Introduction
Fluorescence-based real-time reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) is a type of nucleic acid quantification technology developed on the basis of conventional PCR and realized a leap from the qualitative to quantitative analysis. As we all know, it has been widely used in the fields of molecular diagnostics, life sciences, agriculture, and medicine (Heid et al., Reference Heid, Stevens, Livak and Williams1996; Kubista et al., Reference Kubista, Andrade, Bengtsson, Forootan, Jona´k, Lind, Sindelka, Sjöback, Sjögreen, Strömbom, Stählberg and Zoric2006; Bustin et al., Reference Bustin, Benes, Garson, Hellemans, Huggett, Kubista, Mueller, Nolan, Pfaffl, Shipley, Vandesompele and Wittwer2009). It not only achieves a rapid, sensitive, specific, and efficient detection of nucleic acids, but also accurately quantifies the initial dose of target genes. Recently, RT-qPCR has become a major technology and an effective tool for gene expression analysis due to its sensitivity, accuracy, and timeliness (Pfaffl, Reference Pfaffl2001; Ginzinger, Reference Ginzinger2002; Bustin et al., Reference Bustin, Benes, Nolan and Pfaffl2005; VanGuilder et al., Reference VanGuilder, Vrana and Freeman2008; Schmittgen & Livak, Reference Schmittgen and Livak2008). RT-qPCR provides a relatively simple and intuitive method to assess correlations of development and under stress tolerance conditions with transcriptional regulation (Snell et al., Reference Snell, Brogdon and Morgan2003; De Boer et al., Reference De Boer, Berg, Timmermans, Den Dunnen, Van Straalen, Ellers and Roelofs2011; Tabunoki et al., Reference Tabunoki, Ode, Banno, Katsuma, Shimada, Mita, Yamamoto, Sato, Ishii-Nozawa and Satoh2011). Thus, in short, it is a key method for the quantitative detection of mRNA expression at the transcriptional level (Bustin, Reference Bustin2002).
To accurately determine the expression levels of target genes, reference genes need to be standardized to eliminate the biases of quality and yield of RNA in different samples. Moreover, to obtain precise differences in the expression of target genes, standardization is necessary to correct the transcription efficiency and the amount of cDNA templates (Ginzinger, Reference Ginzinger2002; Huggett et al., Reference Huggett, Dheda, Bustin and Zumla2005; Steinau et al., Reference Steinau, Rajeevan and Unger2006; Derveaux et al., Reference Derveaux, Vandesompele and Hellemans2010; Tunbridge et al., Reference Tunbridge, Eastwood and Harrison2011). Actin, glyceraldehyde-3 phosphate dehydrogenase (GAPDH), tubulin, and elongation factor-1α (EF1α) have frequently been used as reference genes in some studies (Scharlaken et al., Reference Scharlaken, De Graaf, Goossens, Brunain, Peelman and Jacobs2008; Shen et al., Reference Shen, Jiang, Wang and Wang2010; Chapuis et al., Reference Chapuis, Tohidi-Esfahani, Dodgson, Blondin, Ponton, Cullen, Simpson and Sword2011; Feng et al., Reference Feng, Yu, Li, Ning, Wang, Zou, Zhang, Wang, Hu, Hu and Bao2013). Ideal reference genes are stably expressed in organisms, tissues, and cells, irrespective of the developmental stage or various stresses (Radonić et al., Reference Radonić, Thulke, Mackay, Landt, Siegert and Nitsche2004; Li et al., Reference Li, Xie, Wang, Wu, Yang, Yang, Pan, Zhou, Bai, Xu, Zhou and Zhang2013). However, different reference genes may lead to different results in the standardization of the expression levels of the target genes, primarily because the expression levels of reference genes are, in fact, not identical in different tissues or under different experimental conditions, for example, among various cell types, developmental stages, feeding conditions, acaricide exposure, or other environmental stresses (Jiang et al., Reference Jiang, Liu, Tang, Zhou and Wang2010; Ponton et al., Reference Ponton, Chapuis, Pernice, Sword and Simpson2011; Niu et al., Reference Niu, Dou, Ding, Yang, Shen and Wang2012; Peng et al., Reference Peng, Zhai, Ding, Di, Zhang, Li, Shen and Wei2012). In addition, using a single reference gene as a standard may lead to increased errors, whereas the use of at least 2–3 reference genes for standardization can avoid these errors (Thellin et al., Reference Thellin, Zorzi, Lakaye, Hennen, Coumans, Hennen, Grisar, Lgout and Heinen1999; Vandesompele et al., Reference Vandesompele, De Preter, Pattyn, Poppe, Roy, De Paepe and Speleman2002; Chervoneva et al., Reference Chervoneva, Li, Schulz, Croker, Wilson, Waldman and Hyslop2010; Li et al., Reference Li, Xie, Wang, Wu, Yang, Yang, Pan, Zhou, Bai, Xu, Zhou and Zhang2013). A stable reference gene is the most important and the basic condition for real-time quantitative analysis (Shen et al., Reference Shen, Huang, Jiang, Dou and Wang2013). Therefore, in the use of RT-qPCR technology to research the expression levels of target gene, a systematic analysis is needed according to various experimental conditions, that is, to determine appropriate and stable reference genes with increasing the accuracy of the test results for RT-qPCR.
