Introduction
In West Africa, cereals are attacked by a complex of lepidoteran stemborers (Bosque-Pérez & Schulthess, Reference Bosque-Pérez, Schulthess and Polaszek1998). While economic losses to rice and sorghum are rare, severe infestations occur on maize, particularly in areas with a bimodal rainfall distribution (Schulthess et al., Reference Schulthess, Bosque-Perez, Chabi-Olaye, Gounou, Ndemah and Goergen1997a). Yield reductions of 10–70% due to the noctuid Sesamia calamistis Hampson and the pyralid Eldana saccharina (Walker) occur as a result of leaf feeding, stem tunnelling and direct damage to grain (Bosque-Pérez & Mareck, Reference Bosque-Pérez and Mareck1991; Cardwell et al., Reference Cardwell, Schulthess, Ndemah and Ngoko1997; Sétamou et al., Reference Sétamou, Schulthess, Poehling and Borgemeister2000; Chabi-Olaye et al., Reference Chabi-Olaye, Nolte, Schulthess and Borgemeister2005a). Losses are aggravated by poor soil fertility (Sétamou et al., Reference Sétamou, Schulthess, Bosque-Pérez and Thomas-Odjo1995; Chabi-Olaye et al., Reference Chabi-Olaye, Nolte, Schulthess and Borgemeister2005b).
Biological control (BC) of lepidopterous stem and cob borers has been proposed as a major component of integrated pest management (IPM) strategies in Africa (Greathead, Reference Greathead1971; Mohyuddin et al., Reference Mohyuddin, Inayatullah and King1981; Mohyuddin, Reference Mohyuddin1991). For the indigenous pest species, the exchange (or ‘redistribution’) of natural enemies between regions has been proposed (Rao, Reference Rao1965; Schulthess et al., Reference Schulthess, Bosque-Perez, Chabi-Olaye, Gounou, Ndemah and Goergen1997a) in addition to ‘new association’ BC, which entails the use of non-coevolved natural enemy species from closely related hosts occupying similar ecological niches in different geographic areas (Hokkanen & Pimentel, Reference Hokkanen and Pimentel1984; Schulthess et al., Reference Schulthess, Bosque-Perez, Chabi-Olaye, Gounou, Ndemah and Goergen1997a). These approaches have led to the identification of several promising biocontrol candidates. For example, the braconid Cotesia sesamiae (Cameron) is the most common parasitoid of B. fusca and S. calamistis larvae in East and southern Africa (Greathead et al., Reference Greathead, Cock and Girling1986; Kfir & Bell, Reference Kfir and Bell1993; Kfir, Reference Kfir1995, Reference Kfir and Polaszek1998; Zhou et al., Reference Zhou, Overholt and Kimani-Nnjogu2003). In contrast, in surveys in West African countries and Cameroon, C. sesamiae was very rarely found (Bosque-Pérez et al., Reference Bosque-Pérez, Ubeku and Polaszek1994; Moyal, Reference Moyal1998; Conlong, Reference Conlong2001; Ndemah et al., Reference Ndemah, Schulthess, Poehling and Borgemeister2001). It is not known if this is due to differences in host suitability. For example, in Kenya and Zimbabwe, C. sesamiae has been reported to exist as two biotypes, one of which sucessfully parasitizes both B. fusca and S. calamistis (virulent) while the eggs of the other are completely encapsulated in B. fusca (avirulent) (Ngi-Song et al., Reference Ngi-Song, Overholt and Stouthamer1998; Mochiah et al., Reference Mochiah, Ngi-Song, Overholt and Stouthamer2002; Chinwada et al., Reference Chinwada, Overholt, Omwega and Mueke2003).
Three Cotesia species, the exotic C. chilonis (Matsumura) and C. flavipes Cameron and an avirulent coastal Kenyan strain of the native African C. sesamiae, were introduced from the International Centre of Insect Physiology and Ecology (ICIPE), Nairobi, Kenya, into the laboratories of the International Institute of Tropical Agriculture (IITA) in Benin for suitability testing on West African stemborer species. Characteristics that determine whether biological control agents have an impact on the population dynamics of pests and make them efficient natural enemies for biological control programs have often been discussed (Doutt & DeBach, Reference Doutt, DeBach and DeBach1964; Waage & Greathead, Reference Waage and Greathead1998). In the present study, we investigated the resource allocation and bionomics of C. chilonis, C. flavipes and C. sesamiae using S. calamistis as host. More precisely, the study addressed the question of how much of the variation in parasitoid survival, brood size, sex ratio, developmental time and life table parameters could be explained by host size and quality.
