukherjee et al., Reference Mukherjee, Sarkar and Barik2014; Sarkar and Barik, Reference Sarkar and Barik2015). Helium was the carrier gas. The flow rate of helium was 1 ml min−1. The MS temperature parameter was 280°C at the interface, ionization energy 70 eV, scan speed ca. 5 scans/sec, and scanned over the mass range of 40–600 mass units. Fatty acids were verified by comparing the diagnostic ions and GC retention times with those of respective standard esterified fatty acids [methyl laurate (C12:0), methyl tridecanoate (C13:0), methyl tetradecanoate (C14:0), methyl pentadecanoate (C15:0), methyl palmitate (C16:0), methyl palmitoleate (C16:1), methyl stearate (C18:0), methyl oleate (C18:1), methyl linoleate (C18:2), methyl linolenate (C18:3), methyl nonadecanoate (C19:0), methyl arachidate (C20:0), methyl heneicosanoate (C21:0) and methyl docosanoate (C22:0)]. All standard esterified fatty acids (fatty acid methyl esters) were purchased from Sigma-Aldrich, Steinheim, Germany.
The remaining portion of esterified fatty acids (five separate samples obtained from five different batches of leaves of either R. dentatus or P. glabrum) were analyzed using a Techcomp Gas Chromatograph model 7900 fitted with a SE-30 capillary column (Agilent, USA; length: 30 m × 0.32 mm × 0.25-μm film thickness) and a flame ionization detector which was run under the same temperature conditions as described for GC-MS analysis. The injector port temperature was 280°C. The carrier gas was nitrogen with a flow rate of 20 ml min−1 (Mukherjee et al., Reference Mukherjee, Sarkar and Barik2014; Sarkar and Barik, Reference Sarkar and Barik2015). The volume of the sample injected was 1 μl with a split ratio of 1:5. The peaks were identified by comparing retention times with those of standard esterified fatty acids. The amount of individual free fatty acids was computed from the GC peak areas and the areas of all peaks were converted into quantities of fatty acids based on reference standard methyl tricosanoate (C23:0). Solvents (n-hexane, chloroform, n-butanol, acetic acid and methanol) used were of analytical grade and purchased from E. Merck (Mumbai, India).
Olfactometer bioassays
Prior to use in olfactometer bioassays, mated G. placida females (4–6 days old) were provided with water but without food for 6 h. Females were used in bioassays as they employ olfactory cues both for feeding and egg laying. A Y-tube olfactometer (internal radius of 0.6 cm, length of stem or each arm is 5 cm, two lateral arms at an angle of 45°) was used to measure the attractiveness of G. placida (Supplementary fig. 1). A glass-made micro kit adapter was attached with the end of each arm of the Y followed by attachment of a glass vial (1 cm radius × 3 cm long) with the micro kit adapter. Each adapter had two entrances – one air inlet tube for approaching air into the glass vial and another one as outlet tube connecting the glass vial to an arm of the olfactometer. A piece (2 × 2 cm2) of Whatman No. 41 filter paper moistened with 1 ml of the test compounds was placed in one glass vial, while the other glass vial contained a filter paper of same size moistened with 1 ml of the control solvent (petroleum ether). Charcoal-filtered air was pushed into the system at 200 ml min−1. All the connections between different parts of the set-up consisted of Teflon tubing.
The olfactometer bioassays were performed in the laboratory at 21 ± 1°C, 70 ± 5% RH and light intensity 150 lux. To test any intrinsic bias of G. placida within the Y-tube olfactometer, Whatman No. 41 filter papers without test stimuli were provided in the glass vials (1 cm radius × 3 cm long) at the commencement of the experiment. Females did not display directional bias in the Y-tube olfactometer. In preliminary assays, females did not show attraction to the control solvent (petroleum ether). One ml of the test sample and the control solvent were applied to the separate filter paper pieces followed by evaporation of the control solvent in open space under laboratory condition, and these filter papers were placed into the glass vials prior to release of the first insect into the olfactometer for each experiment. One adult G. placida female was placed into the porous glass vial (1 cm radius × 3 cm long), which was then attached with the stem of olfactometer and exposed to a particular odor, consisting of 1 ml of the control solvent (petroleum ether) in one glass vial and 1 ml of the test sample (leaf surface waxes, individual synthetic alkanes and fatty acids or synthetic blends consisting of alkanes and fatty acids) in another glass vial. The behavior of each female was observed for 2 min such as wandering in the Y-tube. The choice of each female was recorded when a female reached at the end of one arm followed by removal of the female from the Y-tube, and the choice of female was recorded as a positive (showed attraction toward test samples) or negative (did not show attraction toward test samples) response, respectively. A female was rejected when the female did not make a choice within 2 min (non-responding, i.e., the insect was in the stem of Y-tube or did not exhibit any movement until the end of observation period), and was substituted by a new one (Mukherjee et al., Reference Mukherjee, Sarkar and Barik2015; Sarkar et al., Reference Sarkar, Mukherjee and Barik2015). Each experiment with one test sample was performed until 60 naïve females had responded (each insect was tested once throughout olfactory bioassays). After testing five insects, the olfactometer set-up was washed with petroleum ether followed by acetone and the position of two arms was systematically changed in order to avoid positional bias.
Dual choice bioassays with female G. placida toward crude surface waxes
The behavioral responses of females toward one leaf equivalent surface wax (crude extract) of either R. dentatus or P. glabrum were tested against the control solvent (petroleum ether) (Supplementary tables 1 and 2). Further, the behavioral responses of females toward one leaf equivalent surface wax (crude extract) of R. dentatus and P. glabrum were tested against each other (Supplementary table 1).
Dual choice bioassays with female G. placida toward synthetic compounds or blends
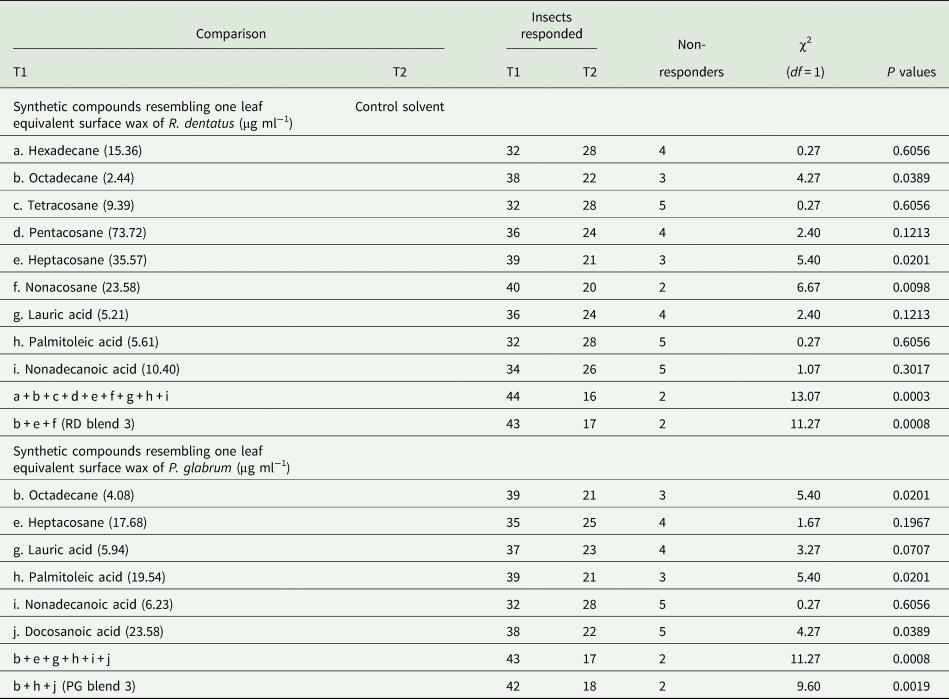
The behavioral responses of females toward individual synthetic compounds (alkanes and fatty acids) resembling the amounts present in one leaf equivalent surface wax of R. dentatus or P. glabrum were tested against the control solvent to observe a response of the insect toward individual compounds. Individual synthetic compounds were dissolved in 1 ml petroleum ether and were tested against 1 ml control solvent (Supplementary tables 3a and 3b). Females showed behavioral responses to nine individual compounds (hexadecane, octadecane, tetracosane, pentacosane, heptacosane, nonacosane, lauric acid, palmitoleic acid and nonadecanoic acid) resembling the amounts present in one leaf equivalent surface wax of R. dentatus. Females exhibited behavioral responses to six individual compounds (octadecane, heptacosane, lauric acid, palmitoleic acid, nonadecanoic acid and docosanoic acid) resembling the amounts present in one leaf equivalent surface wax of P. glabrum. The insect did not indicate any response (the insect remained in the stem of the olfactometer) to the rest of identified individual alkane and fatty acid compounds resembling the amounts present in one leaf equivalent surface wax of R. dentatus and P. glabrum. The standard synthetic n-alkanes and fatty acids identified in this study were all purchased from Sigma Aldrich, Steinheim, Germany.
The insect showed behavioral response to those individual synthetic compounds (the amounts present in one leaf equivalent surface wax) were combined comparable to one leaf equivalent surface wax of either R. dentatus or P. glabrum and were tested against the control solvent. Further, the insect showed attraction to those individual synthetic compounds (comparable to the amounts present in one leaf equivalent surface wax) were combined resembling one leaf equivalent surface wax of either R. dentatus or P. glabrum [R. dentatus : 2.44 μg octadecane + 35.57 μg heptacosane + 23.58 μg nonacosane were dissolved in 1 ml petroleum ether, hereafter will be denoted as RD blend 3; P. glabrum: 4.08 μg octadecane + 19.54 μg palmitoleic acid + 23.58 μg docosanoic acid were dissolved in 1 ml petroleum ether, hereafter will be denoted as PG blend 3] and were tested against the control solvent.
