Introduction
The great capricorn beetle, Cerambyx cerdo L. (Cc; Coleoptera: Cerambycidae) is one of the largest and most striking coleopterans in the western Palaearctic. Adults are conspicuous and popular insects for amateur and professional entomologists (and even for the general public), and their distribution and abundance are therefore fairly well documented in studies of local and regional faunas. Cc occurs throughout much of Europe, northern Africa, the Caucasus and Asia Minor, but, the species is much more prevalent in the southern part of its distribution range, being widespread in Iberia (Bense, Reference Bense1995; Vives, Reference Vives2000; EC, 2007; González-Peña et al., Reference González-Peña, Vives-Noguera and de Sousa-Zuzarte2007; Sama, Reference Sama and Audisio2013). Cc larvae are xylophagous on deciduous, marcescent and evergreen oak species, but, reports on other broadleaf trees are not rare albeit sometimes considered doubtful (Duffy, Reference Duffy1953; Bense, Reference Bense1995; Vives, Reference Vives2000; EC, 2007; González-Peña et al., Reference González-Peña, Vives-Noguera and de Sousa-Zuzarte2007; Sama et al., Reference Sama, Buse, Orbach, Friedman, Rittner and Chikatunov2010). In southern Spain, the main hosts are holm oak (Quercus ilex L.) and cork oak (Quercus suber L.) typically forming part of the Mediterranean dehesa ecosystem, a savannah-like open woodland protected under the EU Habitats Directive (CEC, 1992). The dehesa ecosystem is protected because of its high environmental, biodiversity and socio-economic value (Montero et al., Reference Montero, San Miguel, Cañellas, Jiménez-Díaz and Lamo de Espinosa1998; Bugalho et al., Reference Bugalho, Caldeira, Pereira, Aronson and Pausas2011; Ramírez-Hernández et al., Reference Ramírez-Hernández, Micó, Marcos-García, Brustel and Galante2014).
Cc is a keystone and internationally protected beetle that has been sometimes utilized as an umbrella species in conservation programmes (Albert et al., Reference Albert, Platek and Cizek2012; Drag & Cizek, Reference Drag and Cizek2015). Cc is considered a threatened species in central Europe and was listed in the Bern Convention (CE, 1979), catalogued in Annexes II and IV of the EU Habitats Directive (CEC, 1992) and red-listed as near threatened by the IUCN (2010). Moreover, Cc is currently protected in several European countries by specific national and regional regulations. It is worth noting the potential conflict of interests (legal and ecological) that may arise from the aforementioned Habitats Directive (CEC, 1992) if Cc becomes a harmful ‘pest’ species (see below), given the simultaneous protection of both the beetle and the dehesa ecosystem. Like most longhorn beetles, Cc belongs to the highly diverse assemblage of saproxylic (wood dwelling) insects. This functional group is essential in the creation of hollows in trees later used as shelters for other species, so their activity greatly enhances biodiversity in forest ecosystems (Speight, Reference Speight1989; Grove, Reference Grove2002; Buse et al., Reference Buse, Ranius and Assmann2008a). Short-term threats for Cc are mainly related to loss of open-grown veteran trees, felling of decayed/dying trees and main branches, cutting standing dying/dead trees (snags), recently dead wood removal and uncontrolled pesticide use in farmed areas. Long-term threats in turn are habitat loss (including fragmentation and degradation), poor natural or artificial regeneration and therefore lack of habitat replacement (Grove, Reference Grove2002; MMA, 2006; EC, 2007; Albert et al., Reference Albert, Platek and Cizek2012). Other potential threats for Cc derive from tree canopy closure and excessive understory growth limiting trunk insolation (an important heat source for developing larvae) and adult flight towards trees (Buse et al., Reference Buse, Schröder and Assmann2007). Note, however, that these constraint factors are not a concern in dehesa ecosystems because typical management practices preclude both canopy closure and understory growth as they reduce the production of acorns, pasture and forage for livestock.