Materials and methods
Parasitoid and host culture
The parasitoids were reared using S. calamistis as hosts. S. calamistis larvae were obtained from laboratory cultures maintained on artificial diet for 5–6 generations according to protocols described by Bosque-Pérez & Dabrowski (Reference Bosque-Pérez and Dabrowski1989).
C. chilonis originated from field-collected Chilo suppressalis (Walker) (Lepidoptera: Crambidae) from rice, Oryza sativa L., in Niigata, Japan, that were transferred to ICIPE via Texas A&M University Entomology Quarantine (Okech & Overholt, Reference Okech and Overholt1996). C. flavipes originated from C. partellus on maize, in Rawalpindi, Pakistan (Overholt et al., Reference Overholt, Ochieng, Lammers and Ogedah1994). C. sesamiae was reared from S. calamistis collected on maize from the coastal zone of Kenya (Ngi-Song et al., Reference Ngi-Song, Overholt and Ayertey1995). Prior to shipment to IITA, laboratory cultures of C. chilonis and C. flavipes were maintained on C. partellus, and C. sesamiae on S. calamistis at ICIPE for several generations. In Benin, all three parasitoids were cultured on S. calamistis. Larvae were allowed to feed and produce frass on fresh maize stem for 0.5 h prior to parasitoid exposure. Details of the parasitoid rearing protocol are as described by Ngi-Song et al. (Reference Ngi-Song, Overholt and Ayertey1995).
Identification of host instar larvae and parameters used to characterize successful parasitism
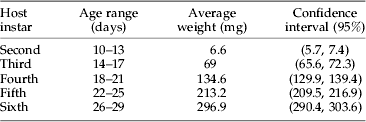
Preliminary experiments showed that the parasitoids did not accept first instar larvae. Thus, 2nd through 6th instar larvae were used in the study. Larvae were harvested from the stock colony and sorted using size and head capsule widths to identify larval instars (Bonato & Schulthess, Reference Bonato and Schulthess1999). Each individual was weighed. Weight groups within an instar were selected and then individuals within an age group were chosen at random for experimental use (table 1). Selected larvae were maintained separately in Petri dishes.
Table 1. Age range and weight of 2nd to 6th instar of Sesamia calamistis.
To study the oviposition behaviour of Cotesia species and confirm successful parasitism, a hand-stinging method was used (Overholt et al., Reference Overholt, Ochieng, Lammers and Ogedah1994) in which 30 female parasitoids were transferred separately into transparent plastic Petri dishes (100×15 mm) arenas, and each offered a host larva for parasitism. Stung larvae were immediately removed and placed on an artificial diet for parasitoid development. Oviposition was considered successful when the Cotesia female inserted her ovipositor, raised her wings perpendicular to her body and the antennae were extended parallel to her body. Twenty-three hosts were dissected subsequently to exhibiting such behaviour by an ovipositing female. The behaviour was positive for parasitoid eggs in 97% of cases. This stereotyped parasitization behaviour and subsequent oviposition success were similar across parasitoid species. Thus, in subsequent experiments a host was considered ‘parasitized’ if the parasitoid expressed this behaviour.
Except for temperature-dependent survival and development, all experiments were conducted at 27±1°C, 50–90% RH and a photoperiod of 12:12 (L:D).
Effect of host age on parasitoid survival, brood size, sex allocation and developmental time
Second to 6th instar larvae were offered individually for parasitism to a single, naïve, mated Cotesia female in a transparent Petri dish (10 cm height×1.5 cm diameter). The host-parasitoid interaction was continuously observed for parasitism. Parasitized larvae were removed from the arena and individually reared in plastic vials containing an artificial diet. For each host age, 30–40 parasitized and 20 unparasitized larvae were reared. Data gathered were: % parasitized hosts that successfully produced parasitoid cocoons, % parasitized hosts that died, % parasitized hosts that pupated, % successfully emergent parasitoid adults, parasitoid brood size, sex ratio (% females in the total brood) and developmental time (days). Mortality was estimated as % hosts that died as larvae or pupae after being parasitized by a female parasitoid
Effect of parasitism on larval growth
This experiment was conducted with second instar larvae confined with either C. chilonis or C. sesamiae. Weights of parasitized and unparasitized second instar larvae were recorded at 0, 24, 48, 72, 120, 168, 216 and 264 h post parasitism. Thereafter, individual larvae were immediately transferred to a fresh artificial diet. Measurements were terminated when either the host pupated, died or parasitoid cocoons were produced.