The behavioral responses of female G. placida toward one leaf equivalent surface wax of R. dentatus were tested against nine individual compounds (hexadecane, octadecane, tetracosane, pentacosane, heptacosane, nonacosane, lauric acid, palmitoleic acid and nonadecanoic acid) or synthetic blends (a synthetic blend of above nine compounds or a synthetic blend of three compounds, i.e., RD blend 3) comparable to the amounts present in one leaf equivalent surface wax of R. dentatus to observe the role of individual synthetic compounds or synthetic blends against one leaf equivalent surface wax of R. dentatus. Similarly, the behavioral responses of female G. placida toward one leaf equivalent surface wax extracted from P. glabrum were tested against six individual compounds (octadecane, heptacosane, lauric acid, palmitoleic acid, nonadecanoic acid and docosanoic acid) or synthetic blends (a synthetic blend of above six compounds or a synthetic blend of three compounds, i.e., PG blend 3) comparable to the amounts present in one leaf equivalent surface wax of P. glabrum to observe the role of individual synthetic compounds or synthetic blends against one leaf equivalent surface wax of P. glabrum. A synthetic blend of three compounds resembling one leaf equivalent surface wax of R. dentatus (RD blend 3) was tested against a synthetic blend of three compounds resembling one leaf equivalent surface wax of P. glabrum (PG blend 3) to observe whether females preferred a particular synthetic blend.
Dose responses of female G. placida toward synthetic compounds against the control solvent
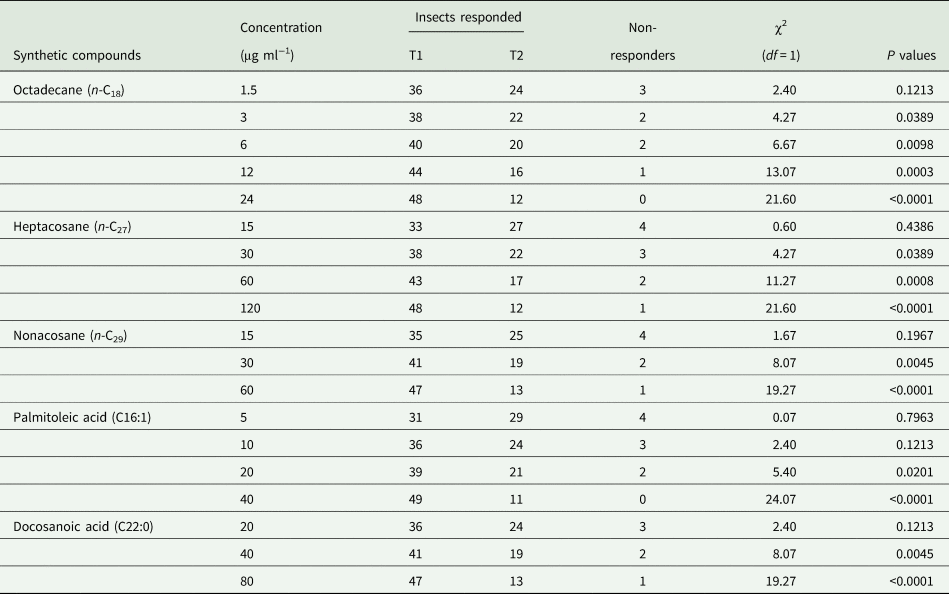
The insect displayed attraction toward five individual compounds (octadecane, heptacosane, nonacosane, palmitoleic acid and docosanoic acid) resembling the amounts present in one leaf equivalent surface wax of both weeds. So, dose responses of female G. placida toward these five compounds were tested at different doses (octadecane : 1.5, 3, 6, 12 and 24 μg were separately dissolved in 1 ml petroleum ether, respectively; heptacosane: 15, 30, 60 and 120 μg were separately dissolved in 1 ml petroleum ether, respectively; nonacosane: 15, 30 and 60 μg were separately dissolved in 1 ml petroleum ether, respectively; palmitoleic acid: 5, 10, 20 and 40 μg were separately dissolved in 1 ml petroleum ether, respectively; docosanoic acid: 20, 40 and 80 μg were separately dissolved in 1 ml petroleum ether, respectively).
Oviposition assay
Three-day-old mated females were employed for oviposition assays. For oviposition assay, 10 square glass chambers (15 × 15 cm2) were used, and coarse grade emery papers were placed along the sides of each glass chamber to avoid egg laying on the wall as well as on the floor of glass chamber. During oviposition assay, females were provided a cotton piece moistened with sucrose solution in a Petri dish (3 cm diameter). Primarily, females did not show any bias for egg laying on the filter paper (Whatman No. 41, 2 × 2 cm2) moistened with the control solvent (petroleum ether). One ml of the test sample and the control solvent were applied on two separate filter paper pieces and permitted to evaporate the solvent in open space followed by placing of these filter papers in two round Petri dishes (each Petri dish 3 cm diameter). One filter paper with the test sample in a Petri dish and the other filter paper with the control solvent in another Petri dish were positioned with a gap of 8 cm inside a glass chamber (15 × 15 cm2), and a mated female was released in the experimental glass chamber. Ten mated females were separately tested for each experiment excluding the number of insects that did not show egg laying behavior. Each mated female was observed at 24 h interval after releasing in a square glass chamber (15 × 15 cm2), and when the mated females laid eggs for the first time then the mated female was discarded and eggs were counted.
Oviposition assays with female G. placida toward crude surface waxes
A single R. dentatus leaf vs a single dewaxed R. dentatus leaf, and a single P. glabrum leaf vs a single P. glabrum dewaxed leaf (for dewaxing of leaves, surface waxes were removed by gum arabic method) were tested to find whether leaf surface waxes of both weeds stimulated females to lay eggs (Supplementary table 4). Further, a single R. dentatus and P. glabrum leaf was tested against each other to find whether a single leaf of a particular weed was preferred by females to lay more eggs (Supplementary table 4). One leaf equivalent surface wax of R. dentatus or P. glabrum was tested against the control solvent to observe whether crude leaf surface waxes of both weeds stimulated females to lay eggs (Supplementary table 4). In addition, one leaf equivalent surface wax of R. dentatus and P. glabrum was tested against each other to find whether leaf surface wax of a particular weed was preferred by females to lay more eggs (Supplementary table 4).
Oviposition assays of G. placida females toward synthetic compounds and blends
Three individual synthetic compounds, octadecane, heptacosane and nonacosane (as G. placida showed significant attraction responses toward these three individual compounds in olfactometer bioassays) at similar amounts present in one leaf equivalent surface wax of R. dentatus were tested against the control solvent to observe whether these three compounds individually stimulated females to lay eggs. Similarly, three individual synthetic compounds, octadecane, palmitoleic acid and docosanoic acid (as G. placida showed significant attraction responses toward these three individual compounds in olfactometer bioassays) at similar amounts present in one leaf equivalent surface wax of P. glabrum were tested against the control solvent to observe whether individual compounds could stimulate females to lay eggs.
RD blend 3 or PG blend 3 was tested against the control solvent to find whether synthetic blends at similar amounts present in one leaf equivalent surface wax of R. dentatus or P. glabrum stimulated females to lay eggs (Supplementary table 4). One leaf equivalent surface wax of R. dentatus vs RD blend 3, and one leaf equivalent surface wax of P. glabrum vs PG blend 3 were conducted to find whether synthetic blends and leaf surface waxes of both weeds were equally stimulated females to lay eggs (Supplementary table 4). RD blend 3 was tested against PG blend 3 to find whether a particular synthetic blend stimulated females to lay more eggs (Supplementary table 4).
Statistical analyses
Student's t test was applied on the data of total amounts of alkanes and free fatty acids, and amounts of individual alkanes and free fatty acids present in R. dentatus and P. glabrum leaf surface waxes (Zar, Reference Zar1999). The Principal component analysis (PCA) was performed to show differences between the chemical composition of R. dentatus and P. glabrum using individual alkanes and free fatty acids as variables (XLSTAT version 13). The data recorded on olfactometer bioassays and oviposition assays of G. placida to the test samples were analyzed based on the null hypothesis that the probability of scores for the test compound(s) or control solvent is equal to 50%, i.e., by a Chi-square test (H0: P = 50%) (Karmakar et al., Reference Karmakar, Mitra, Koner, Das and Barik2020; Mitra et al., Reference Mitra, Das and Barik2020). Insects that did not respond by choosing either arm of the olfactometer were not included in the analyses.
Results
Leaf surface waxes
Total amount of crude surface waxes from two types of weed leaves were homogeneously distributed as revealed by Levene's test (W) of homogeneity of variance (W = 0.370; df = 8; P = 0.560). Total amounts of crude leaf surface waxes were higher in R. dentatus (48.30 ± 1.30 mg, mean ± SE) compared to P. glabrum (43.14 ± 1.55 mg, mean ± SE) (t = −2.547; df = 8; P < 0.05). In R. dentatus, alkanes and free fatty acids represented for 29.33 ± 1.33 and 4.24 ± 0.25 mg (mean ± SE), respectively; whereas in P. glabrum, alkanes and free fatty acids accounted for 23.97 ± 0.89 and 5.34 ± 0.15 mg (mean ± SE), respectively, with the balance consisting of unidentified surface wax compounds.