Neoseiulus barkeri Hughes (Acari, Parasitiformes, Gamasid, Phytoseiidae, Neoseiulus) inhabits plants such as Citrus, Fructus Caricae and Artemisia annua L. and their stored products. This predatory mite has a variety of feeding habits, with its natural prey including spider mites, whiteflies and thrips, and it is widely distributed across China, Japan, Thailand, and the USA, as well as in some countries of South Africa and Europe (Hessein & Parrella, Reference Hessein and Parrella1990; Moraes et al., Reference Moraes, McMurtry, Denmark and Campos2004; Xia et al., Reference Xia, Zou, Li and Lin2012). Because N. barkeri has a short development stage, low natural mortality, high spawning rate, and strong ability to spread, it is considered to be one of the best biological control products (Bonde, Reference Bonde1989). Previous studies showed that environmental conditions such as temperature, illumination, and acaricide significantly influence the growth and reproduction of this mite; however, the acaricide resistance is also easily developed in mites (Petrushov, Reference Petrushov1992; Veerman, Reference Veerman1992; Xia et al., Reference Xia, Zou, Li and Lin2012). To conduct in-depth studies on the effects of external conditions and food on the growth and reproduction of N. barkeri and to clarify the molecular mechanisms for the development of acaricide resistance in this mite, it is necessary to analyze the expression levels of related functional genes. Although, as discussed above, stable reference genes play a key role in accurate gene expression analysis; now, no reports examining reference genes for N. barkeri are available. In view of this, the present study evaluated the stability for the expression of 11 commonly used candidate reference genes in N. barkeri at different developmental stages and under various stresses using RT-qPCR; thus, we provide empirical evidence for selecting optimal reference genes for use in quantitative gene analysis studies of N. barkeri.
Materials and methods
Origin and rearing of mites
The N. barkeri were provided by the Citrus Research Institute at the Chinese Academy of Agricultural Sciences. It is derived from the leaves of Citrus sinensis Osbeck which was drug-free in citrus orchards and fed Aleurolyphus ovatus mixture with a sterilized wheat bran and maintained a surrounding that was at 25 ± 1°C with a relative humidity of 80 ± 5% in a 14:10 h light:dark cycle in the laboratory for 3 years. During these years, it did not contact any acaricide that is an ideal experimental material.
Special culture dishes were prepared by overlaying sponges, filter paper, and the leaves of C. sinensis Osbeck. Water was injected to be made from water-isolated platforms. The leaves were surrounded with cotton thread to prevent the mites from escaping.
Individual feeding rooms were prepared using a 2.5 cm × 2.5 cm glass plate containing a groove with a diameter of 1.5 cm. Circular leaves of C. sinensis Osbeck with a diameter of 1.0 cm were placed in it. Hatched mites were placed on the leaves and covered with coverslips. The coverslips were fixed with rubber bands to prevent the mites from escaping.
Developmental stages
A large number (10,000 or more) of adult N. barkeri without eggs were fed artificial diets. Their eggs were collected 2 h later and placed in culture dishes (prepared as described above). The purpose of this step is to ensure all the eggs come from the same period. After these eggs hatched, the larval mites were collected. The nymphs and adult mites were collected when they are still larval mites. Three hundred female adults were selected at each developmental stage, frozen in liquid nitrogen, and immediately placed at −80°C. Each treatment was performed three times.
Acaricide stress
Avermectin (1.8% emulsifiable concentrate, Liuzhou Huinong Chemical Co. Ltd Liuzhou, Guangxi, China), fenpropathrin (20% emulsifiable concentrate, Sumitomo Chemical Co. Ltd, Japan) and chlorpyrifos (48% emulsifiable concentrate, Jiangsu Bailing Agrochemical Co. Ltd, Jiangyin, Jiangsu, China) were used for the experiments. They are commonly used acaricides in field experiment. Avermectin and fenpropathrin were diluted 1000, 2000, and 4000 times and chlorpyrifos was diluted 500, 1000, and 2000 times with water. The middle concentrations are also commonly used in field. N. barkeri was treated at each concentration of each acaricide for 1, 24, and 48 h. Adult mites were placed in prepared culture devices for 5 s of pharmaceutical treatment. After the treatment, 300 surviving female mites were collected, frozen in liquid nitrogen, and placed at −80°C. Each treatment was performed three times. Mites handled similarly but treated with clear water instead of an acaricide served as controls.
Temperature stress
The mites were placed in incubators set at 35 and 4°C for 1, 2, and 4 h consecutively. The processing temperature can cause a higher mortality of mites. After the temperature treatments, 300 surviving female adults were immediately frozen in liquid nitrogen and placed at −80°C. Each treatment was performed three times. Control mites did not receive temperature stress. Culture dishes for treating mites were prepared as described above.