Temperature dependent survival and development
The effect of constant temperature regimes (19, 25, 28, 30, 31, 32 and 34°C) on the developmental rates and survival of C. chilonis, C. flavipes and C. sesamiae were tested using fourth instar S. calamistis as hosts. For each temperature, the relative humidity varied between 50–90%.
A host larva was continuously observed for parasitization by each naïve, mated female of Cotesia species in a Petri dish (10 cm height×1.5 cm diameter) at 28°C. Host larvae scored positive for parasitoid attack were transferred to a fresh artificial diet and randomly assigned to one of the seven constant temperature regimes. For each temperature regime, 50–65 parasitized and 30 unparasitized larvae were reared. Unparasitized larvae were used as a control to estimate the background mortality. Percentage of parasitized hosts that successfully produced parasitoid cocoons, % parasitized hosts that died, % parasitized hosts that pupated, % successfully emergent parasitoid adults, parasitoid brood size, sex ratio and egg-to-adult developmental time (days) were recorded. Data gathered on the egg-to-adult developmental time were used to estimate the lower developmental threshold for each Cotesia species and to describe the relationship between developmental rate and temperature.
Effect of constant temperature on the reproductive potential of three Cotesia species
Life table studies of C. chilonis, C. flavipes and C. sesamiae were carried out at four constant temperatures (19, 25, 28 and 30°C) using S. calamistis as the host. Naïve, mated parasitoid females were offered fourth instar larvae in a short tunnel bored into one end of a piece of maize stem with a 1:1 parasitoid to host ratio. Parasitism was expected when the frass was thrown out of the tunnel by the struggling larvae. Thereafter, larvae were removed and a new infested stem piece was offered until the parasitoid died. Parasitized larvae were reared on an artificial diet until the production of parasitoid cocoons or host pupae. Parasitoid females used for this experiment were continuously fed honey streaks in a plastic container (11.6 cm length×4.6 cm diameter). The procedure was replicated four times with a cohort of 20 females for each parasitoid-host pairing. Female longevity was determined and for each parasitoid female, the total progeny produced was counted and sexed. Data gathered were used to compute the life table statistics of parasitoid species.
Statistical analyses
Differences in parasitism, developmental time, mortality, total progeny, sex ratio and life history parameters between host instar larvae, Cotesia species or temperature were analysed by analysis of variance (ANOVA) using the general linear model (GLM) procedure of SAS for PC (SAS Institute, 1989). Percentages were transformed to arcsine values before analysis. F statistics from type III sums of squares were used for tests of significance and means were separated using the Tukey test. The significance level was set at P=0.05.
For estimation of the lower developmental threshold (T0=intercept/slope) and the thermal constant (K=slope−1=the number of day-degrees to complete the pre-reproductive phase), a simple regression over the linear range of the relationship between temperature (T) and developmental rates [(R(T)=(developmental time)−1] of the parasitoids was done (Campbell et al., Reference Campbell, Frazer, Gilbert, Gutierrez and Mackauer1974).
A modified Logan model (Logan et al., Reference Logan, Wolikind and Hoyt1976) by Lactin et al. (Reference Lactin, Holliday, Johnson and Craigen1995) was used to describe the relationship between temperature and developmental rate:
where T is the temperature in (°C), and ρ, Tmax, Δ and λ are fitted coefficients.
Life table statistics were calculated according to Hulting et al. (Reference Hulting, Orr and Obrycki1990) using the Jackknife program. Differences in rm (intrinsic rate of increase) and Ro (net reproduction rate) values among populations were calculated following the protocol of Dixon (Reference Dixon, Minks and Harrewijn1987) and compared with Newman-Keuls tests (Sokal & Rohlf, Reference Sokal and Rolf1995) based on jackknife estimates of variance for rm values (Meyer et al., Reference Meyer, Ingersoll, McDonald and Boyce1986). Growth of parasitized and unparasitized larva was described by fitting the data to the equation of Sequeira & Mackauer (Reference Sequeira and Mackauer1992) using nonlinear least squares regression:
where, H is the live mass in milligrams (mg) of parasitized and unparasitized larvae, t is days after parasitisation and a, b and c are fitted coefficients. The cumulative growth index was determined by dividing the change in growth by the time interval:
where, Hi is the weight of the second instar larva (prior to parasitism), Hf is the maximum weight prior to parasitoid cocoon formation and G is the cumulative growth index for the immature parasitoid stage. The relationship between days after treatment and cumulative growth index was analyzed using linear least squares regression. Spearman's correlation was used to describe the relationship between cumulative growth index and parasitoid brood and sex ratio.
