Introduction
Grain legumes play a crucial role in agricultural areas with semi-arid climate. They are not only a major source of protein for humans but also a source of fodder and help maintain soil fertility of cereal-based cropping systems because of their ability to fix nitrogen from the atmosphere and tolerance to heat and drought (Graham & Vance, Reference Graham and Vance2003). Subsistence farmers in developing countries profit from the fact that dry grain legume seeds are storable over extended periods and are thus available for consumption or sale throughout the year. Storability is important because dramatic seasonal price variation of many grain legumes means farmer returns can be substantially higher when sales are commenced off-season, when the prices are considerably higher than at harvest (Moussa et al., Reference Moussa, Lowenberg-DeBoer, Fulton and Boys2011).
The most important pests of stored grain legume seeds are bruchid beetles (Coleoptera: Chrysomelidae: Bruchinae). Even low initial infestation rates can cause tremendous damage because of the high fertility and short generation times of bruchid beetles (Southgate, Reference Southgate1979). Most subsistence farmers in developing countries rely on traditional storage structures, which are especially vulnerable to bruchid attacks (van Huis, Reference van Huis1991; Nukenine, Reference Nukenine2010). Application of residual insecticides or fumigants to protect the seeds from bruchids is not reasonable under such circumstances for economic and health reasons (Keneni et al., Reference Keneni, Bekele, Getu, Imtiaz, Damte, Mulatu and Dagne2011). Alternative controls that can be applied include cultural, physical, biological, biorational and genetic measures (van Huis, Reference van Huis1991; Murdock et al., Reference Murdock, Seck, Ntoukam, Kitch and Shade2003; Phillips & Throne, Reference Phillips and Throne2010). However, their effective implementation is often hindered by the lack of equipment and expertise of the farmers, and non-acceptance of newly proposed techniques (van Huis, Reference van Huis1991). The protection against bruchids could be improved by growing varieties featuring an inherent seed resistance to bruchid beetles. Despite intensive conventional breeding efforts, bruchid-resistant varieties of chickpea (Cicer arietinum L.) and cowpea (Vigna unguiculata L.), the predominant grain legumes in the Indian subcontinent and the savanna of tropical Africa, respectively, have not been achieved (Keneni et al., Reference Keneni, Bekele, Getu, Imtiaz, Damte, Mulatu and Dagne2011). Screening of more than 5000 chickpea lines for resistance to Callosobruchus chinensis L. did not reveal any useful resistance (Singh, Reference Singh1997). A similar screening of more than 8000 cowpea lines for resistance to the bruchid Callosobruchus maculatus F. revealed only three lines with moderate resistance, including the promising landrace TVu 2027 (Singh, Reference Singh1977; Singh et al., Reference Singh, Singh and Adjadi1985). However, the moderate resistance of this particular line, for example, lasted only for about 90 days post-infestation (Murdock et al., Reference Murdock, Coulibaly, Higgins, Huesing, Ishiyaku, Sithole-Niang, Kole and Hall2008). This does not meet the requirements of subsistence farmers who need to store the seeds at least until the next sowing season, i.e., for about nine months. Transgenic approaches offer a capable path to obtain varieties with substantially higher resistance than that available in the crop germplasm resources.