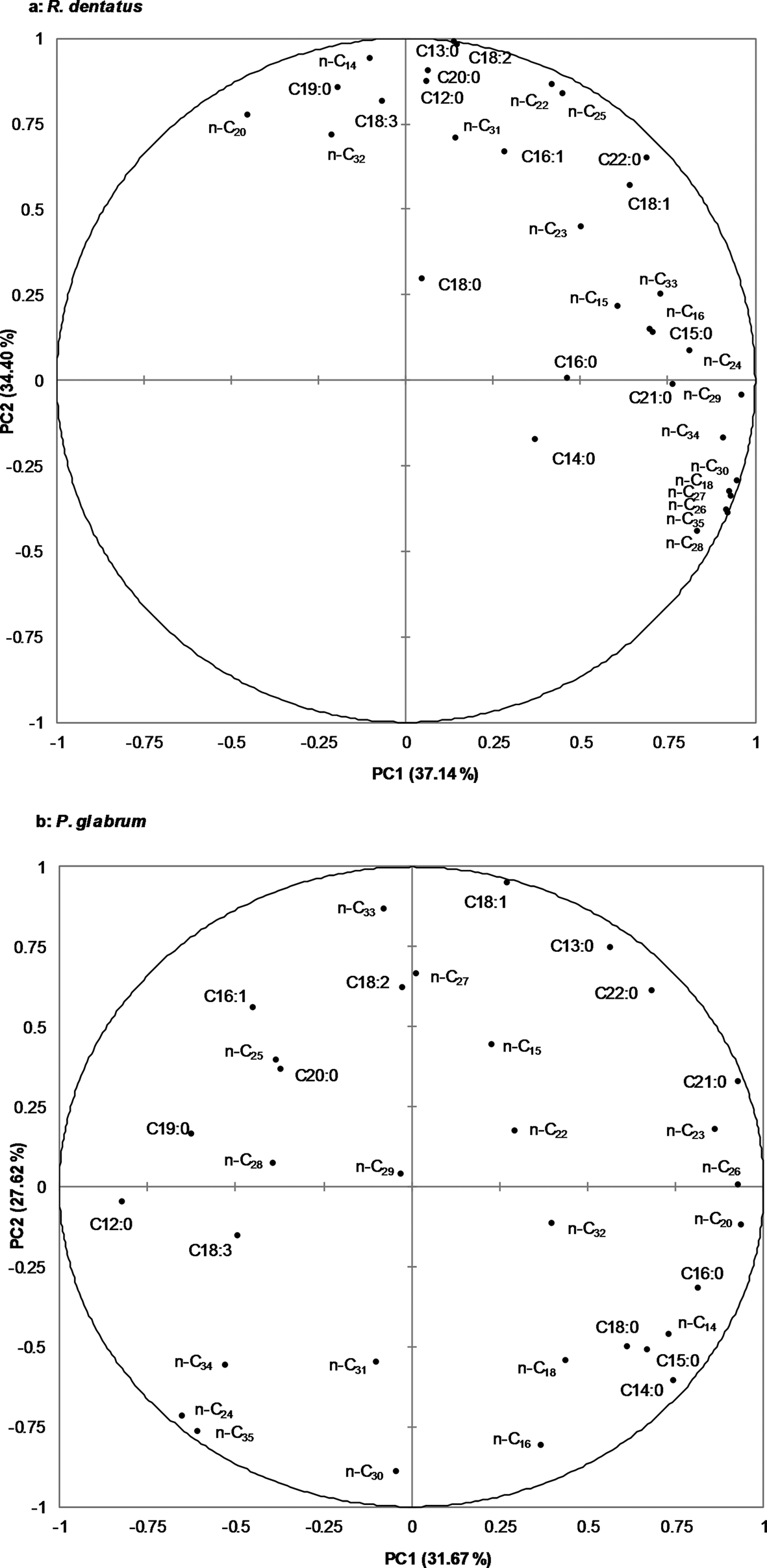
For R. dentatus, the first two principal components, PC1 and PC2, represented for 37.14% and 34.40% of the total variance, respectively (fig. 1a); whereas for P. glabrum, PC1 and PC2, represented for 31.67% and 27.62% of the total variance, respectively (fig. 1b). Except for pentadecane (n-C15), octadecane (n-C18), docosane (n-C22), tricosane (n-C23), tridecanoic acid (C13:0), tetradecanoic acid (C14:0), oleic acid (C18:1), nonadecanoic acid (C19:0) and docosanoic acid (C22:0), where no differences in terms of their relative abundance were observed in biplots between R. dentatus and P. glabrum, rest of the alkanes and free fatty acids were different in terms of their relative abundances between R. dentatus and P. glabrum (fig. 1a, b).
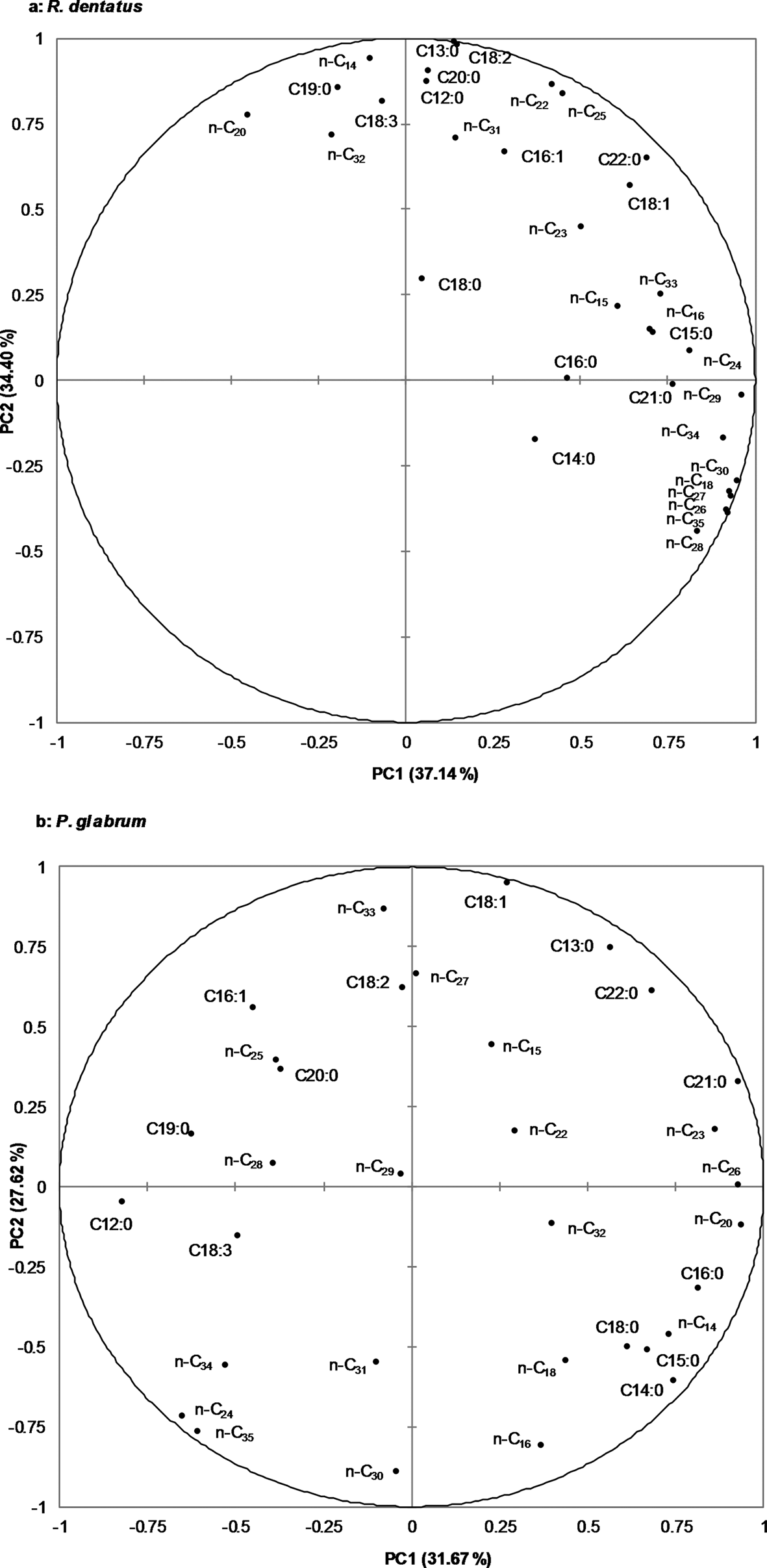
Figure 1. Principal component analysis (PCA) of n-alkanes (n-C14 to n-C35) and free fatty acids (C12:0 to C22:0) from leaf surface waxes of two weeds, a: Rumex dentatus, b: Polygonum glabrum.