All the fitted coefficients were estimated using the linear or non linear model (proc reg and proc nlr, respectively) procedure of SAS for PC (SAS Institute, 1989).
Results
Effect of host age on parasitoid survival, brood size, sex allocation and developmental time
Parasitoid-induced host mortality varied with parasitoid species (F=5.38; df=2, 69; P=0.0068) and host instars (F=5.94; df=4, 69; P=0.0004), but the parasitoid species×host instars interaction was not significant (F=1.9; df=8, 69; P=0.0743) (fig. 1). Second instars appeared to be more affected by parasitoid-induced mortality than the other host instars (fig. 1). For all Cotesia species, average host mortality was >35% with second instar larvae, whereas for the 3rd–6th host instars, the highest mortality was <38%. C. flavipes induced host mortality in all instars, but no mortality was found in the third instar with C. chilonis and C. sesamiae (fig. 1). Percentage of hosts that produced parasitoid cocoons varied significantly among parasitoid species (F=7.37; df=2, 107; P=0.001) and host instars (F=9.55; df=4, 107; P=0.0001). Average % host larvae that yielded cocoons after parasitism by C. chilonis, C. flavipes and C. sesamiae was 47.1, 62.7 and 74.5%, respectively, using the second instar larvae as host. Thereafter, the parasitism increased and peaked at the third instar at 62.7, 74.5 and 82.4%, respectively, and subsequently decreased linearly (YCc=–6.3∗X+75.9, r2=0.97; YCf=–8.4∗X+86.9, r2=0.65; and YCs=–13.7∗X+111.8, r2=0.98; P<0.01) to the sixth instar larvae.

Fig. 1. Effect of S. calamistis instars of larvae on % parasitism, % larvae pupated and larval mortality (%). (□, C. chilonis; ![]() , C. flavipes;
, C. flavipes; ![]() , C. sesamiae.)
, C. sesamiae.)
The percentage of parasitized hosts that failed to produce cocoons but yielded host pupae varied significantly among parasitoid species (F=6.97; df=2, 107; P=0.0014). For all three Cotesia species, the percentage of parasitized hosts that yielded host pupae increased linearly with host instar (X): YCc=6.2∗X+21.6, r2=0.94; YCf=7.1∗X+12.4, r2=0.97; and YCs=11.2∗X–2.2, r2=0.94; P<0.001).
The total brood size varied significantly with Cotesia species (F=39; df=2, 613; P=0.0001) and host instars (F=12.8; df=4, 613; P=0.0001) with significant interactions between parasitoid species and host instars (F=4.4; df=8, 613; P=0.0001). Across all host instars, C. sesamiae and C. flavipes produced larger broods than C. chilonis, except with the fourth instar larvae, where there was no significant difference in brood size between C. flavipes and C. chilonis (P>0.05) (fig. 2). The third instar larvae yielded the highest brood size of C. flavipes (80.4±7.8) and C. sesamiae (66.7±5.9).

Fig. 2. Effect of S. calamistis instars larvae on total brood size, sex ratio and developmental time. (![]() , C. chilonis;
, C. chilonis; ![]() , C. flavipes;
, C. flavipes; ![]() , C. sesamiae.)
, C. sesamiae.)
Sex ratio of progeny varied significantly among parasitoid species (F=14.72; df=2, 604; P=0.0001) and host instars (F=10.33; df=4, 604; P=0.0001) with a significant interaction between parasitoid and host instars (F=2.26; df=8, 604; P=0.0223). Second instar larvae parasitized by C. chilonis larvae yielded a significantly (P<0.001) lower sex ratio than those parasitized by C. flavipes and C. sesamiae (fig. 2). C. flavipes had the highest sex ratio when parasitizing fourth instar larvae. For C. sesamiae and C. chilonis, the highest sex ratio was recorded with the 5th and 6th instar larvae, respectively. But, there was no significant difference in sex ratio among parasitoids species with 3rd, 5th and 6th instars larvae (fig. 2).