Genetic engineering has been used to transfer the gene coding for the α-amylase inhibitor αAI-1, a bruchid resistance factor from the common bean (Phaseolus vulgaris L.), into other grain legumes including pea (Pisum sativum L.), azuki bean (Vigna angularis (Wildenow)), chickpea and cowpea (Ishimoto et al., Reference Ishimoto, Sato, Chrispeels and Kitamura1996; Sarmah et al., Reference Sarmah, Moore, Tate, Molvig, Morton, Rees, Chiaiese, Chrispeels, Tabe and Higgins2004; Ignacimuthu & Prakash, Reference Ignacimuthu and Prakash2006; Solleti et al., Reference Solleti, Bakshi, Purkayastha, Panda and Sahoo2008). α-Amylases, the target of αAI-1, are key enzymes for starch digestion and have been shown to be vital for bruchid development. The gene construct transferred to the transgenic legumes is regulated by the seed-specific promoter phytohemagglutinin-L gene (dlec2) of P. vulgaris, resulting in expression restricted to the cotyledon and embryonic axis of the developing seeds (Altabella & Chrispeels, Reference Altabella and Chrispeels1990). Following egg-hatch, bruchid larvae chew into the seed on which they are laid until completion of development. In seeds expressing αAI-1, bruchid infestation of transgenic seeds proceeds normally until the larvae are exposed to αAI-1 in the cotyledons. The development of susceptible bruchid species ceases rapidly and the larvae starve in the first or second instar. At this early stage of development, physical damage and weight loss of the seed is minimal (Schroeder et al., Reference Schroeder, Gollasch, Moore, Tabe, Craig, Hardie, Chrispeels, Spencer and Higgins1995). In all the grain legumes expressing αAI-1 in their cotyledons, there is high resistance to the bruchid species C. chinensis, C. maculatus, Callosobruchus analis and Bruchus pisorum (L.) (Shade et al., Reference Shade, Schroeder, Pueyo, Tabe, Murdock, Higgins and Chrispeels1994; Schroeder et al., Reference Schroeder, Gollasch, Moore, Tabe, Craig, Hardie, Chrispeels, Spencer and Higgins1995; Ishimoto et al., Reference Ishimoto, Sato, Chrispeels and Kitamura1996; Morton et al., Reference Morton, Schroeder, Bateman, Chrispeels, Armstrong and Higgins2000; Sarmah et al., Reference Sarmah, Moore, Tate, Molvig, Morton, Rees, Chiaiese, Chrispeels, Tabe and Higgins2004; Ignacimuthu & Prakash, Reference Ignacimuthu and Prakash2006; De Sousa-Majer et al., Reference De Sousa-Majer, Hardie, Turner and Higgins2007; Solleti et al., Reference Solleti, Bakshi, Purkayastha, Panda and Sahoo2008). The potential of this approach has been demonstrated even under field conditions, where transgenic pea seeds were completely resistant to B. pisorum (Morton et al., Reference Morton, Schroeder, Bateman, Chrispeels, Armstrong and Higgins2000).
In the present study, we assessed the resistance of αAI-1 transgenic cowpea and chickpea lines and several conventional chickpea cultivars to the bruchid species C. chinensis, C. maculatus and Acanthoscelides obtectus (Say). The latter is known to be tolerant to αAI-1 (Ishimoto & Kitamura, Reference Ishimoto and Kitamura1992). The two Callosobruchus species are considered to be major pests of chickpea and cowpea. A. obtectus has spread throughout the world and, although primarily attacking the common bean, it has also become a pest of both cowpea and chickpea (CABI crop protection compendium, available on http:/www.cabi.org/cpc). For the first time, we did a simultaneous evaluation of the resistance of different αAI-1-expressing legume species, lines and/or cultivars to three major bruchid pests providing a thorough assessment of the potential of this resistance trait.
Experimental methods
Insects
The experiments were carried out with three bruchid species, all provided by C. Adler (Julius Kühn-Institut, Germany): A. obtectus, C. chinensis and C. maculatus. The strains are colonized in the laboratory since 1967 (A. obtectus and C. chinensis) or 1998 (C. maculatus), their geographical origin is unknown. Colonies were maintained on both C. arietinum and V. unguiculata seeds for the respective experiments for at least five generations in a climate chamber at 25 °C, 50% RH and total darkness.