It has been widely accepted that the Cc life cycle is almost exclusively restricted to old, decayed or diseased host trees, and so many authors consider that this longhorn cannot become harmful in natural conditions. However, Cc needs living and fresh wood to feed on and properly develop (Buse et al., Reference Buse, Schröder and Assmann2007, Reference Buse, Ranius and Assmann2008a; Sallé et al., Reference Sallé, Nageleisen and Lieutier2014; Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz and Sánchez-González2017), so the beetle is potentially able to colonize and attack healthy living trees. When Cc populations are large, initial tunnelling activity of small larvae into cambium and xylem can alter sap flow and trigger wilting, die-back, leaf fall, vigour loss and tree decay. As the larvae grow, they bore increasingly wider and longer galleries into the inner wood and may cause huge physiological, mechanical and structural damage. Exit holes and larval activity may also favour the spread of oak pathogens such as the charcoal disease (Martín et al., Reference Martín, Cabezas, Buyolo and Patón2005). Inhabited trees may be recognized by the presence of 15–20 mm oval exit holes on the bark with red-coloured interior sides, often together with abundant presence of frass. Hence, Cc is included among the primary saproxylic beetles which, under certain selective pressures, could turn into a harmful or pest species (Speight, Reference Speight1989). This situation is often observed in southern Spain in mismanaged oak forests, especially in those suffering inappropriate pruning or aggressive cork stripping, as large wounds inflicted to trees are extremely attractive for ovipositing Cc females. A similar scenario has been reported for the sympatric and congeneric species Cerambyx welensii Küster (=Cerambyx velutinus Brullé) (Cw), currently considered an emerging pest (Torres-Vila et al., Reference Torres-Vila, Sánchez-González, Ponce-Escudero, Martín-Vertedor and Ferrero-García2012a, Reference Torres-Vila, Sánchez-González, Merino-Martínez, Ponce-Escudero, Conejo-Rodríguez, Martín-Vertedor and Ferrero-García2013).
All the above reflect the somewhat controversial ecological status of Cc in the western Palaearctic depending on latitude and, in particular, mediterraneity. Despite being widely considered an endangered species in northern and most of central Europe, Cc has been increasingly reported in recent decades as a harmful or pest species on oak trees, a trend that could be linked to global warming (Allen et al., Reference Allen, Macalady, Chenchouni, Bachelet, McDowell, Vennetier, Kitzberger, Rigling, Breshears, Hogg, Gonzalez, Fensham, Zhang, Castro, Demidova, Lim, Allard, Running, Semerci and Cobb2010; Wilson & Maclean, Reference Wilson and Maclean2011; Sallé et al., Reference Sallé, Nageleisen and Lieutier2014). Cc was mentioned early as a common or harmful oak borer in Germany (Ratzeburg, Reference Ratzeburg1839 (as Cerambyx heros Fab.); Escherich, Reference Escherich1923), and was first considered a true oak pest in Ukraine and Georgia (Rudnev, Reference Rudnev1936, Reference Rudnev1957). In the 1960s, Cc was reported as an orchard pest of pome and stone fruit trees in Lebanon, Turkey, Egypt and other middle and southern European countries (Gentry, Reference Gentry1965), although this report should be taken with caution as it could derive from a confusion with the similar congeneric species Cerambyx dux Faldermann, a serious pest of rosaceous fruit trees in the same area. More recently, Cc has been described as a harmful or pest species of oak trees in a large array of circum-Mediterranean countries, including Spain (EC, 2007; González et al., Reference González, Gallego, Lencina, Closa, Muntaner and Núñez2010, Reference González, Núñez, Lencina and Gallego2013; Peris-Felipo et al., Reference Peris-Felipo, Bernués-Bañeres, Pérez-Laorga-Arias and Jiménez-Peydró2010), Morocco (Villemant & Fraval, Reference Villemant and Fraval1993; El