UV irradiation stress
Adult mites were placed in culture dishes and subjected to 260 nm UV irradiation treatments for 1, 2, and 4 h consecutively. After UV treatments, 300 surviving female mites were immediately frozen in liquid nitrogen and placed at −80°C. Each treatment was performed three times. Mites not receiving UV treatments served as controls.
Prey stress
The eggs collected as is shown in developmental stages section. After these eggs hatched, the larval mites were placed into individual feeding rooms containing sufficient numbers of larval Panonychus citri and Eotetranychus kankitus Ehera, respectively, until the next generation of N. barkeri was produced. The P. citri and E. kankitus are the main pest in citrus orchard and are the favorite prey of N. barkeri. Surviving female mites (n = 300) were collected, immediately frozen in liquid nitrogen and kept at −80°C. Each treatment was performed three times. Mites reared in the absence of the prey served as controls.
RNA extraction and cDNA synthesis
For RNA extraction, an RNeasy plus Micro kit (Qiagen, Germany) was used according to the manufacturer's instructions, and the genomic DNA was removed via a DNA elimination step which is supplied by the kit. A NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA) and agarose gel electrophoresis were used to evaluate the RNA quality. Qualified samples were stored at −80°C.
The obtained RNA was reverse-transcribed into cDNA using a PrimeScript® RT reagent Kit (Takara, Japan) according to the manufacturer's instructions. The mixtures were placed in a professional thermocycler (Biometra, Germany) for reactions at 37°C for 15 min and at 85°C for 5 s. After the reverse transcription was completed, the synthesized cDNA was stored at −20°C.
Reference gene selection and primer design
A total of 11 candidate reference genes (http://www.ncbi.nlm.nih.gov/nuccore/ KP310113-KP310123) were evaluated, including EF1α, tubulin α (α-TUB), β-actin (β-ACT), 28S ribosomal RNA (28S rRNA), RNA polymerase II subunit (RNAP II), heat shock protein 90 (Hsp90), Hsp70, Hsp60, Hsp40, GAPDH, and ubiquitin conjugating enzyme (UBC). Amplification primers were designed based on the transcriptome database of N. barkeri in our laboratory using Primer 3.0, and the specificity of the primers was analyzed using the melting curves from RT-qPCR.
Real-time quantitative PCR
All real-time quantitative operations in the present experiment were performed using an iCycler iQ Multicolor Real-Time PCR Detection System (Bio-RAD, USA). A 20.0 µl reaction system was used for RT-qPCR, including 2 µl of diluted cDNA, 1 µl of the forward and reverse primers, 10.0 µl of SYBR Premix Ex Taq II (TaKaRa, Japan), and 6.0 µl of ddH2O. The reaction conditions were as follows: predegeneration at 95°C for 2 m; followed by 40 cycles of 95°C for 30 s and 60°C for 30 s. Upon completion of the amplification program, 60–95°C was performed to ensure the specificity of the amplified product. Then, a threefold dilution series of cDNA was used to construct the standard curve and to determine the efficiency of various primers for PCR amplification. The results showed that the amplification efficiencies of the 11 candidate reference genes were 91.10–115.70%, and the coefficients of determination were between 0.994 and 0.997. This indicated that the primers of these genes were consistent with the requirements of RT-qPCR analysis. The gene accession numbers, primer sequences, amplification lengths, amplification efficiencies, and coefficients of determination are presented in Table 1.
Table 1. Details of the 11 candidate reference genes of N. barkeri for real-time qPCR.

R 2, coefficient of determination.
Data analyses
Analysis of the expression stability for each candidate reference gene was conducted using GeNorm (Vandesompele et al., Reference Vandesompele, De Preter, Pattyn, Poppe, Roy, De Paepe and Speleman2002), NormFinder (Andersen et al., Reference Andersen, Jensen and Ørntoft2004), and BestKeeper (Pfaffl et al., Reference Pfaffl, Tichopad, Prgomet and Neuvians2004) as well as the online tool RefFinder (Xie et al., Reference Xie, Sun, Stiller and Zhang2011) (http://www.leonxie.com/referencegene.php). The GeNorm software was used to determine the M value of expression stability for the candidate reference genes. These genes whose value of M <0.5 are considered to be stable. The M value is defined as the pairwise variation of a reference gene with all other reference genes. To get a rank, gene with the highest M value (the worst reference gene) is eliminated and the new M values for the other candidate genes are recalculated, until only two genes are remained as the most stable ones. For the minimum number of genes used to get accurate result, GeNorm calculates the pairwise variation Vn/Vn + 1 which represents the variation between using n most stable genes and using n + 1 most stable genes. This evaluation uses 0.150 as the cut-off, below which the inclusion of an additional reference gene is not required, that is, n reference genes are sufficient for accurate normalization (Feng et al., Reference Feng, Yu, Li, Ning, Wang, Zou, Zhang, Wang, Hu, Hu and Bao2013). NormFinder software was used for comparisons based on calculated stability values, the lower stability values the more stable. BestKeeper software analysis was based on the C t values of the candidate reference genes of the samples in each group and the calculated standard deviation (SD). The gene with the lower value of SD is considered to be more stable. The online RefFinder analysis integrated these three analytical methods to obtain a comprehensive ranking index. The gene with a smaller index value indicates a higher stability.