Egg-adult developmental time of parasitoids species varied significantly among Cotesia species (F=135.8; df=2, 322; P=0.0001) and host instars (F=99.3; df=4, 322; P=0.0001). Across host age, C. chilonis developed faster than the other two Cotesia species (fig. 2). Using the second instar larvae, the developmental time of C. chilonis was 1.5 times faster than that of C. flavipes and C. sesamiae; while with 3rd–6th instar larvae, the difference in developmental time between C. chilonis and the other two Cotesia species was reduced by 23–28% (fig. 2).
Influence of parasitism on larval growth
The wet mass of live unparasitized and parasitized larvae taken at different days after parasitization was fitted to a non-linear regression. (For unparasitized larvae, Y=234/(1+exp(3.981–0.51∗tH)); for larvae parasitized by C. chilonis, YCC=92/(1+exp(3.566–0.72∗tH)); for larvae parasitized by C. sesamiae, YCS=345/(1+exp(3.883–0.33∗tH)); fig. 3). Comparisons of weight gain by parasitized and unparasitized larvae revealed that larvae parasitized by C. sesamiae reached a greater weight than unparasitized larvae (P<0.05, one-tailed t-test); whereas, larvae parasitized by C. chilonis exhibited lower weight compared to the unparasitized control (P<0.05, one-tailed t-test). This observation was consistent with the number of molts of larvae parasitized by each parasitoid; second instar larvae parasitized by C. sesamiae molted to the sixth instar and attained an average larval weight of 353 mg within 17 days after parasitization. Second instar larvae parasitized by C. chilonis only reached the fourth instar and attained a maximum weight of 107 mg within ten days after parasitization.

Fig. 3. Growth curve of nonparasitized S. calamistis larvae and parasitized larvae by C. chilonis and C. sesamiae.
The cumulative growth index (Y) of larvae parasitized by C. sesamiae increased linearly with days after parasitization (X) (YCS=3.12+0.812∗X, r 2=0.88, P=0.0002); for C. chilonis YCC=5.40+0.297∗X, r 2=0.24, P=0.330.
The net weight gain by parasitized larvae was significantly positively correlated with parasitoid brood size (r=0.68 and 0.89 for C. chilonis and C. sesamiae, respectively, P<0.02). There was no relationship between larval weight gain and sex ratio (r=0.31, P=0.35 for C. chilonis; and r=0.20, P=0.38 for C. sesamiae).
Effect of constant temperature on the parasitism, survival and development of three Cotesia species
The percentage of parasitized hosts that produced parasitoid cocoons, died or pupated varied significantly (P<0.05) with temperature (fig. 4). For all Cotesia species, % parasitized hosts that produced parasitoid cocoons were >50% at all temperatures except 32°C, and the highest rate was recorded at 28°C. At 32°C, host larvae parasitized by C. chilonis and C. sesamiae did not produce any cocoons, but <30% of host larvae produced cocoons for larvae parasitized by C. flavipes (fig. 4).

Fig. 4. Effect of constant temperature on the parasitization of S. calamistis larvae. (![]() , % Parasitized; ■, % Died; □, % Pupated.)
, % Parasitized; ■, % Died; □, % Pupated.)
Between 19–30°C, larval mortality after parasitization was higher with C. flavipes compared to C. chilonis and C. sesamiae (fig. 4). For all parasitoids species, the highest % parasitized hosts that died was found at 32°C. Between 28–30°C, no mortality was recorded for larvae parasitized by C. chilonis and C. sesamiae.
For all parasitoid species, the highest % parasitized hosts that pupated was found at 32°C; pupation was higher with C. chilonis and C. sesamiae than with C. flavipes between 19–30°C.
There were no significant differences (P>0.05) in developmental time of male and female parasitoids, thus data gathered on developmental time were pooled across sexes for further analyses. Egg-adult developmental time decreased significantly (table 2) as temperature increased (between 19–30°C for C. chilonis and C. sesamiae and 19–31°C for C. flavipes). Only C. flavipes completed development at 32°C (table 2).
Table 2. Effect of constant temperature on the emergence and egg-adult developmental time (mean±SE) of three Cotesia species.