Antry, Reference El Antry1999; El Boukhari et al., Reference El Boukhari, Brhadda and Gmira2015), France (Aberlench & Lentenois, Reference Aberlench, Lentenois, Holthof and Schnetzler2003; Dupont & Zagatti, Reference Dupont and Zagatti2005; EC, 2007), Algeria (Bouhraoua et al., Reference Bouhraoua, Villemant, Khelil and Bouchaour2002; Chakali et al., Reference Chakali, Attal-Bedreddine and Ouzani2002), Italy (Contarini, Reference Contarini1984; EC, 2007), Tunisia (Pujade-Villar et al., Reference Pujade-Villar, Grami and Ben Jamâa2010), Albania (Anon., 2015), Greece (Avtzis, Reference Avtzis, Liebhold, McManus, Otvos and Fosbroke2001) and Turkey (IUCN, 2010); in Black Sea countries, including Bulgaria (Stalev & Aleksandrov, Reference Stalev and Aleksandrov2014), Romania (Antonie, Reference Antonie2015), Moldova (Gavrilita & Druta, Reference Gavrilita, Druta and Mátyás2010) and Georgia (Matsiakh, Reference Matsiakh2014); as well as in other countries such as Poland (Szujecki, Reference Szujecki1987) and Iran (Farashiani et al., Reference Farashiani, Yarmand, Tavakoli, Sedghian, Al-Mansoor and Ahmadi2005). Nowadays, Cc's impact has been reported as especially worrying on cork oaks in the Mamora Forest in Morocco, on several cork and holm oak open forests in northern Algeria and on holm oaks in the Serra de Tramuntana (Mallorca island) in Spain (see the above references). Note that in these three areas the congeneric species Cw does not occur, so there is no possible confusion between damage caused by Cc and Cw.
Knowledge of the ecology of Cc has considerably improved recently, particularly from studies dealing with saproxylic activity, spatial distribution, microhabitat selection, population genetics, reintroduction events or flight behaviour (Buse et al., Reference Buse, Schröder and Assmann2007, Reference Buse, Ranius and Assmann2008a, Reference Buse, Zábranský and Assmannb; Albert et al., Reference Albert, Platek and Cizek2012; Drag et al., Reference Drag, Kosnar and Cizek2013; Drag & Cizek, Reference Drag and Cizek2015; Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz and Sánchez-González2017). In contrast, comprehensive studies addressing the reproductive biology of Cc are surprisingly very limited (El Antry, Reference El Antry1999). Such basic biological information is crucial to improve Cc conservation guidelines in ecosystems in which the species is threatened, but also to enhance species-specific management tactics in those forest contexts in which larval damage becomes excessive. Acquiring knowledge about the reproductive output, mating behaviour, egg-laying pattern, longevity and other species-specific fitness components of Cc is relevant to an array of genetic, demographic and ecological aspects. These aspects include gene flow, genetic drift, inbreeding depression, ‘bottleneck proneness’, local extinction risk, metapopulation dynamics, founder events, reintroduction attempts or species occupancy, all of which are important to adopt strategies for improving management and conservation biology (Thomas, Reference Thomas2000; Ranius, Reference Ranius2006; Buse et al., Reference Buse, Schröder and Assmann2007; Holland, Reference Holland2009; Drag et al., Reference Drag, Hauck, Pokluda, Zimmermann and Cizek2011; Clobert et al., Reference Clobert, Baguette, Benton and Bullock2012; Drag & Cizek, Reference Drag and Cizek2015). Understanding Cc reproductive biology is particularly necessary in a framework in which tools to manage bark and borer insects associated with oaks are almost non-existent (Evans et al., Reference Evans, Moraal, Pajares, Lieutier, Day, Battisti, Grégoire and Evans2004). To address this shortcoming, the main goals of this study were to investigate in Cc: (i) the reproductive output and related biological variables, (ii) the oviposition pattern and its relationship with egg size variation and (iii) the number of matings in both females (polyandry) and males (polygyny) and their relationship with reproductive output.