Results
Expression profiles of candidate reference genes
The expression levels of these candidate reference genes were great difference in all samples that the C t values ranged from 14.30 to 35.40 (Table S1). In addition, the variation of C t values of the candidate reference genes in this study was exhibited by the box plot graph (fig. 1). It can be seen from the figure that the median C t values were reflected from lowest 18.43 (EF1α) to highest 26.44 (Hsp40), with a large variation. The EF1α was considered to be a highly expressed gene since its median C t value is lesser than 20. Moreover, the gene owned the lowest ranges of C t value. However, the genes with higher C t ranges than others were Hsp90, Hsp70, Hsp60, especially Hsp70, in which Delta-C t value reached 17.58. Thus, these data imply that the expression levels for genes might be more easily affected by external environment. But only via the raw C t values, the evaluation of the expression stability of candidate reference is meaningless. So, we will utilize different statistical algorithms to validation.
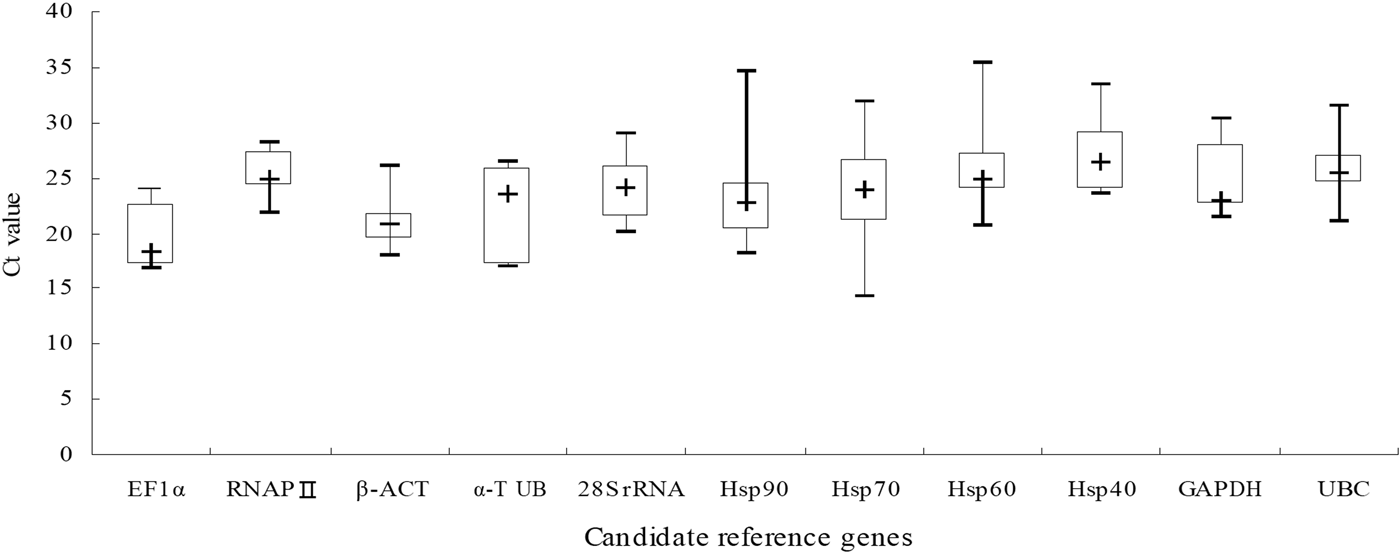
Fig. 1. C t value of 11 candidate reference genes in all experiment stress conditions for N. barkeri.
Analysis of expression stability for candidate reference genes
The 11 candidate reference genes of N. barkeri in developmental stages were analyzed by GeNorm. The results showed that the M values of the Hsp40 and Hsp90 genes were less than 0.5 which indicated that these genes could be used as reference genes. The BestKeeper analysis showed that the SD of all genes was >1.0, which was incompatible with the requirements of a single reference gene. However, the α-TUB and β-ACT genes were relatively stable. The NormFinder indicated that β-ACT and Hsp70 were the most stable genes. As shown in Table 2, the stability of the 11 candidate reference genes was not high throughout developmental stages in the mites. In addition, the optimal number of genes required for accurate normalization in RT-qPCR was also presented by GeNorm, through calculating pairwise variation Vn/n + 1 which uses 0.150 as the proposed cut-off value. A Vn/n + 1 <0.150 means that the top n reference genes are adequate for accurate RT-qPCR normalization. Here the V2/V3 value was <0.150 (fig. 2a), thus the top two reference genes would be adequate in RT-qPCR normalization for developmental stages in N. barkeri, and the addition of the third gene is not necessary. So, according to the rank of RefFinder and pairwise variation values of the GeNorm (fig. 2a), β-ACT and Hsp40 were recommended as reference genes in various developmental stages in N. barkeri.