Alkanes in leaf surface waxes
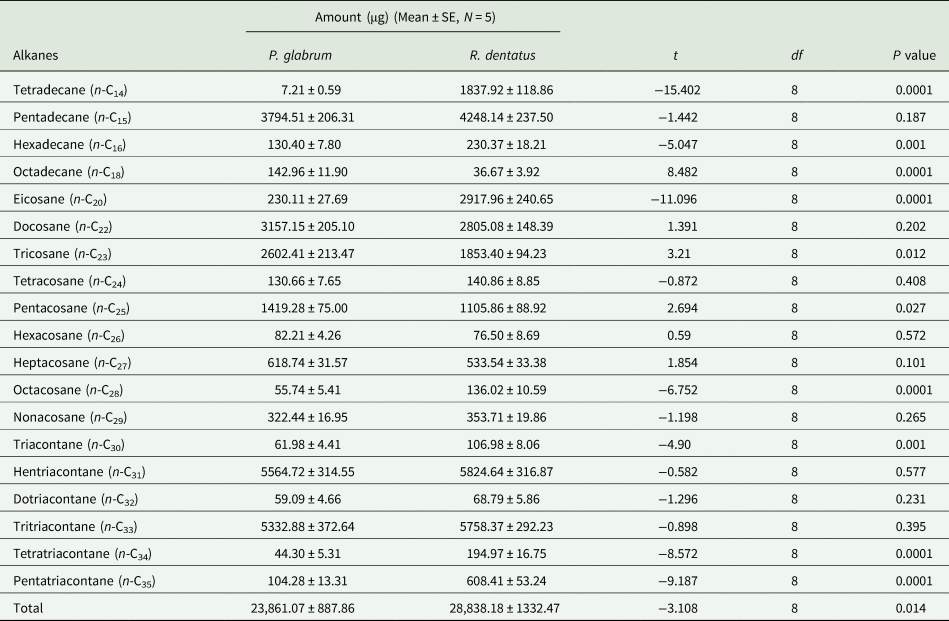
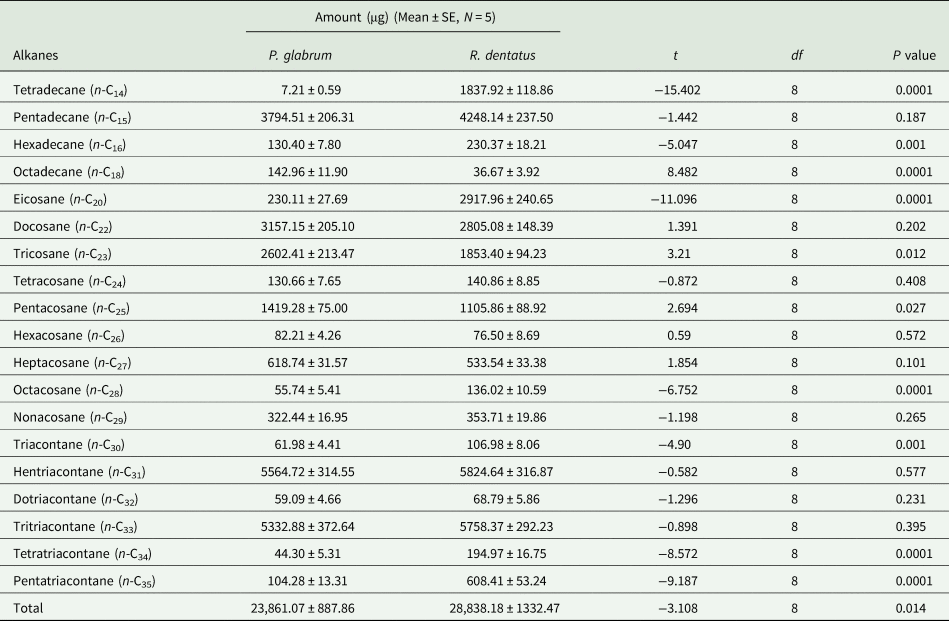
Total amount of alkanes was higher in the leaf surface waxes of R. dentatus compared to P. glabrum (t = −3.320; df = 8; P < 0.05). The identified n-alkanes and unidentified branched-chain alkanes in the leaf surface waxes of R. dentatus represented for 28.84 ± 1.33 and 0.49 ± 0.02 mg (mean ± SE), respectively. In P. glabrum, identified n-alkanes and unidentified branched-chain alkanes accounted for 23.86 ± 0.89 and 0.11 ± 0.01 mg (mean ± SE), respectively. Nineteen n-alkanes in the series of n-C14 to n-C35 were detected in the leaf surface waxes of both weeds (table 1, Supplementary fig. 2). Heptadecane, nonadecane and heneicosane were absent between n-C14 and n-C35 alkanes in the leaf surface waxes of both weeds. The amounts of n-C15, n-C22, n-C24, n-C26, n-C27, n-C29, n-C31, n-C32 and n-C33 did not significantly differ between R. dentatus and P. glabrum leaf surface waxes. The amounts of alkanes, n-C14, n-C16, n-C20, n-C28, n-C30, n-C34 and n-C35 were higher in the leaf surface waxes of R. dentatus compared to P. glabrum, while n-C18, n-C23 and n-C25 were higher in the leaf surface waxes of P. glabrum compared to R. dentatus (table 1). Hentriacontane (n-C31) was predominant followed by tritriacontane (n-C33) in the leaf surface waxes of both weeds; whereas n-C18 and n-C14 were the least abundant in the leaf surface waxes of R. dentatus and P. glabrum, respectively.
Table 1. Composition of alkanes (μg/25 g leaf) in Polygonum glabrum and Rumex dentatus leaves.
Free fatty acids in leaf surface waxes
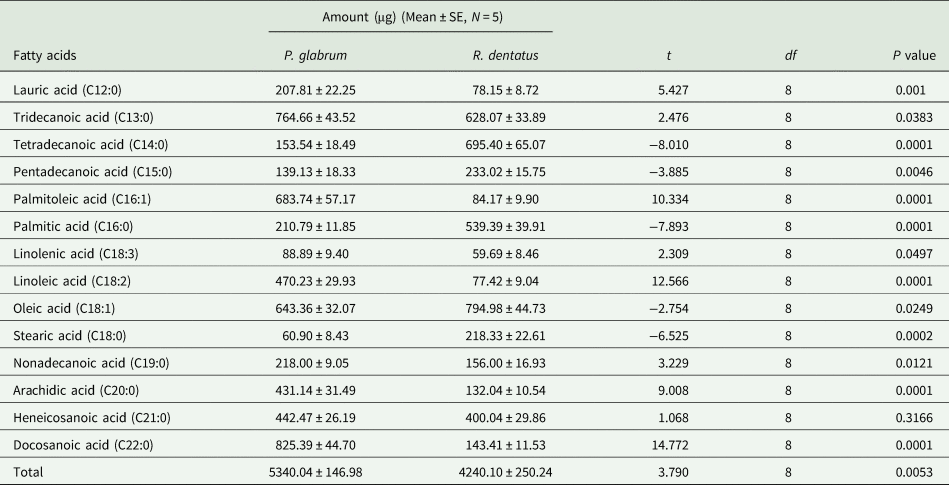
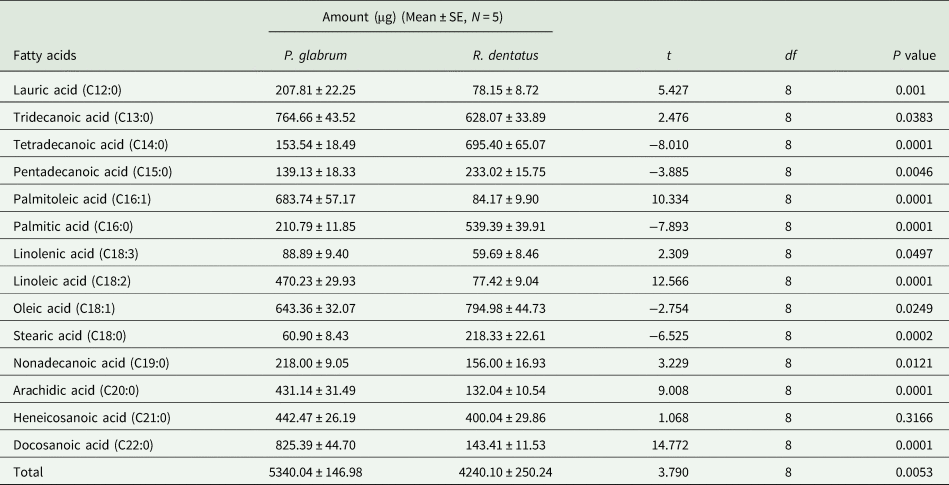
Total amount of free fatty acids was higher in the leaf surface waxes of P. glabrum compared to R. dentatus (t = 3.790; df = 8; P = 0.0053). Fourteen free fatty acids from C12:0 to C22:0 were detected in the leaf surface waxes of both weeds (table 2, Supplementary fig. 3). Docosanoic acid (C22:0) and oleic acid (C18:1) predominated among all free fatty acids present in P. glabrum and R. dentatus, respectively; whereas stearic acid (C18:0) and linolenic acid (C18:3) were the least abundant in P. glabrum and R. dentatus, respectively (table 2). The amounts of eight free fatty acids, C12:0, C13:0, C16:1, C18:3, C18:2, C19:0, C20:0 and C22:0 were significantly (P < 0.05) higher in P. glabrum compared to R. dentatus; whereas the amounts of five free fatty acids, C14:0, C15:0, C16:0, C18:1 and C18:0 were significantly higher in R. dentatus compared to P. glabrum. There was no significant difference in the amount of C21:0 fatty acid between P. glabrum and R. dentatus (table 2).
Table 2. Composition of free fatty acids (μg/25 g leaf) in Polygonum glabrum and Rumex dentatus leaves.