Seeds
Twelve chickpea and ten cowpea genotypes were included in this study (table 1). Transgenic chickpea seeds of the cultivar Semsen expressing αAI-1 have been described (Sarmah et al., Reference Sarmah, Moore, Tate, Molvig, Morton, Rees, Chiaiese, Chrispeels, Tabe and Higgins2004). The corresponding Semsen non-transgenic parental line was included as a control. In addition, the following conventional chickpea cultivars were included: the Desi type cultivars ‘Vijay’ (a high-yielding cultivar released in central India; resistant to Fusarium oxysporum and Helicoverpa armigera), ICCC 37 (a high-yielding cultivar released in Andhra Pradesh, India; resistant to F. oxysporum, moderately resistant to H. armigera and moderately tolerant to root rot), ICCV 10 (a high-yielding cultivar released in southern and central India; resistant to F. oxysporum and drought tolerant), ICC 506 (a cultivar resistant to H. armigera) and the Kabuli type ICCV 2 (a cultivar resistant to F. oxysporum and tolerant to drought, salinity and heat stress), with hitherto unknown resistance to bruchids, as well as four Desi cultivars with reported resistance to C. maculatus (ICC 12422, ICC 4969, ICC 14336 and ICC 4957) (Erler et al., Reference Erler, Ceylan, Erdemir and Toker2009). These conventional cultivars and the respective information were provided by the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) in India.
Table 1. Chickpea and cowpea genotypes included in the experiment, their plant background, average seed weight and seed coat thickness. Transgenic lines are indicated with an asterisk.

Transgenic cowpea lines expressing αAI-1 were developed in two diverse cowpea genotypes (Popelka et al., Reference Popelka, Gollasch, Moore, Molvig and Higgins2006; Higgins et al., Reference Higgins, Gollasch, Molvig, Moore, Popelka, Watkins, Armstrong, Mahon, Ehlers, Huesing, Margam, Shade and Murdock2013). The cowpea parental genotypes from which the transgenic lines were developed were breeding line IT86D-1010, developed at the International Institute of Tropical Agriculture (IITA), Nigeria, and the Japanese cultivar ‘Sasaque’. The transgenic αAI-1 expressing and non-expressing lines developed from IT86D-1010 were TCP 14A and NTCP 14A, respectively, whereas from cultivar Sasaque, three independently transformed lines expressing αAI-1 (T 170, T 239 and T 310) and their corresponding non-transformed null-pair lines (NT 170, NT 239 and NT 310) were assayed.
As an additional control, the commercially available chickpea and cowpea seeds used to rear the bruchids, purchased from a local supermarket, were also included in the experiments.
Physical characteristics of the seeds are presented in table 1. Seed weight is the average of 100 seeds. To determine the average seed coat thickness, ten dried seeds per genotype were peeled and the thickness of the coat at the side of the seed was measured using an Absolute Digimatic 500-181U micrometer (Mitutoyo, Urdorf, Switzerland).
Experimental setup
Experimental conditions were identical to the rearing conditions, i.e., 25 °C, 50% RH and total darkness. The experiments were carried out with each combination of bruchid species and seeds from chickpea and cowpea separately. For the experiments with C. chinensis and C. maculatus, 30 seeds of each genotype were placed individually in an open Petri dish (2.2×2.2×1 cm) and arranged randomly in a large box (100×50×20 cm). Approximately 2000 newly emerged adult beetles were released into the box and allowed to oviposit for 24 h. Embryonic development, which is visible through the egg chorion, was inspected on a daily basis and as soon as the first larva started chewing into the seed, all other larvae on the same seed were removed with a scalpel to avoid interference among multiple larvae developing in a single seed.
Given that A. obtectus does not attach the eggs to the seeds, eggs were collected by carefully sieving the seeds on which adult beetles had been depositing eggs for 24 h. Into each seed, a hole of 1 mm depth was pierced with a needle. After hatching, one larva per seed was carefully introduced into the hole with a fine brush, checked for physical integrity, and observed until it started chewing into the seed. Emerging adults were collected daily from individual seeds, transferred into a 0.2-ml cup and immediately frozen and stored at −20 °C until they were dried and weighed.
Chickpea and cowpea resistance to bruchids and stage-specific mortality
Resistance of each chickpea and cowpea genotype to bruchids was calculated as the percentage of seeds in which no adult bruchid emerged. Seeds where no adult bruchid emerged were dissected and the stage-specific mortality determined. We distinguished whether the bruchid (i) failed to perforate the seed coat and enter the cotyledons; (ii) died inside the seed in the larval (further referred to as within-seed larval mortality) or (iii) pupal stage; or (iv) failed to emerge from the seed after successfully completing development. As only bruchid larvae feeding on the cotyledons and embryonic axis of a seed are exposed to αAI-1, within-seed larval mortality in the different transgenic and corresponding non-transgenic chickpea and cowpea genotypes was analyzed.