Within a column, means followed by different lower case letters are significantly different (comparison between temperatures). Within a row, means followed by different capital letters are significantly different (comparison between species), P⩽0.05 (t-test).
Effect of constant temperature on the developmental rate of three Cotesia species
Developmental rates increased linearly between 19–30°C for C. chilonis and C. sesamiae and between 19–31°C for C. flavipes (fig. 5). The lower developmental threshold calculated from the linear regression of temperature (T) on developmental rate was 14.3, 13.8 and 13.8°C for C. chilonis, C. sesamiae and C. flavipes, respectively; and the thermal requirement for completion of the pre-reproductive phase was 212.8, 238.1 and 222.2 degree-days above the lower developmental threshold.

Fig. 5. Effect of temperature on the developmental rate of C. chilonis, C. flavipes and C. sesamiae, using S. calamistis as host.
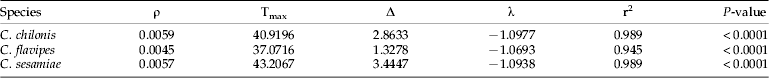
The modified Logan model gave a good fit to the data sets for all parasitoid species within the lower and upper threshold (r 2>0.94, P<0.0001; fig. 5, table 3). The fitted parameters of the model were estimated and presented in table 3. The lower developmental threshold estimated from the non-linear regression of temperature (T) on developmental rate was 15.9, 15.9 and 14.9°C for C. chilonis, C. sesamiae and C. flavipes, respectively; and the upper lethal temperature was 34.2, 35.2 and 33.8°C for C. chilonis, C. sesamiae and C. flavipes, respectively.
Table 3. Fitted coefficients of a modified Logan model (Logan et al., Reference Logan, Wolikind and Hoyt1976) by Lactin et al. (Reference Lactin, Holliday, Johnson and Craigen1995).
Effect of constant temperature on the reproductive potentials of three Cotesia species, using S. calamistis as host
There was no significant (P>0.05) difference in the number of larvae attacked among Cotesia species. A maximum of four ovipositions were observed per female during her entire life span ranging from 1.3 days for C. chilonis and C. flavipes to 1.6 days for C. sesamiae. The highest number of adult progeny was recorded at 28°C for C. chilonis and C. flavipes, while the numbers of offspring for C. sesamiae were the same at all temperatures tested (table 4). Across temperatures, C. flavipes yielded the highest offspring, following by C. sesamiae and C. chilonis. The sex ratios did not vary significantly with species and temperature (table 4). Across temperatures, the first brood of C. chilonis, C. flavipes and C. sesamiae accounted for, respectively, 66.6±4.0, 67.7±3.9, 50.9±3.5% of the total; and the sex ratio was, respectively, 41.7±3.3, 60.4±3.2 and 66.4±3.7% for C. chilonis, C. flavipes and C. sesamiae.
Table 4. Effect of constant temperature on the total progeny and sex ratio (mean±SE) of three Cotesia species.
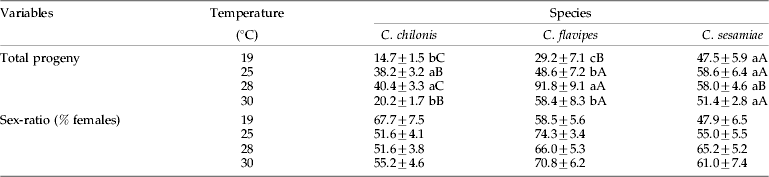
Within a column, means followed by different lower case letters are significantly different (comparison between temperatures); within row, means followed by different capital letters are significantly different (comparison between species), P⩽0.05 (t-test).
The life table parameters varied significantly with temperatures and among parasitoids species (table 5). The intrinsic rate of increase (rm) and net reproductive rate (Ro) increased significantly with temperature and peaked at 28°C. Mean generation time and doubling time decreased linearly between 19–30°C.
Table 5. Effect of temperature on average (±SE) of life table parameters of three Cotesia species.

Within a row, means followed by different lower case letters are significantly different (comparison between temperatures); within column, means followed by different capital letters are significantly different (comparison between species), P⩽0.05 (Student-Newman-Keul test); rm, intrinsic rate of natural increase; R0, net reproductive rate; G, mean generation time (days); DT, doubling time (days); λ, finite rate of increase.