Olfactometer bioassays with G. placida females toward crude leaf surface waxes
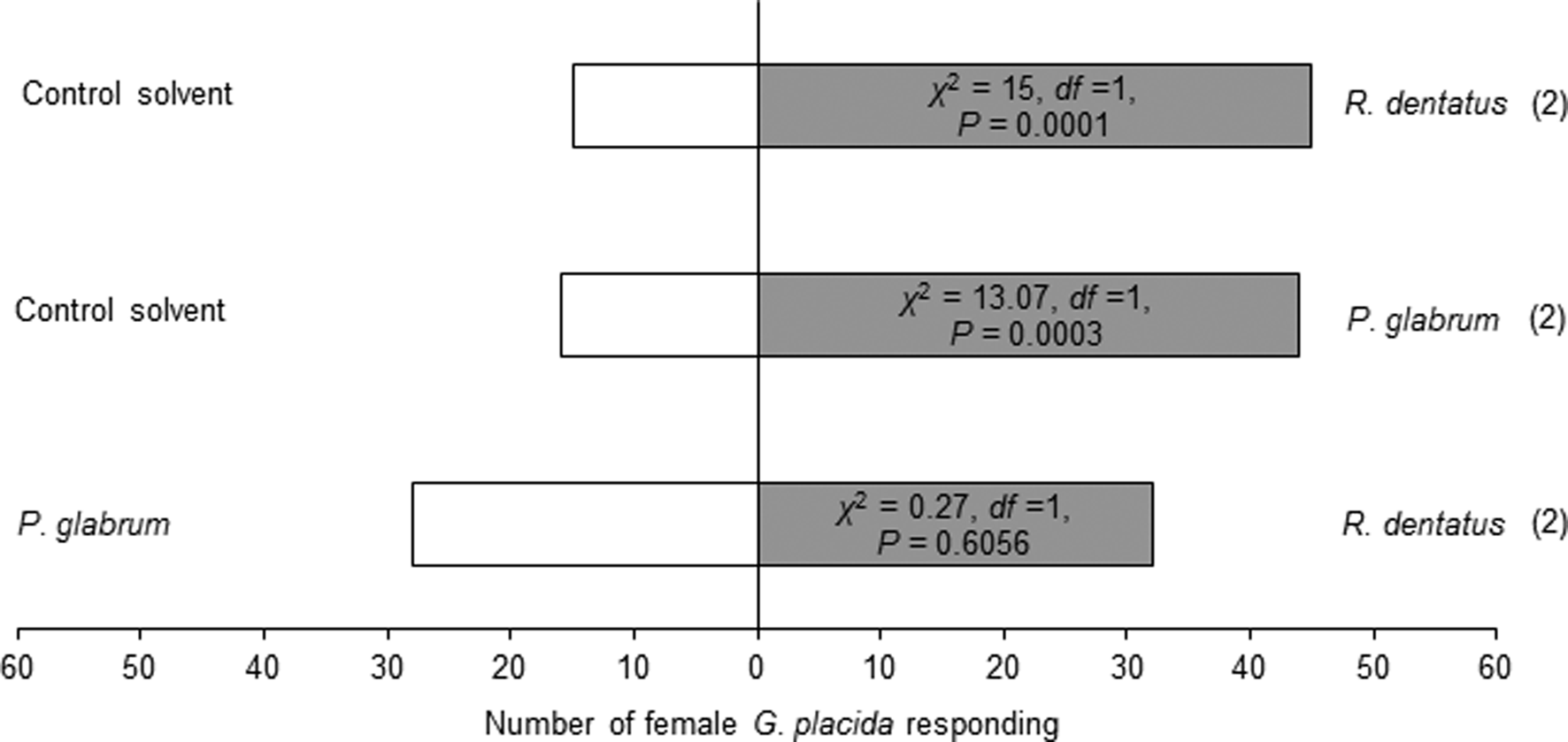
Females showed attraction toward one leaf equivalent surface wax of R. dentatus (χ2 = 15, df = 1, P = 0.0001) or P. glabrum (χ2 = 13.07, df = 1, P = 0.0003) compared to the control solvent (fig. 2). But, females could not discriminate between one leaf equivalent surface wax of R. dentatus and P. glabrum (χ2 = 0.27, df = 1, P = 0.6056) (fig. 2).
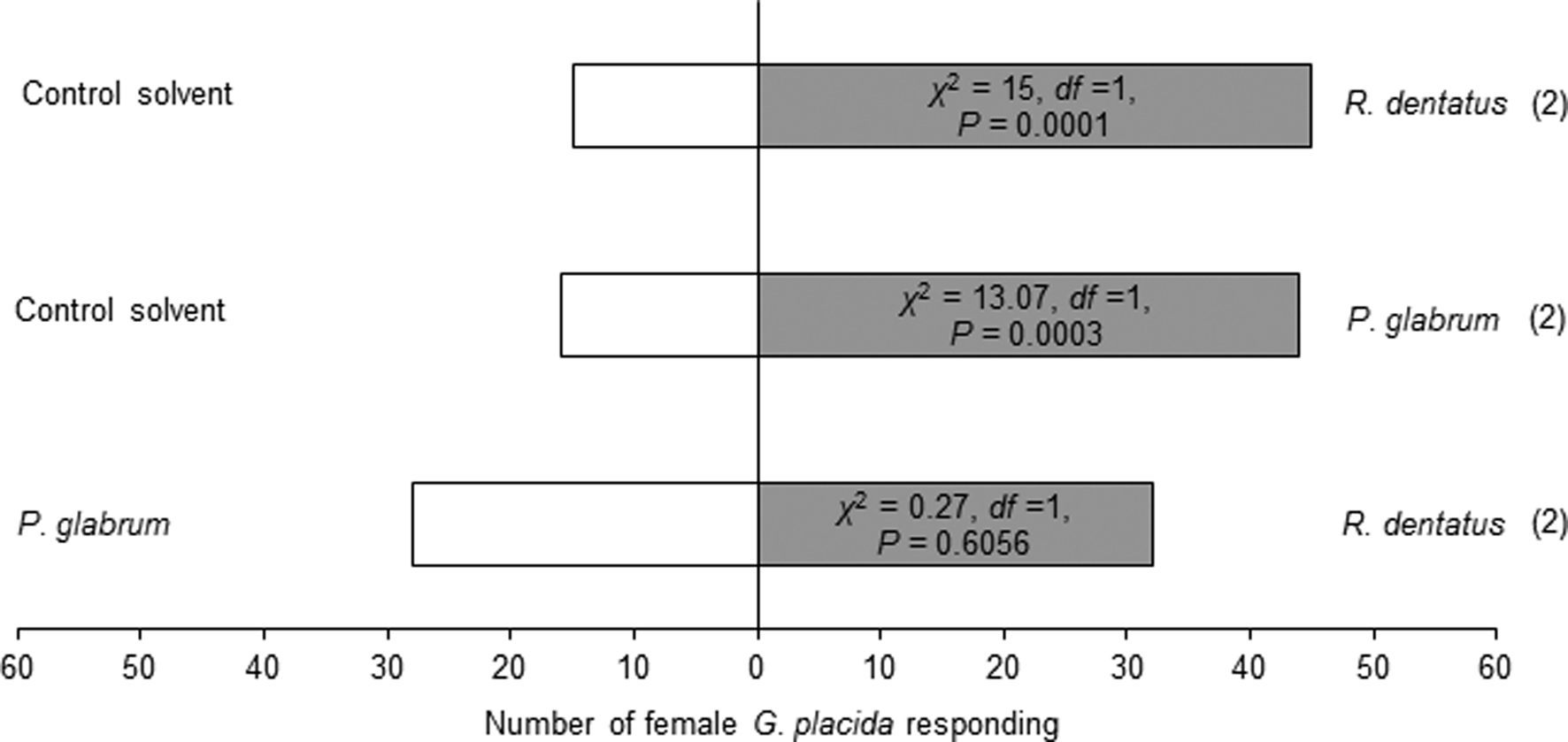
Figure 2. Behavioral responses of Galerucella placida females toward one leaf equivalent surface wax of Rumex dentatus and Polygonum glabrum against the control solvent (petroleum ether), and R. dentatus vs. P. glabrum in the Y-tube olfactometer bioassay. Each bioassay was performed until 60 naïve females had responded. Numbers within parentheses are the number of insects that did not respond to either treatment.
Olfactometer bioassays with G. placida females toward synthetic compounds or blends
Among the identified 19 alkanes and 14 free fatty acids present in the leaf surface waxes of R. dentatus, females showed responses toward nine individual synthetic compounds (hexadecane, octadecane, tetracosane, pentacosane, heptacosane, nonacosane, lauric acid, palmitoleic acid and nonadecanoic acid) at similar amounts present in one leaf equivalent surface wax of R. dentatus compared to the control solvent (table 3). Females showed attraction toward a synthetic blend of above nine compounds at similar amounts present in one leaf equivalent surface wax of R. dentatus compared to the control solvent (χ2 = 13.07, df = 1, P = 0.0003). Among nine individual compounds, females showed significant attraction responses toward three compounds, octadecane (χ2 = 4.27, df = 1, P = 0.0389) or heptacosane (χ2 = 5.40, df = 1, P = 0.0201) or nonacosane (χ2 = 6.67, df = 1, P = 0.0098) at similar amounts present in one leaf equivalent surface wax of R. dentatus compared to the control solvent (table 3). The insect showed significant attraction responses toward a synthetic blend of above three compounds, i.e., RD blend 3 (octadecane, heptacosane and nonacosane) (χ2 = 11.27, df = 1, P = 0.0008) at similar amounts present in one leaf equivalent surface wax of R. dentatus compared to the control solvent (table 3).
Table 3. Behavioral responses of Galerucella placida females toward individual synthetic compounds or synthetic blends at similar amounts present in one leaf equivalent wax of Rumex dentatus or Polygonum glabrum vs the control solvent (petroleum ether) in the Y-tube olfactometer bioassay (N = 60 in each bioassay).