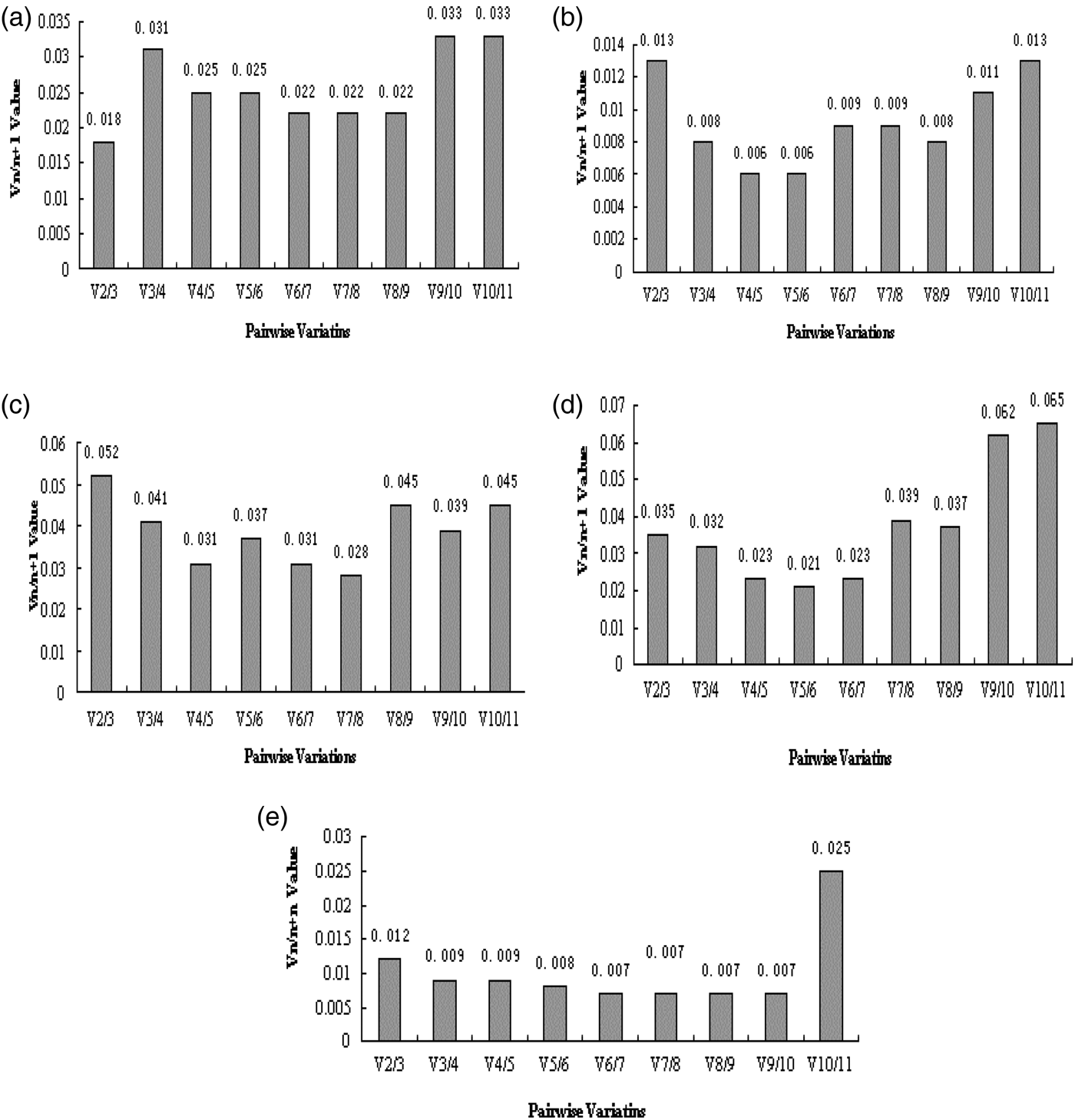
Fig. 2. Optimal number of reference genes for pairwise variation analysis (Vn/n + 1) of N. barkeri under different experimental conditions by GeNorm. (a) Developmental stages; (b) acaricides stress; (c) temperature stress; (d) UV irradiation stress; (e) prey stress.
Table 2. The expression stability ranking of candidate reference genes of N. barkeri in developmental stages.