Discussion
Host size has long been recognized as a major attribute of host quality (Salt, Reference Salt1940). Because large hosts contain more resources, they are considered to be of higher quality than small ones (Charnov et al., Reference Charnov, Los-den Hartogh, Jones and van den Assem1981; Charnov, Reference Charnov1982; King, Reference King1987). In the present study, the three Cotesia species attacked and successfully completed development in all larval stages of S. calamistis except for first instars. However, second instars suffered more from parasitoid-induced mortality than 3rd–5th instars. Similarly, Jiang et al. (Reference Jiang, Sétamou, Ngi-Song and Omwega2004) found that parasitoid-induced mortality was higher in third than the fourth larval instar of C. partellus when the host larva was attacked by C. flavipes. In the present study, the proportion of instars that pupated after being parasitized increased linearly with host age at parasitization, indicating encapsulation of parasitoid progeny by the host immune system when older host instars were attacked. According to van Alphen & Drijver (Reference van Alphen and Drijver1982), in younger larvae, the risk of encapsulation is lower because their immune system is not as advanced as that of older larvae. Thus, the high larval mortality, following attack by parasitoid females, observed in second instar hosts is probably due to oviposition associated trauma or from complications that arise subsequent to physiological interaction between the host and the parasitoid (Vinson & lwantsch, Reference Vinson and Iwantsch1980; Boultreau, Reference Boultreau, Waage and Greathead1986). For example, Harvey et al. (Reference Harvey, Harvey and Thompson1994) showed that, when the koinobiont ichneumonid larval parasitoid Venturia canescens (Gravenhorst) attacked second instars of the pyralid Plodia interpunctella Hübner, up to 50% of the larvae died, which was attributed to mutilation of the larval host with insertion and removal of the ovipositor during parasitism. Furthermore, Kajita & Drake (Reference Kajita and Drake1969) and Ngi-Song et al. (Reference Ngi-Song, Overholt and Ayertey1995) reported that host mortality from the act of parasitism was greater when young larval instars were attacked. When assessing the impact of a natural enemy, field parasitism, usually calculated as the percentage of suitable life stages yielding parasitoids, is the most widely used parameter. C. flavipes was released in coastal Kenya for control of the invasive C. partellus in 1993. Borer densities started to decline rapidly at parasitization rates of less than 10%, and they never exceeded 30% (Jiang et al., Reference Jiang, Zhou, Overholt and Schulthess2006). By 2004, pest densities were reduced by 75%. These low parasitism rates cannot explain the impact the parasitoid had on pest populations. However, in view of the high mortality occurring in younger larval instars when attacked by Cotesia spp. found in the present study, basing the success of a parasitoid on parasitism of suitable life stages only would highly underestimate the possible impact a parasitoid can have in the field. Young C. partellus larvae move to the whorl where they either feed on the leaves or disperse to other plants (Päts & Ekbom, Reference Päts and Ekbom1992). Thus, it is hypothesized that in the field, C. flavipes is frequently attacking exposed young larval instars, most of which die or probably drop from the plant. As a result, the cumulative mortality of C. partellus is higher than estimated from successful parasitism alone.
In the present study, larvae parasitized by C. chilonis exhibited a reduced growth trajectory compared to unparasitized larvae. This was also reported by Sequeira & Mackauer (Reference Sequeira and Mackauer1992) for the pea aphid parasitized by Aphidius ervi Halyday. This apparent inability to regulate continued host growth was not evident for larvae parasitized by C. sesamiae. Second instar larvae parasitized by C. sesamiae first continued to grow at the same rate and then surpassed growth of unparasitized larvae, suggesting that the immature parasitoid remained quiescent until the host attained a suitable size before it commenced its destructive attack on the tissue. Polydnaviruses (PDVs) are known to cause developmental arrest in hosts parasitized by braconid parasitoids (Beckage et al., Reference Beckage, Tan, Schleifer, Lane and Cherubin1994; Beckage & Gelman, Reference Beckage and Gelman2004). Recent studies revealed two types of PDV in C. sesamiae in Kenya, one associated with the coastal and one with the inland strain of the parasitoid (Gitau, Reference Gitau2006). The differences in the growth trajectory of S. calamistis caused by the two Cotesia species might have been due to differences in the functional role of PDVs.