Impact of host seeds on bruchid life-history parameters
For all bruchid adults emerging from the different non-transgenic chickpea cultivars, within-seed developmental time (WSD) and adult dry weight (ADW) were determined to assess sublethal effects on the bruchids. The WSD was calculated by measuring the time from a larva starting to chew into the seed until the emergence of the adult. Emerged adults were sexed and their ADW determined after drying them at 60 °C for 72 h using a MX5 microbalance (Mettler Toledo, Greifensee, Switzerland). For both sexes of each bruchid species, the impact when feeding on the different chickpea cultivars was evaluated by correlating WSD and ADW with mean seed weight, seed coat thickness, resistance and the within-seed larval mortality rate.
We did not assess the impact of the seed characteristics for cowpea because the parental lines came from only two genetic backgrounds.
Data analyses
All data were analyzed using the software R (version 2.13.2). Resistance and within-seed larval mortality rates of the transgenic and corresponding null-pair chickpea and cowpea lines, respectively, were analyzed pairwise using Fisher's exact test. In the case of the cowpea breeding line IT86D-1010, three pairwise comparisons between the parental, transgenic and null-pair lines were conducted and the α-level adjusted according to the Bonferroni method, resulting in α=0.017.
Results
Chickpea genotypes
Results for the chickpea genotypes with respect to resistance and within-seed larval mortality of the three bruchid species are presented in fig. 1. The parental Semsen line had, compared to the other non-transgenic cultivars, a relatively high resistance to all three bruchid species. The transgenic Semsen line was completely resistant to C. maculatus and nearly so to C. chinensis. However, the difference to the parental line was only significantly different for the latter bruchid species (P<0.01). As expected, resistance to A. obtectus was not increased in the transgenic line. Resistance of the other non-transgenic cultivars was not only highly variable within each of the three bruchid species but also varied among species. Resistance was highest against C. maculatus (10–87%), followed by A. obtectus (3–67%) and C. chinensis (0–33%).

Fig. 1. Resistance (percentage of seeds from which no adult beetle emerged) and within-seed larval mortality in different chickpea genotypes for A. obtectus, C. chinensis and C. maculatus. A pairwise comparison was made among the transgenic (TG, bar in gray) and parental (PL) Semsen line using Fisher's exact test (*P<0.05, ***P<0.01, n.s.=not significant).
Within-seed larval mortality of all bruchid species was low in the non-transgenic chickpea cultivars. Highest mortality rates for A. obtectus, C. chinensis and C. maculatus were 20%, 13% and 26%, respectively. In contrast, within-seed larval mortality in the transgenic line was 100% and 97% for C. maculatus and C. chinensis, respectively, and, in both cases, significantly higher than the parental Semsen line (P<0.001). The resistance of the transgenic Semsen line to C. chinensis was exclusively owing to within-seed larval mortality. In the case of C. maculatus, some mortality was caused by the fact that larvae failed to perforate the seed coat. However, all larvae reaching the cotyledon subsequently died in the larval stage. There was no difference in within-seed larval mortality of A. obtectus between the transgenic line and its control.
In all species, there were differences between the overall resistance (i.e., total mortality rate) and the within-seed larval mortality rate in most genotypes (results are illustrated in Supplementary Fig. 1). For A. obtectus and C. chinensis, this difference was exclusively because of adults failing to emerge from the seed after successfully completing their development. In contrast, C. maculatus larvae frequently failed to perforate the seed coat. In addition, there was a single case of mortality in the pupal stage in the latter species.