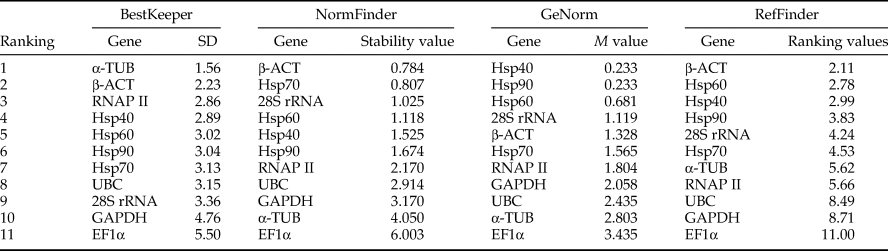
The 11 candidate reference genes of N. barkeri exposed in the acaricides analyzed by GeNorm showed that the mean stability values (M values) from the lowest to the highest was: RNAP II = Hsp40 > Hsp90 > Hsp60 > GAPDH > Hsp70 > UBC > EF1α > β-ACT > α-TUB > 28S rRNA. These results indicated that the RNAP II and Hsp40 genes were most stable, followed by the Hsp90 and Hsp60 genes, whereas the 28S rRNA gene was least stable. When we use NormFinder to analyze genes expression stability, it indicates that the lower the stable value the more the stability of genes. The top five genes of stable expression were Hsp60, Hsp90, GAPDH, Hsp70, and RNAP II, but the most stable genes were Hsp60 and Hsp90. In addition, BestKeeper analysis determined that the SD of the candidate reference genes, except 28S rRNA (SD = 1.86), was <1.0, consistent with the requirements of a single reference gene. Among them, Hsp60 was the most stable (Table 3). According to the rank of RefFinder and pairwise variation V2/3(0.013) in GeNorm, as shown in fig. 2b, which was significantly below the critical cut-off value of 0.15, two of the candidate reference genes were suitable for RT-qPCR. Introduction of a third reference for standardization was unnecessary because it did not significantly increase the statistical reliability. Therefore, Hsp60 and Hsp90 were recommended as reference genes in studies of acaricides stress in N. barkeri.
Table 3. The expression stability ranking of candidate reference genes of N. barkeri under acaricides stress.

For temperature stress, GeNorm showed that the M values from the lowest to the highest was: Hsp40 = α-TUB > β-ACT > EF1α > Hsp70 > Hsp90 > GAPDH > Hsp60 > UBC > RNAP II > 28S rRNA. This result indicated that Hsp40 and α-TUB were relatively stable. Meanwhile, the NormFinder obtained a similar result to those of the GeNorm analysis (Table 4). BestKeeper indicated that the Hsp60 and UBC may be used as a single reference gene. In addition, according to the rank of RefFinder and pairwise variation V2/3(0.052) of GeNorm, as shown in fig. 2c, which was significantly below the critical cut-off value of 0.15, Hsp40 and α-TUB were recommended for use as reference genes in gene expression studies with temperature stress in N. barkeri.
Table 4. The expression stability ranking of candidate reference genes of N. barkeri under temperature stress.

For UV irradiation stress, GeNorm analysis showed that the M values for all of the genes were >0.5, which were not consistent with the requirements of a single reference gene. However, EF1α and β-ACT demonstrated minimal M values. BestKeeper and NormFinder obtained similar results that the genes EF1α, β-ACT, and Hsp60 were relatively stable as single reference genes (Table 5). Finally, we utilized an online analysis system RefFinder to evaluate the expression stable in UV irradiation stress. It also found out that β-ACT and EF1α were the most two stable genes. As shown in fig. 2d, the pairwise variation from GeNorm showed us V2/3 (0.035) which was significantly below the critical cut-off value of 0.15. Therefore, EF1α and β-ACT were recommended the most two stable reference genes under UV stress in N. barkeri.
Table 5. The expression stability ranking of candidate reference genes of N. barkeri under UV irradiation stress.

For prey treatments, GeNorm analysis showed that the M values for genes except β-ACT and α-TUB were <0.5, which did meet the requirements of a single reference gene. However, BestKeeper pointed out the SD values for all the genes except UBC and RNAP II were <1.0, β-ACT and α-TUB owned the least stability values. As shown in Table 6, NormFinder analysis results also showed that β-ACT and α-TUB were the two most stable genes. Finally, utilizing the rank of RefFinder and pairwise variation values V2/3 (0.012) of the GeNorm (fig. 2e), β-ACT and TUB are recommended as reference genes in studies of the effects of prey stress in gene expression for N. barkeri.
Table 6. The expression stability ranking of candidate reference genes of N. barkeri under prey stress.