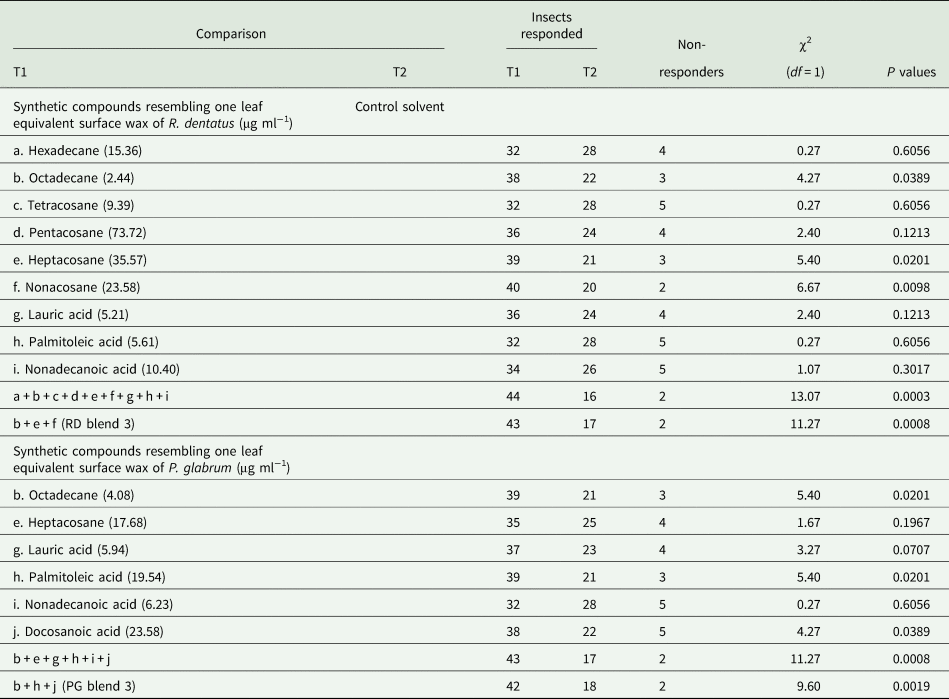
Among a total of 33 compounds (19 alkanes and 14 free fatty acids) present in the leaf surface waxes of P. glabrum, females displayed responses to six individual synthetic compounds (octadecane, heptacosane, lauric acid, palmitoleic acid, nonadecanoic acid and docosanoic acid) at similar amounts present in one leaf equivalent surface wax of P. glabrum compared to the control solvent (table 3). Females showed attraction toward a synthetic blend of above six compounds at similar amounts present in one leaf equivalent surface wax of P. glabrum compared to the control solvent (χ2 = 11.27, df = 1, P = 0.0008) (table 3). Among six individual compounds, females showed significant attraction responses toward three compounds, octadecane (χ2 = 5.40, df = 1, P = 0.0201) or palmitoleic acid (χ2 = 5.40, df = 1, P = 0.0201) or docosanoic acid (χ2 = 4.27, df = 1, P = 0.0389) at similar amounts present in one leaf equivalent surface wax of P. glabrum compared to the control solvent (table 3). The insect showed significant attraction responses toward a synthetic blend of above three compounds, i.e., PG blend 3 (octadecane, palmitoleic acid and docosanoic acid) (χ2 = 9.60, df = 1, P = 0.0019) at similar amounts present in one leaf equivalent surface wax of P. glabrum compared to the control solvent (table 3).
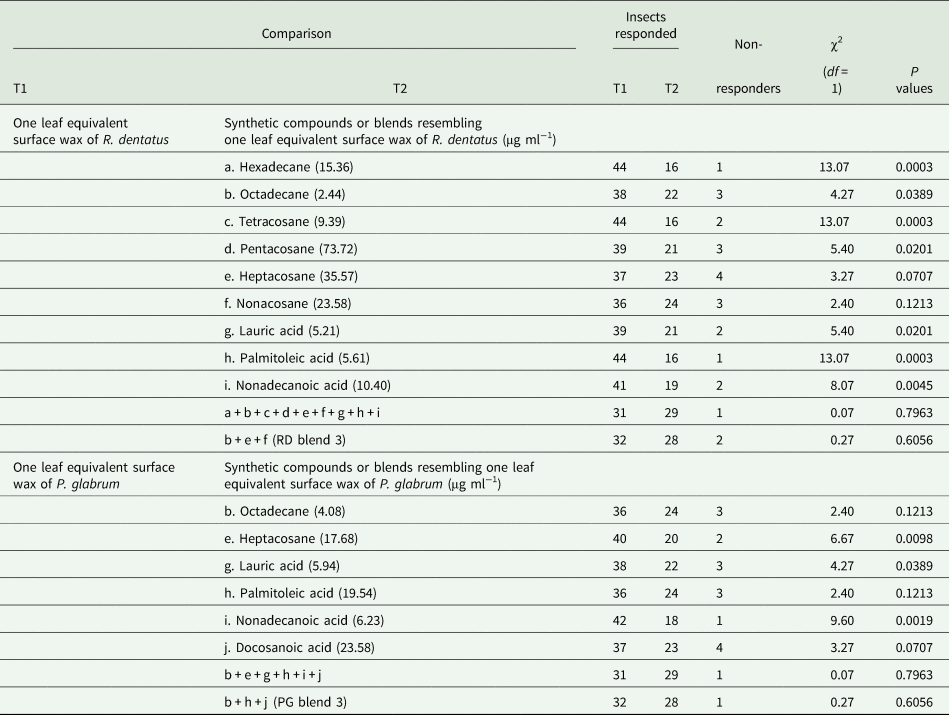
Females were attracted toward one leaf equivalent surface wax of R. dentatus when tested against individual hexadecane or octadecane or tetracosane or pentacosane or lauric acid or palmitoleic acid or nonadecanoic acid at similar amounts present in one leaf equivalent surface wax of R. dentatus (table 4). But, females could not distinguish between one leaf equivalent surface wax of R. dentatus and individual heptacosane or nonacosane at similar amounts present in one leaf equivalent surface wax of R. dentatus (table 4). Females could not differentiate between one leaf equivalent surface wax of R. dentatus and a synthetic blend of nine compounds (hexadecane, octadecane, tetracosane, pentacosane, heptacosane, nonacosane, lauric acid, palmitoleic acid and nonadecanoic acid) (χ2 = 0.07, df = 1, P = 0.7963) or RD blend 3 (octadecane, heptacosane and nonacosane) (χ2 = 0.27, df = 1, P = 0.6056) (table 4).
Table 4. Behavioral responses of Galerucella placida females to one leaf equivalent wax of Rumex dentatus and Polygonum glabrum vs individual synthetic compounds or synthetic blends at similar amounts present in one leaf equivalent wax of R. dentatus and P. glabrum in the Y-tube olfactometer bioassay (N = 60 in each bioassay).
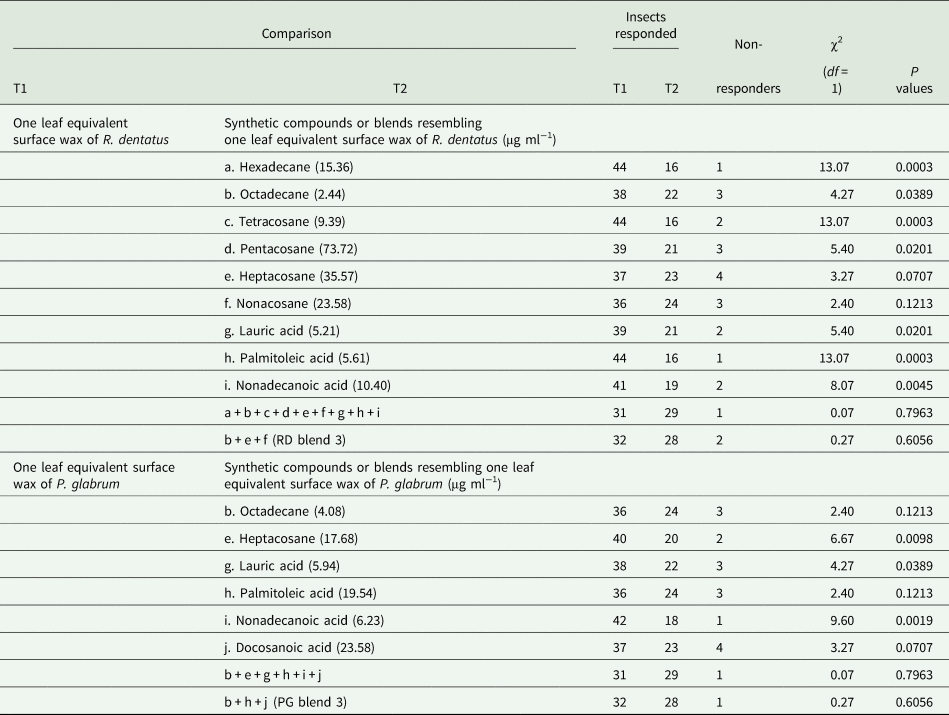
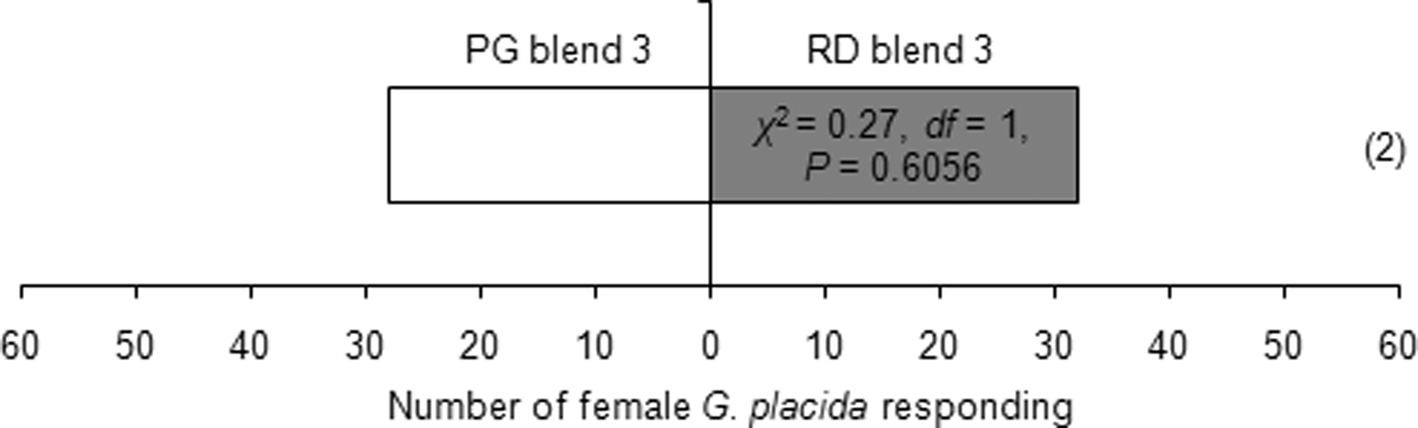
Females showed attraction toward one leaf equivalent surface wax of P. glabrum compared to individual heptacosane or lauric acid or nonadecanoic acid at similar amounts present in one leaf equivalent surface wax of P. glabrum (table 4). But, females could not differentiate between one leaf equivalent surface wax of P. glabrum and individual octadecane or palmitoleic acid or docosanoic acid at similar amounts present in one leaf equivalent surface wax of P. glabrum (table 4). Females could not discriminate between one leaf equivalent surface wax of P. glabrum and a synthetic blend of six compounds (octadecane, heptacosane, lauric acid, palmitoleic acid, nonadecanoic acid and docosanoic acid) (χ2 = 0.07, df = 1, P = 0.7963) or PG blend 3 (octadecane, palmitoleic acid and docosanoic acid) (χ2 = 0.27, df = 1, P = 0.6056) (table 4). Females also could not distinguish between RD blend 3 and PG blend 3 (χ2 = 0.27, df = 1, P = 0.6056) (fig. 3).