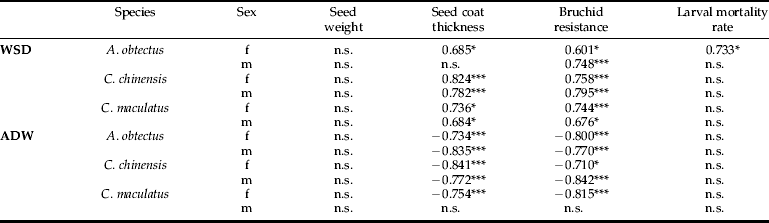
In the case of the chickpea seeds, the WSD of the emerging beetles was positively correlated with seed coat thickness (except for A. obtectus males) and the overall resistance (table 2). The ADW was negatively correlated with seed coat thickness and resistance (except for C. maculatus males in both cases) (table 2). With one exception, neither the WSD nor the ADW data correlated with the within-seed larval mortality rate. The WSD and ADW data are provided in detail in Supplementary Table 1.

Fig. 2. Resistance (percentage of seeds from which no adult beetle emerged) and within-seed larval mortality in different cowpea genotypes for A. obtectus, C. chinensis and C. maculatus. Comparison was made among the three IT86D lines (IT86D: parental line; TCP14A: transgenic line; NTCP14A: null-pair line) and pairwise among the transformed (T, bar in gray) and respective non-transformed (NT) Sasaque lines 170, 239 and 310 using Fisher's exact test (*P<0.05, ***P<0.01, n.s.=not significant; for the IT86D lines, the α level was adjusted for three pairwise comparisons using the Bonferroni method, resulting in α=0.017).
Table 2. Pearson correlation coefficients for mean WSD and mean ADW of females (f) and males (m) of the bruchid species A. obtectus, C. chinensis and C. maculatus emerged from different non-transgenic chickpea cultivars correlated to seed weight, seed coat thickness (see table 1), bruchid-resistance (see fig. 1) and the within-seed larval mortality rate (see fig. 2). *P<0.05, ***P<0.01, ‘n.s.’ indicates that the correlation was not significant.
Cowpea genotypes
Results for the cowpea genotypes with respect to resistance and within-seed larval mortality of the three bruchid species are presented in fig. 2. The transgenic IT86D-1010 line (TCP 14A) was completely resistant to the two susceptible Callosobruchus species, significantly more than both the corresponding null-pair line (NTCP 14A) and the parental line (IT86D-1010) (for both, P<0.001). In contrast, the transgenic line was significantly more susceptible to A. obtectus than the parental line (P=0.007), but did not differ from the null-pair line. Furthermore, the null-pair line was significantly more susceptible to all bruchid species than the parental line (for all, P<0.001). All transgenic Sasaque lines were completely resistant to both Callosobruchus species, significantly more than their corresponding null-pair lines (for all, P<0.001). As expected, none of the transgenic lines were completely resistant to A. obtectus. However, the transgenic line T 170 was more resistant (P=0.030) and the transgenic line T 310 was more susceptible (P<0.001) than their corresponding null-pair lines.
Within-seed larval mortality of the Callosobruchus species was 100% in all transgenic lines, significantly higher than in their corresponding null-pair lines and the parental IT86D-1010 line, respectively (for both, P<0.001). No significant differences could be detected between the parental IT86D-1010 line and its null-pair line for these two bruchids. Mortality of A. obtectus was mostly because of within-seed larval mortality (Supplementary Fig. 1). Mortality was significantly higher in the parental IT86D-1010 line compared to the corresponding transgenic line (P=0.015) and the null-pair line (P<0.001). The two latter lines did not differ significantly from each other. In the Sasaque lines, a significantly higher within-seed larval mortality was observed in the transgenic line T 170 compared to the corresponding null-pair line (P=0.030) and in the null-pair line NT 310 compared to the corresponding transgenic line (P=0.005).
In cowpea, few adults from A. obtectus and C. maculatus failed to emerge from the seeds, while this was not observed at all for C. chinensis (Supplementary Fig. 1). A. obtectus mainly died in the larval stage inside the seeds. In contrast, larvae of the two Callosobruchus species frequently failed to enter the seeds. Mortality in the pupal stage was observed only once in C. chinensis.