Discussion
Identification of suitable reference genes is a very important process for studying gene expression in N. barkeri under different external stimuli conditions. In this study, the four evaluation methods, GeNorm (Vandesompele et al., Reference Vandesompele, De Preter, Pattyn, Poppe, Roy, De Paepe and Speleman2002), NormFinder (Andersen et al., Reference Andersen, Jensen and Ørntoft2004), BestKeeper (Pfaffl et al., Reference Pfaffl, Tichopad, Prgomet and Neuvians2004), and RefFinder (Xie et al., Reference Xie, Sun, Stiller and Zhang2011), were used to systematically evaluate the expression stability of 11 candidate reference genes in N. barkeri at different developmental stages and under abiotic stresses. Meanwhile, this work was the first systematic analysis on reference genes selection for RT-qPCR normalization in N. barkeri under these conditions. However, the present and previous experiments found that some of the different results generated by various software were connected with their different calculation methods. NormFinder uses the intra-group and inter-group variance to calculate the stable value, and GeNorm ranks candidate reference genes according to the highest degree of expression similarity in their expression profile. In a sense, the method of NormFinder is to cover the shortage of co-regulation for GeNorm. Whereas BestKeeper analysis is a pairwise correlational analysis based on C t values. BestKeeper can be used to reflect the original data, but it is less effective than either GeNorm or NormFinder analysis in ranking the reference genes. Based on the stabilities it is usually used in preliminary screening. RefFinder is a web-based calculation tool comprehensively ranking the genes using the above-mentioned algorithms. By analyzing with above tools, we found that β-ACT and Hsp40 were the top two stable reference genes in different developmental stages of mites. We also concluded that Hsp60 and Hsp90 were the most stable reference genes for proper RT-qPCR analysis of gene expression under various acaricides stresses. For alterations in temperature, Hsp40 and α-TUB were the most suitable reference genes. About UV stress, EF1α and α-TUB were the best choice, and for different prey stresses, β-ACT and α-TUB were best suited. Further comprehensive analysis found that β-ACT and α-TUB were the two of the highest stable reference genes to respond to all kinds of stresses, which were recommend reference genes under different conditions. More and more evidence shows that there are no universal reference genes that are suitable for all the experimental conditions of all species. For example, GAPDH and Ribosomal protein 49 were found to be the best reference genes in investigation of gene expression across both experimental conditions of Tetranychus urticae (Mariany et al., Reference Mariany, Bianca, Laura, Mark, Douglas and Zhu2016); for Tetranychus cinnabarinus, ribosomal protein S18 and 5.8S ribosomal RNA can be considered as the best reference genes in different acaricides resistant strains, RPS18 and α-TUB as in different developmental stages (Sun et al., Reference Sun, Jin, He, Lu and Li2010); EF1α, RNAP II,α-TUB, and GAPDH are the most stable reference genes in various developmental stages of P. citri; meanwhile, EF1α and GAPDH were the most stable reference genes under various abiotic stresses (Niu et al., Reference Niu, Dou, Ding, Yang, Shen and Wang2012). Furthermore, in the same species, the best reference gene sets may vary significantly under different biotic and abiotic conditions (De Boer et al., Reference De Boer, De Boer, Mariën, Timmermans, Nota, Van Straalen, Ellers and Roelofs2009; Niu et al., Reference Niu, Dou, Ding, Yang, Shen and Wang2012; Mariany et al., Reference Mariany, Bianca, Laura, Mark, Douglas and Zhu2016).
EF1α is a protein factor promoting the elongation of the polypeptide chain during mRNA translation. It was identified as one of the most stably expressed reference genes in several other arthropod species, including Apis mellifera, P. citri, Orchesella cincta, and Leptinotarsa decemlineata (Lourenco et al., Reference Lourenco, Mackert, Cristino and Simoes2008; De Boer et al., Reference De Boer, De Boer, Mariën, Timmermans, Nota, Van Straalen, Ellers and Roelofs2009; Zhu et al., Reference Zhu, Xu, Palli, Ferguson and Palli2011; Niu et al., Reference Niu, Dou, Ding, Yang, Shen and Wang2012). It is stably expressed in most organisms and under different treatment conditions, and thus is considered an ideal reference gene (Ponton et al., Reference Ponton, Chapuis, Pernice, Sword and Simpson2011; Lopez-Pardo et al., Reference Lopez-Pardo, de Galarreta and Ritter2013). Through the use of several different calculation methods and algorithms, the present study found that EF1α had the least stability throughout the developmental stages of mites. Although inconsistent with most of the reports about EF1α relatively stable in developmental stages (Mamidala et al., Reference Mamidala, Rajarapu, Jones and Mittapalli2011; Bansal et al., Reference Bansal, Mamidala, Mian, Mittapalli and Michel2012; Niu et al., Reference Niu, Dou, Ding, Yang, Shen and Wang2012), we found that EF1α was highly stable under UV stress and moderately expressed under other treatments. Therefore, it is speculated that EF1α in N. barkeri is relatively stable under various environmental stresses. However, it is not suitable for use as a reference gene in mites across different developmental stages.
β-ACT is a widely used reference gene and is considered to be more stable than other internal controls in Aphis gossypii, Spodoptera litura, and L. decemlineata (Zhu et al., Reference Zhu, Xu, Palli, Ferguson and Palli2011; Lu et al., Reference Lu, Yuan, Gao, Kang, Zhan, Hu and Li2013; Ma et al., Reference Ma, Li, Liang, Chen, Liu and Gao2016). But, it is also proposed that β-ACT is not suitable as an endogenous control for gene expression analysis in some treatment conditions (Shen et al., Reference Shen, Huang, Jiang, Dou and Wang2013). Toutges et al. and Lord et al. also reported similar results for β-ACT (Lord et al., Reference Lord, Hartzer, Toutges and Oppert2010; Toutges et al., Reference Toutges, Hartzer, Lord and Oppert2010). By contrast, the present study found that β-ACT was most stably expressed in N. barkeri under various stresses. It was highly expressed at different developmental stages and under temperature, UV, and prey treatments, and therefore could function as a reference gene for study about gene expression in N. barkeri at different developmental stages and under various environmental stresses.