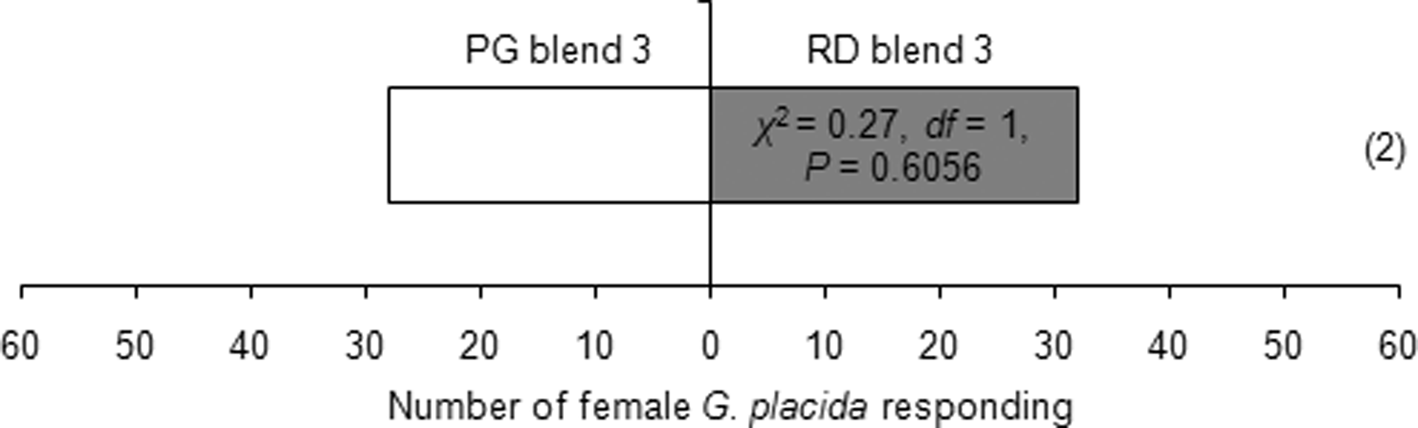
Figure 3. Behavioral responses of Galerucella placida females toward RD (Rumex dentatus) blend 3 (2.44, 35.57 and 23.58 μg ml−1 of octadecane, heptacosane and nonacosane, respectively) against PG (Polygonum glabrum) blend 3 (4.08, 19.54 and 23.58 μg ml−1 of octadecane, palmitoleic acid and docosanoic acid, respectively) in the Y-tube olfactometer bioassay. Each bioassay was performed until 60 naïve females had responded. Numbers within parentheses are the number of insects that did not respond to either treatment.
In dose response bioassays, females started to show positive responses toward octadecane at 3 μg ml−1 (χ2 = 4.27, df = 1, P = 0.0389) and showed the highest positive responses at 24 μg ml−1 (χ2 = 21.60, df = 1, P < 0.0001) (table 5). Females started to display positive responses toward heptacosane at 30 μg ml−1 (χ2 = 4.27, df = 1, P = 0.0389) and showed the highest positive responses at 120 μg ml−1 (χ2 = 21.60, df = 1, P < 0.0001) (table 5). Females started to show positive responses toward nonacosane at 30 μg ml−1 (χ2 = 8.07, df = 1, P = 0.0045) and showed the highest positive responses at 60 μg ml−1 (χ2 = 19.27, df = 1, P < 0.0001). Females started to exhibit positive responses toward palmitoleic acid at 20 μg ml−1 (χ2 = 5.40, df = 1, P = 0.0201) and showed the highest positive responses at 40 μg ml−1 (χ2 = 24.07, df = 1, P < 0.0001) (table 5). Females started to display positive responses toward docosanoic acid at 40 μg ml−1 (χ2 = 8.07, df = 1, P = 0.0045) and showed the highest positive responses at 80 μg ml−1 (χ2 = 19.27, df = 1, P < 0.0001) (table 5).
Table 5. Responses of Galerucella placida females toward individual synthetic compound (T1) vs the control solvent (T2) (petroleum ether) in the Y-tube olfactometer bioassay (N = 60 in each concentration bioassay).
Oviposition assays with G. placida females toward leaf surface waxes
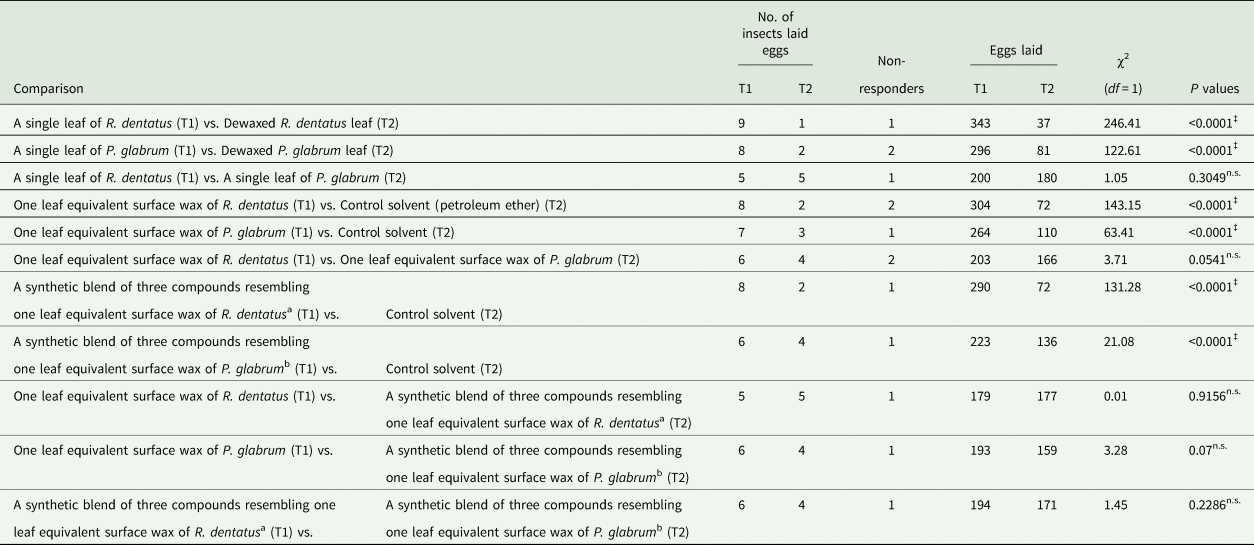
Females laid significantly more eggs on the intact leaf of each weed than the dewaxed leaf of same weed (R. dentatus: χ2 = 246.41, df = 1, P < 0.0001; P. glabrum: χ2 = 122.61, df = 1, P < 0.0001) (table 6). Females could not distinguish between R. dentatus and P. glabrum leaves for egg laying (χ2 = 1.05, df = 1, P = 0.3049) (table 6). Females laid significantly more eggs on one leaf equivalent surface wax of R. dentatus (χ2 = 143.15, df = 1, P < 0.0001) or P. glabrum (χ2 = 63.41, df = 1, P < 0.0001) compared to the control solvent (table 6). Females could not distinguish between one leaf equivalent surface wax of R. dentatus and P. glabrum for egg laying (χ2 = 3.71, df = 1, P = 0.0541) (table 6).
Table 6. Oviposition assays of Galerucella placida females toward leaf surface waxes of Rumex dentatus or Polygonum glabrum plants and synthetic blends at similar amounts present in one leaf equivalent surface wax of R. dentatus or P. glabrum (N = 10 in each bioassay, number of eggs laid by insects were used for χ2 test).

a Indicates RD blend 3: a synthetic blend of three compounds (2.44 μg octadecane + 35.57 μg heptacosane + 23.58 μg nonacosane) at similar amounts present in one leaf equivalent surface wax of R. dentatus.
b Indicates PG blend 3: a synthetic blend of three compounds (4.08 μg octadecane + 19.54 μg palmitoleic acid + 23.58 μg docosanoic acid) at similar amounts present in one leaf equivalent surface wax of P. glabrum.
‡ Indicates P value is significant.
n.s. Indicates P value is not significant.
Oviposition assays with G. placida females toward synthetic compounds or blends
Individual synthetic octadecane (χ2 = 0.10, df = 1, P = 0.7532) or heptacosane (χ2 = 3.34, df = 1, P = 0.0677) or nonacosane (χ2 = 2.66, df = 1, P = 0.1028) at similar amounts present in one leaf equivalent surface wax of R. dentatus could not significantly stimulate females to lay more eggs compared to the control solvent (Supplementary table 5). Similarly, individual synthetic octadecane (χ2 = 3.41, df = 1, P = 0.0647) or palmitoleic acid (χ2 = 2.19, df = 1, P = 0.1390) or docosanoic acid (χ2 = 0.17, df = 1, P = 0.6799) at similar amounts present in one leaf equivalent surface wax of P. glabrum could not significantly stimulate females to lay more eggs compared to the control solvent (Supplementary table 5).