Discussion
All transgenic cowpea lines expressing αAI-1 were completely protected from the bruchid species C. chinensis and C. maculatus. The single chickpea line expressing the inhibitor was also completely resistant to C. maculatus and highly resistant to C. chinensis. This is not surprising, as the two Callosobruchus species are known to be susceptible to αAI-1 (Ishimoto & Kitamura, Reference Ishimoto and Kitamura1989) and it confirms earlier reports of increased resistance of αAI-1 transgenic legumes to these bruchids (Ishimoto et al., Reference Ishimoto, Sato, Chrispeels and Kitamura1996; Sarmah et al., Reference Sarmah, Moore, Tate, Molvig, Morton, Rees, Chiaiese, Chrispeels, Tabe and Higgins2004; Ignacimuthu & Prakash, Reference Ignacimuthu and Prakash2006; Solleti et al., Reference Solleti, Bakshi, Purkayastha, Panda and Sahoo2008). The significant increase in within-seed larval mortality clearly demonstrates that αAI-1 was the cause of this effect. The fact that the transgenic chickpea line was not completely resistant to C. chinensis is likely to be because of a lower expression level of αAI-1 in the transgenic chickpea line compared to the cowpea lines tested (T.J.V. Higgins, unpublished results). It is a common observation that independent transgenic legume lines display varying levels of transgene expression (Shade et al., Reference Shade, Schroeder, Pueyo, Tabe, Murdock, Higgins and Chrispeels1994; Sarmah et al., Reference Sarmah, Moore, Tate, Molvig, Morton, Rees, Chiaiese, Chrispeels, Tabe and Higgins2004; Solleti et al., Reference Solleti, Bakshi, Purkayastha, Panda and Sahoo2008). αAI-1 expression level-dependent resistance of susceptible bruchids has been reported for pea (Shade et al., Reference Shade, Schroeder, Pueyo, Tabe, Murdock, Higgins and Chrispeels1994; Morton et al., Reference Morton, Schroeder, Bateman, Chrispeels, Armstrong and Higgins2000), and other transgenic chickpea and cowpea lines had only detrimental, but not lethal impacts on C. chinensis and C. maculatus (Sarmah et al., Reference Sarmah, Moore, Tate, Molvig, Morton, Rees, Chiaiese, Chrispeels, Tabe and Higgins2004; Ignacimuthu & Prakash, Reference Ignacimuthu and Prakash2006; Solleti et al., Reference Solleti, Bakshi, Purkayastha, Panda and Sahoo2008). Although the experiment was conducted only for a single bruchid generation, we can assume that the initial level of resistance would not decrease during seed storage. αAI-1 is a seed storage protein, which are known to be highly stable, not likely to be changed in dry mature seeds, and only broken down during germination and seedling growth (Ladizinsky & Hymowitz, Reference Ladizinsky and Hymowitz1979; Chrispeels & Raikhel, Reference Chrispeels and Raikhel1991). Nevertheless, a high expression level is required to achieve a complete protection of the stored seeds and to prevent development of resistance in susceptible species, but also that αAI-1 has a significant detrimental effect on the survival of susceptible bruchid larvae. The recent finding that bruchids rely heavily on water produced during carbohydrate metabolism (Murdock et al., Reference Murdock, Margam, Baoua, Balfe and Shade2012), in combination with the fact that grain legume seeds are also rich in proteins, implies that αAI-1 not only limits energy production in susceptible larvae but also deprives them of water.
As expected, the transgenic chickpea and cowpea lines were not resistant to A. obtectus, whose α-amylase is not inhibited by αAI-1 (Ishimoto & Kitamura, Reference Ishimoto and Kitamura1992). However, in two out of four cowpea lines there were significant differences in both resistance and within-seed larval mortality between the transgenic lines and their respective control lines. While the cause of the observed differences remains subject to speculation the results suggest that the transformation procedure has caused some changes to the cowpea seed that affect the bruchids. For example, it is known that the process of tissue culture, used in the generation of transgenic plants, can lead to phenotypic changes often called somaclonal variation (Larkin & Scowcroft, Reference Larkin and Scowcroft1981; Pellegrineschi, Reference Pellegrineschi1997).