Tubulin is a widely distributed globular protein functioning as structural protein to maintain the integrity of the cytoskeletal structure (Scharlaken et al., Reference Scharlaken, De Graaf, Goossens, Brunain, Peelman and Jacobs2008; De Boer et al., Reference De Boer, De Boer, Mariën, Timmermans, Nota, Van Straalen, Ellers and Roelofs2009). Tubulin is widely used as a reference gene in qPCR studies because it is one of the basic components of the cells (Shen et al., Reference Shen, Jiang, Wang and Wang2010; Wan et al., Reference Wan, Zhao, Qian, Sui, Malik and Chen2010). We found that the expression stability of α-TUB and β-ACT were relatively similar. They both demonstrated well stability under all various stresses, except the acaricides stress. They were the most stable genes under prey stress. Meanwhile, they were also stable reference genes in developmental stages and under various environmental stresses. However, some studies have found that α-TUB expression is not stable in different tissues or in different environments for some species. For example, α-TUB is very unstable in the lower lip line and fat body of Bombus terrestris and Bombus lucorum (Horňáková et al., Reference Horňáková, Matoušková, Kindl, Valterová and Pichová2010), in potato tubers under cold stress (Lopez-Pardo et al., Reference Lopez-Pardo, de Galarreta and Ritter2013), and in different tissues of tilapia and under different treatments (Yang et al., Reference Yang, Wang, Tian, Liu, Wu, Jiang and Wen2013). Taken together, these observations indicate that α-TUB is susceptible to various factors. Therefore, the stability of α-TUB should be verified when it is selected as a reference gene in other species.
In the Hsp family, Hsp60 and Hsp90 under acaricides stress as well as Hsp40 under temperature stress were stably expressed which can be used as reference genes for these stress conditions. The other genes of this family showed low or moderate expression under all various stresses and, therefore, were not suitable as reference genes for these stresses, especially the Hsp70. Our results for Hsp70 were similar to those of De Boer et al. (Reference De Boer, De Boer, Mariën, Timmermans, Nota, Van Straalen, Ellers and Roelofs2009) and Veazey & Golding (Reference Veazey and Golding2011).
The 28S rRNA demonstrated low stability under various stress conditions and was least stable under acaricides and temperature stress with C t values of 16.45–37.79 and 14.19–37.22, respectively. The result indicated that the stability of gene expression has some difference and might not be an ideal reference gene. These findings were consistent with that of Xu et al. (Xu et al., Reference Xu, Xu, Zhu, Luo, Qian, Ji, Hu, Sun, Wang, Song, Sun and Chen2014). It was also reported that 18S rRNA and 5.8S rRNA were unstably expressed under specific experimental conditions (Bas et al., Reference Bas, Forsberg, Hammarström and Hammarström2004; Mehta et al., Reference Mehta, Birerdinc, Hossain, Afendy, Chandhoke, Younossi and Baranova2010; Niu et al., Reference Niu, Dou, Ding, Yang, Shen and Wang2012; Li et al., Reference Li, Xie, Wang, Wu, Yang, Yang, Pan, Zhou, Bai, Xu, Zhou and Zhang2013). Shen et al. reported in 2013 that the rRNA genes might not be the best choice as reference genes and suggested that if rRNA is used as a reference gene to standardize qPCR data, its stability should be evaluated first (Shen et al., Reference Shen, Huang, Jiang, Dou and Wang2013).
GAPDH is considered by some reports to be a stable reference gene (Scharlaken et al., Reference Scharlaken, De Graaf, Goossens, Brunain, Peelman and Jacobs2008; Niu et al., Reference Niu, Dou, Ding, Yang, Shen and Wang2012). In the present study, the results of BestKeeper and NormFinder showed that GAPDH could be used as a single reference gene for studies utilizing acaricide or prey stress. However, the results of other analyses indicated that GAPDH would not be an ideal reference gene under the other stresses. These results are consistent with those of Bagnall and Kotze and Tong et al. (Tong et al., Reference Tong, Gao, Wang, Zhou and Zhang2009; Bagnall & Kotze, Reference Bagnall and Kotze2010). In general, RNAP II and UBC showed low expression stability for all stresses in this study, demonstrated the least stability with prey stresses. Therefore, these two genes were not considered as stable reference genes. Their low stability may be species-specific, some species showing unique physiological characteristics so that the same stress herein leads to different results. The previous reports showed that these genes have different expression levels even within the same organism at different developmental stages (De Boer et al., Reference De Boer, De Boer, Mariën, Timmermans, Nota, Van Straalen, Ellers and Roelofs2009). Thellin et al. and Bustin also believe that the differential expression of these candidate reference genes is related to the tissue type and physiological status (Thellin et al., Reference Thellin, Zorzi, Lakaye, Hennen, Coumans, Hennen, Grisar, Lgout and Heinen1999; Bustin, Reference Bustin2000).
Through this research, we not only selected the most stable reference genes for RT-qPCR analysis of gene expression in N. barkeri, but also provided a reliable resource to analyze other mites in gene expression studies. However, we also highly recommend to notarize stable expression of candidate reference genes in order to obtain perfect date of RT-qPCR in further studies for N. barkeri.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S000748531800072X
Acknowledgments
This research was supported by the National Key Research and Development Program (2018YFD0201508), the National Agricultural Innovation Project (CAAS-XTCX-2016013), and the Chongqing Scientific Research Project (cstc2016shms-ztzx80003, cstc2017shms-xdny0443).