In gregarious species, parasitoid fitness is not only affected by host size but also by the number of parasitoid developing in the host (Waage & Godfray, Reference Waage, Godfray, Sibly and Smith1985; Charnov & Skinner, Reference Charnov and Skinner1988; Alleyne & Beckage, Reference Alleyne and Beckage1997). Our findings showed that the net weight gain by parasitized larvae was significantly positively correlated with parasitoid brood size in all the three parasitoids species. However, the second instar host larvae yielded significantly fewer female offspring of C. chilonis compared to C. flavipes and C. sesamiae. Similarly, Wellings et al. (Reference Wellings, Morton and Hart1986) found that the braconid koinobiont A. ervi produced a male-biased sex ratio in smaller hosts. They hypothesed that differential mortality, rather than facultative control of sex ratio, was the main cause of a male-biased sex ratio in smaller hosts, with female offspring less likely to survive in smaller hosts. According to Charnov et al. (Reference Charnov, Los-den Hartogh, Jones and van den Assem1981) and Godfray (Reference Godfray1994), the production of male broods in less fit hosts is an evolutionally adaptation; if small hosts are the result of low plant vigor caused by, for example, low soil fertility or adverse climatic conditions (Schulthess et al., Reference Schulthess, Neuenschwander and Gounou1997b), a female biased sex ratio in small hosts may cause local extinction of both the host and parasitoid. The higher parasitism rates by C. sesamiae compared to C. chilonis may also be a result of a higher acceptance of the host by the former. If this is the case, differences in sex ratio may, indeed, be the results of facultative control of the sex ratio, whereby the parasitoids refuses to oviposit or decides to oviposit mainly unfertilized eggs.
The developmental threshold of C. flavipes was lower than that of C. chilonis and C. sesamiae. However, the percent of adults emerged between 19–30°C was similar in all three Cotesia species. If temperature was the main driving factor determining the performance of a parasitoid, C. flavipes would have a higher ability to adapt to a new area than the other two species. In fact, in Kenya, after its release in 1993, C. flavipes is now recovered from 0 to 2000 m above sea level (Eric Muchugu, ICIPE, Nairobi, Kenya, unpublished data).
The optimal and maximum temperatures obtained from the nonlinear model were very high. However, the thermal constants of the three Cotesia species were considerably (>3 times) shorter than the 761 day-degrees obtained for its host S. calamistis (Shanower et al., Reference Shanower, Schulthess and Bosque-Perez1993). Furthermore, the rm of S. calamistis reported by Sétamou et al. (Reference Sétamou, Schulthess, Bosque-Pérez and Thomas-Odjo1993) were 50–96% at 25°C and 74–97% at 28°C (depending on nitrogen content in stems) of the values calculated for the three Cotesia species. Thus, in terms of thermal requirements and biotic potential, the three Cotesia species are considerably superior to their host S. calamistis. In addition, differences in rm between parasitoid species indicate that C. sesamiae will out-compete both C. flavipes and C. chilonis over the temperature range of 19–30°C with S. calamistis as host. On the other hand, the inability of C. chilonis to regulate continued growth of the parasitized host, coupled with the production of male-biased broods, makes the parasitoid inferior to the other two species, as also indicated by the lowest rm.
All three Cotesia spp. were released in Benin but only C. sesamiae finally established. Based on the results of the present study alone, this could not have been predicted although, in terms of biotic potential only, it was superior to the other two Cotesia species. However, the other main borer species in the region, the pyralid Eldana saccharina Walker, which is unsuitable to all three species (Hailemichael, Reference Hailemichael1998), was accepted for oviposition by both C. flavipes and C. chilonis and, thus, may have acted as a reproductive sink. Consequently, only C. sesamiae is being considered for releases in western Africa. A Kenyan inland strain of C. sesamiae will be released against B. fusca in the humid forest zone and the Western Highlands in Cameroon. The present findings suggest that temperature will not be a limiting factor and that the parasitoid will establish in both ecozones.
The present study investigated the physiological suitability of S. calamistis for the three Cotesia spp., while other studies have examined suitability of other West African stemborers. However, the success of a parasitoid depends, not only on physiological compatibility with its host, but also on the suitability of other acceptable hosts occurring in the system and its ability to find hosts and intrinsic competition within hosts. Additionally, host finding and competition studies may help to better understand the establishment and dynamics of these parasitoids once released in nature.
Acknowledgements
The authors are thankful to Dr G. Goergen, from the Insect Museum of IITA in Cotonou, Benin, who provided identification of the parasitoids.