Comparing the performance of the different bruchid species on the non-transgenic chickpea cultivars, it became evident that certain cultivars were more resistant than others to all three bruchid species. This included the four cultivars reported to be partially resistant to C. maculatus by Erler et al. (Reference Erler, Ceylan, Erdemir and Toker2009). In the previous study, where three out of these four cultivars were completely resistant, none of them was completely resistant to C. maculatus in our experiment and the resistance against C. chinensis and A. obtectus was even lower. In our study, both chickpea and cowpea seeds were more susceptible to C. chinensis than to A. obtectus and C. maculatus. This illustrates the difficulty of extrapolating the results obtained with a single bruchid strain; resistance not only varies among bruchid species but also between strains of a species. For example, the cowpea landrace TVu 2027, denoted as bruchid resistant, was in fact only tested with C. maculatus (Singh, Reference Singh1977; Singh et al., Reference Singh, Singh and Adjadi1985). Whether this landrace is also reasonably resistant to other bruchid species is not known. Furthermore, this genotype was not only shown to be resistant for a limited period only, but was also highly susceptible to another strain of C. maculatus found in Nigeria (Shade et al., Reference Shade, Murdock and Kitch1999; Murdock et al., Reference Murdock, Coulibaly, Higgins, Huesing, Ishiyaku, Sithole-Niang, Kole and Hall2008).
Apart from the expression of αAI-1, the major source of resistance to all three bruchid species was the seed coat. In chickpea, the difference between within-seed larval mortality and resistance for C. chinensis and A. obtectus was exclusively because of adults failing to emerge from the seeds. Larvae failing to enter the seeds occurred regularly in C. maculatus only. The role of the seed coat in defense against bruchids is also supported by the fact that WSD and ADW were significantly correlated with seed coat thickness in chickpea. Unfortunately, a thicker seed coat also makes the chickpea less desirable for human consumption (Moreno & Cubero, Reference Moreno and Cubero1978; Gil & Cubero, Reference Gil and Cubero1993). While there is consensus that the seed coat has an impact on bruchid resistance in chickpea, its value in cowpea is controversial. Although Edde & Amatobi (Reference Edde and Amatobi2003) claim that the seed coat has no value in protecting cowpea against C. maculatus, Lattanzio et al. (Reference Lattanzio, Terzano, Cicco, Cardinali, Di Venere and Linsalata2005) state that resistance factors in the seed coat must also be considered in the biochemical defense of cowpea against C. maculatus. Finally, Souza et al. (Reference Souza, Santos, Pinto, Wermelinger, Ribeiro, Souza, Deus, Souza, Xavier-Filho, Fernandes and Oliveira2011) demonstrated that defense compounds in the seed coat of non-host legumes can significantly contribute to protection against bruchids.
The significant correlation of WSD and ADW with resistance in chickpea indicates that the resistance factor(s) also cause sublethal effects on the surviving beetles, leading to a reduced fitness that will contribute to a delay in bruchid population growth in the stored seeds. But, even though resistance in a range of 80–90%, as observed in those non-transgenic cultivars with the highest resistance, may increase the period until a certain damage threshold is exceeded, multivoltinism, short generation time and high fertility of the bruchid species means significant loses will occur under common storage scenarios, where farmers would store their crops for six months or more (Southgate, Reference Southgate1979). According to Erler et al. (Reference Erler, Ceylan, Erdemir and Toker2009), only genotypes with a resistance higher than 90% can be considered as practically resistant. Hence, none of the non-transgenic chickpea cultivars tested in our study would be considered resistant to any of the three tested bruchid species. Furthermore, the beetles in our experiment developed in the respective seeds only for a single generation. Bruchids are known to be able to quickly adapt to new hosts. This has, for example, been reported for A. obtectus infesting chickpea (Tucić et al., Reference Tucić, Mikuljanac and Stojković1997) and for C. maculatus infesting cowpea (Fricke & Arnqvist, Reference Fricke and Arnqvist2007; Zhu-Salzman & Zeng, Reference Zhu-Salzman and Zeng2008). Especially for the two Callosobruchus species, which have been attacking both chickpea and cowpea for thousands of generations, the efforts to find new resistance traits in wild relatives and transfer them to domesticated legumes, which was already found to be difficult per se (Sarmah et al., Reference Sarmah, Moore, Tate, Molvig, Morton, Rees, Chiaiese, Chrispeels, Tabe and Higgins2004; Murdock et al., Reference Murdock, Coulibaly, Higgins, Huesing, Ishiyaku, Sithole-Niang, Kole and Hall2008), may not provide long-term control. Shade et al. (Reference Shade, Murdock and Kitch1999) argue that it is likely that C. maculatus has encountered most resistance genes present in both wild and domesticated Vigna species, and resistance achieved by conventional breeding will therefore be of low durability. The situation should be different for introduced bruchid species, such as A. obtectus. Pelegrini et al. (Reference Pelegrini, Lay, Murad, Anderson and Franco2008) identified an α-amylase inhibitor in cowpea called VuD1, which efficiently inhibits α-amylases from the αAI-1 tolerant bruchids A. obtectus and Zabrotes subfasciatus, both new world species, but not C. maculatus. The authors suggested that the gene coding for VuD1 could be transferred into other plants to control these bruchids, but it should also be possible to develop cowpea cultivars with a VuD1-based resistance to A. obtectus and Z. subfasciatus by conventional breeding.