Females laid significantly more eggs on RD blend 3 (χ2 = 131.28, df = 1, P < 0.0001) or PG blend 3 (χ2 = 21.08, df = 1, P < 0.0001) compared to the control solvent (table 6). Females could not distinguish between one leaf equivalent surface wax of R. dentatus and RD blend 3 for egg laying (χ2 = 0.01, df = 1, P = 0.9156) (table 6). Females could not discriminate between one leaf equivalent surface wax of P. glabrum and PG blend 3 for egg laying (χ2 = 3.28, df = 1, P = 0.07) (table 6). Females also could not discriminate for egg laying between RD blend 3 and PG blend 3 (χ2 = 1.45, df = 1, P = 0.2286) (table 6).
Discussion
The chemical characteristics of plant leaf surface waxes influence the acceptance or rejection behavior of an insect herbivore. The suitability of a host plant by a monophagous or oligophagous beetle is dependent on the chemical recognition of a plant as the beetle feeds and develops only on one or limited plants within one plant family. The current research demonstrated that G. placida employs leaf surface waxes of Polygonaceae weeds, R. dentatus and P. glabrum for short-range attraction and oviposition stimulation. Insects employ long-range volatile organic compounds as olfactory cues to find a host, and after coming close range of the host, insects also employ leaf surface wax compounds as short-range cues for oviposition (Mitra et al., Reference Mitra, Sarkar and Barik2017; Das et al., Reference Das, Koner and Barik2019b). Leaf surface waxes from the rice-field weed, Ludwigia octovalvis (Jacq.) Raven are used by the biocontrol agent, Altica cyanea Weber (Coleoptera: Chrysomelidae) as short-range attractant and oviposition stimulant (Mitra et al., Reference Mitra, Sarkar and Barik2017). Therefore, this study is in agreement with the hypothesis that the leaf surface wax compounds could serve as short-range attractant and oviposition stimulant (Eigenbrode and Espelie, Reference Eigenbrode and Espelie1995; Mitra et al., Reference Mitra, Sarkar and Barik2017; Das et al., Reference Das, Koner and Barik2019b, Mitra et al., Reference Mitra, Das and Barik2020; Mobarak et al., Reference Mobarak, Koner, Mitra, Mitra and Barik2020).
The present study revealed that long-chain alkanes (R. dentatus – 60.72%, P. glabrum – 55.57%) and free fatty acids (R. dentatus – 8.78%, P. glabrum – 12.38%) are the major constituents of leaf surface waxes of both weed species. In the surface waxes of P. orientale (Polygonaceae) mature leaves, 19 n-alkanes from n-C15 to n-C33 and 15 free fatty acids from C12:0 to C22:0 were identified, and the predominant alkanes and free fatty acids were nonacosane and palmitic acid, respectively (Malik and Barik, Reference Malik and Barik2015; Malik et al., Reference Malik, Mitra and Barik2017). In the current investigation, hentriacontane (n-C31) was the predominant alkane, while docosanoic acid (C22:0) and oleic acid (C18:1) were the most abundant free fatty acids in leaf surface waxes of P. glabrum and R. dentatus, respectively. A number of studies demonstrated that different long-chain alkanes and free fatty acids are predominant in the leaf surface waxes of diverse plant species (Tomasi et al., Reference Tomasi, Dyer, Jenks and Abdel-Haleem2018; Macel et al., Reference Macel, Visschers, Peters, van Dam and de Graaf2020; Weber and Schwark, Reference Weber and Schwark2020).
A body of research indicates that n-alkanes and free fatty acids present in leaf surface waxes play an important role in plant−insect interactions such as short-range attractant (Manosalva et al., Reference Manosalva, Pardo, Perich, Mutis, Parra, Ortega, Isaacs and Quiroz2011; Mukherjee et al., Reference Mukherjee, Sarkar and Barik2013, Reference Mukherjee, Sarkar and Barik2014; Sarkar et al., Reference Sarkar, Mukherjee and Barik2013; Karmakar et al., Reference Karmakar, Malik and Barik2016; Mitra et al., Reference Mitra, Das and Barik2020; Mobarak et al., Reference Mobarak, Koner, Mitra, Mitra and Barik2020) and ovipositional stimulant (Udayagiri and Mason, Reference Udayagiri and Mason1997; Parr et al., Reference Parr, Tran, Simmonds, Kite and Credland1998; Grant et al., Reference Grant, Zhao and Langevin2000; Li and Ishikawa, Reference Li and Ishikawa2006; Das et al., Reference Das, Koner and Barik2019b). In this study, olfactometer bioassay results revealed that females of G. placida showed attraction toward one leaf equivalent surface wax of R. dentatus and P. glabrum weeds against the control solvent (petroleum ether). But, the insect could not make a distinction between one leaf equivalent surface wax of R. dentatus and P. glabrum, indicating that one leaf equivalent surface wax of both weeds equally attracted the insect. A synthetic blend of three compounds (octadecane, heptacosane and nonacosane) comparable to the amounts present in one leaf equivalent surface wax of R. dentatus or another synthetic blend of three compounds (octadecane, palmitoleic acid and docosanoic acid) comparable to the amounts present in one leaf equivalent surface wax of P. glabrum acted as short-range attractant in olfactometer bioassay and stimulated oviposition in G. placida, indicative of that G. placida females could identify R. dentatus and P. glabrum host plants for oviposition by different surface wax profiles. Some of these compounds present in leaf surface waxes of plants might be ubiquitous in leaf surface wax of diverse plants, but the precise qualitative combination and amounts of compounds differ between plants by which an insect can discriminate between surface waxes of host plants and non-host plants (Mitra et al., Reference Mitra, Sarkar and Barik2017). Attraction of insects could fade away when the amounts of key compounds were changed (Bruce et al., Reference Bruce, Wadhams and Woodcock2005; Najar-Rodriguez et al., Reference Najar-Rodriguez, Galizia, Stierle and Dorn2010; Bruce and Pickett, Reference Bruce and Pickett2011). Similarly, olfactory receptor neurons of an insect register fluxes and modulate behavioral responses by tuning the detection of ecologically relevant odors (Riffell et al., Reference Riffell, Lei, Christensen and Hildebrand2009a, Reference Riffell, Lei and Hildebrandb; Galizia and Rӧssler, Reference Galizia and Rӧssler2010). This observation suggests that the amount of compounds present in one leaf equivalent surface wax of R. dentatus and P. glabrum becomes vital components, which act as olfactory cues for oviposition in G. placida (Bruce et al., Reference Bruce, Wadhams and Woodcock2005; Bruce and Pickett, Reference Bruce and Pickett2011).
The term ‘biotype’ refers an infraspecific group of organisms that are not morphologically discernible, but differing by a biological function (Eastop, Reference Eastop1973). In the current investigation, we observed that larvae and adults of G. placida feed on R. dentatus, P. glabrum and P. orientale belonging to Polygonaceae in adjoining areas of the University of Burdwan and different districts of West Bengal, India. However, we did not observe the insect to feed on other crop plants such as rice, wheat, potato, mustard and maize as R. dentatus and P. glabrum grow in association with these crops in crop fields, indicating that G. placida has a particular biotype in this region. In a previous study, Malik and Barik (Reference Malik and Barik2015) demonstrated that a synthetic blend of 3.59, 7.89, 44.82, 9.91, 32.31, 18.33 and 15.88 μg of lauric, myristic, pentadecanoic, palmitoleic, heptadecanoic, nonadecanoic and docosanoic acids comparable to the amounts present in two leaf (mature) equivalent surface waxes of P. orientale showed attraction of G. placida; whereas in another study, a synthetic blend of 1.60, 0.86, 11.90, 17.79, 1.82, 1.54, 12.06, 6.60, 6.55 and 25.45 μg of n-C16, n-C17, n-C18, n-C20, n-C21, n-C23, n-C24, n-C26, n-C28 and n-C31, respectively, comparable to the amounts present in two leaf (mature) equivalent surface waxes of P. orientale exhibited attraction of the G. placida (Malik et al., Reference Malik, Mitra and Barik2017). The effectiveness of alkanes or free fatty acids present in two mature leaf equivalent surface waxes of P. orientale was separately tested as short-range attractant in G. placida, but these two studies did not consider to observe the effectiveness of alkanes and free fatty acids together comparable to the amounts present in one leaf equivalent surface wax of P. orientale toward G. placida. This is the explanation for a higher number of alkanes and fatty acids as well as their increased amounts are required to cause the short-range attraction of G. placida toward leaf surface waxes of P. orientale. Further, the effectiveness of alkanes and free fatty acids present in leaf surface waxes of P. orientale as oviposition stimulant in G. placida was not considered. However, the current research provides a complete knowledge on the effectiveness of alkanes and free fatty acids present in one leaf equivalent surface wax of both weeds, R. dentatus and P. glabrum, which could serve as short-range attractant and oviposition stimulant in G. placida.
This study summarizes that females of G. placida were attracted toward a synthetic blend of either 2.44, 35.57 and 23.58 μg ml−1 of octadecane, heptacosane and nonacosane, respectively, resembling the amounts present in one leaf equivalent surface wax of R. dentatus or 4.08, 19.54 and 23.58 μg ml−1 of octadecane, palmitoleic acid and docosanoic acid, respectively, resembling the amounts present in one leaf equivalent surface wax of P. glabrum, which could serve as close-range olfactory cues both for host recognition and acceptance process as well as for oviposition in G. placida females. This information could be employed to effectively screen surface wax profile of other non-target plants for their susceptibility toward G. placida. Further investigations on behavioral responses of G. placida females toward leaf surface waxes of other plants in the ecological perspective should be a topic for future research.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485321000730.
Acknowledgements
We thank anonymous reviewers for many helpful suggestions of an earlier version of the manuscript. We are thankful to C. L. Staines, National Museum of Natural History, Smithsonian Institution, Washington DC for identifying the insect, and Prof. Ambarish Mukherjee, Department of Botany, of this University for authenticating these weeds. We thank DST PURSE Phase-II for providing necessary instrumental facilities. The financial assistance from Govt. of West Bengal as Swami Vivekananda Merit-cum-Means Scholarship (SVMCM) to Anamika Koner is gratefully acknowledged.