Independent of whether the resistance is achieved by conventional breeding or genetic engineering, bruchid management should not be based on a single resistance factor alone, but a combination of different approaches to maximize efficiency and sustainability of bruchid management (Lüthi et al., Reference Lüthi, Álvarez-Alfageme and Romeis2010). This would not only reduce damage but also prevent or delay development of resistance to αAI-1. Hermetic storage of transgenic seeds in drums or bagging utilizing triple plastic bags (Murdock et al., Reference Murdock, Seck, Ntoukam, Kitch and Shade2003), or releasing natural enemies (Sanon et al., Reference Sanon, Ouedraogo, Tricault, Credland and Huignard1998; Schmale et al., Reference Schmale, Wäckers, Cardona and Dorn2003; Velten et al., Reference Velten, Rott, Conde Petit, Cardona and Dorn2008) are powerful approaches that could be combined with the transgenic seeds. For the combination with natural enemies, this means, however, that the insecticidal trait in the αAI-1 transgenic seeds should not interfere with the biological control services provided by natural enemies, in particular hymenopteran parasitoids (Romeis et al., Reference Romeis, Sharma, Sharma, Das and Sarmah2004). Hosts developing in transgenic seeds have ingested αAI-1, therefore, parasitoids of the larval and pupal stages of bruchids might be exposed to the inhibitor when attacking such hosts. The potential interference with these biological control organisms should thus be considered in the non-target risk assessment of αAI-1 transgenic legumes prior to commercial release (Romeis et al., Reference Romeis, Bartsch, Bigler, Candolfi, Gielkens, Hartley, Hellmich, Huesing, Jepson, Layton, Quemada, Raybould, Rose, Schiemann, Sears, Shelton, Sweet, Vaituzis and Wolt2008). A conceptual model describing how transgenic legume seeds expressing αAI-1 could interfere with bruchid control by parasitoids has been developed (Lüthi et al., Reference Lüthi, Álvarez-Alfageme and Romeis2010). An initial non-target risk assessment of αAI-1 transgenic legumes revealed that harmful effects on the inhibitor on parasitoids cannot be discounted (Álvarez-Alfageme et al., Reference Álvarez-Alfageme, Lüthi and Romeis2012). Further research will be required to determine whether αAI-1 expressing chickpea and cowpea have a negative impact on this important group of non-target organisms. However, if the impact on bruchid parasitoids can be shown to be minimal, we believe that αAI-1 transgenic legumes are a leap in the development of bruchid-resistant legume seeds and could significantly contribute to food security in developing countries.
Supplementary material
The supplementary material for this article can be found at http://www.journals.cambridge.org/BER
Acknowledgements
We are grateful to Dr H.C. Sharma and Dr H.D. Upadhyaya (ICRISAT, Patancheru, India) for providing chickpea seeds and Dr C. Adler (Julius-Kühn-Institut, Berlin, Germany) for providing the original bruchid colonies. This study was funded by the NCCR Plant Survival, a research program of the Swiss National Science Foundation.