Materials and methods
Study species
Cc is univoltine and flies from late May to early August, peaking in late June and early July. Adults are large (28–53 mm long in this study) with a blackish-brown body and a brown-reddish elytral apex. The species has sexual size dimorphism with a slightly larger body in females and with antennae longer in males (about twice as long as the body). Adults feed mainly on sap and tree exudates, while larvae are xylophagous. Mated females wander over the host tree probing the bark with the ovipositor and lay eggs into suitable bark crevices and pruning wounds. After hatching, neonate larvae bore directly into the inner bark and initiate feeding. Larval development usually lasts 2–3 years and pupation occurs in late summer in the sapwood within a pupal cell closed by a calcareous plug. Adults emerge from pupae in late summer or early autumn, but do not leave the pupal cell and overwinter protected within the tree in a pre-reproductive stage until late spring or early summer of the following year. Daily activity of adults (feeding, flight, mating and egg laying) occurs typically at dusk and early evening (Duffy, Reference Duffy1953; Bense, Reference Bense1995; Vives, Reference Vives2000).
Insect origin and adult preparation for tests
Insects for tests were collected in the field during six consecutive years (2011–2016), either in holm oaks at the dehesas La Jara (Badajoz) (38°38′17′′N, 6°51′18′′W, 320–330 m above sea level (a.s.l.)) and La Serrana (Mérida) (39°00′59′′N, 6°37′56′′W, 220–230 m a.s.l.) or in cork oaks at the Cornalvo Natural Park (39°01′22′′N, 6°13′40′′W, 370–380 m a.s.l.). The three sampling sites are located in Badajoz province, Extremadura (southwestern Spain), and form a triangle with a surface area of more than 800 km2.
Artificial rearing in this species is difficult (mainly due to the long larval stage) so most tested individuals were captured as adults very early in the season with baited traps (see Torres-Vila et al., Reference Torres-Vila, Sánchez-González, Ponce-Escudero, Martín-Vertedor and Ferrero-García2012a, Reference Torres-Vila, Sánchez-González, Merino-Martínez, Ponce-Escudero, Conejo-Rodríguez, Martín-Vertedor and Ferrero-García2013 for a description of traps). Field-trapped females were scored as virgins if they did not initiate egg laying on cork oak wood disks after remaining isolated during 4–5 days under optimal laboratory conditions (see below). Females that resumed egg laying within 24–48 h were assumed to be mated and were discarded for tests. Male virginity could clearly not be ascertained in this way. Some tested individuals were also obtained by rearing to adulthood, either as field-derived mature larvae or neonates obtained from field-collected females. Larvae were individually reared at room temperature (22–28°C and 50–70% relative humidity) in well-aerated 140 ml plastic containers on an artificial diet (Morales-Rodríguez et al., Reference Morales-Rodríguez, Sánchez-González, Conejo-Rodríguez and Torres-Vila2015). For more information about the rearing protocol see Torres-Vila et al. (Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016). It is worth nothing that during field surveys we were only able to collect a small number of Cc individuals from inside the wood (either mature larvae or overwintering adults). This occurred despite Cc populations being relatively abundant in the sampled sites, as evidenced by the high number of adult catches in baited traps located in the immediate vicinity. This unexpected outcome requires further research. In short, unsuccessful field sampling and the difficulty of laboratory rearing led us to make use of field-trapped young adults in this study. It was verified that reproductive traits of field-trapped and laboratory-reared adults were similar when corrected for body size and hence both groups were pooled for statistical analyses.
Adults were sexed, measured (body length was used as an estimator of adult size) and individually marked by sex. Reference numbers were written on the elytra either directly with a white permanent marker or with a black permanent marker on a fine layer of white correction fluid previously applied on the elytra (Torres-Vila et al., Reference Torres-Vila, Zugasti, De-Juan, Oliva, Montero, Mendiola, Conejo, Sánchez, Fernández, Ponce and Espárrago2015, Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016). Small white spots were also painted on the adults to readily distinguish both sexes (or species for other trials conducted simultaneously) under the reduced lighting of the artificial dusk used in the experiments (see below).
Laboratory tests: general procedures
Laboratory tests were carried out in the summer when active adults occurred in the field, by using the methodology previously reported with Cw (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016). We used standardized 16 l cardboard cages with a transparent cover as mating and oviposition chambers. Males shared cages with females during tests and were kept in well-aerated 240 ml clear plastic containers during inactivity periods. Caged males and females were regularly sprayed with water and fed ad libitum on a saturated sugar–water paste simulating host tree exudates. Freshly cut cork oak branches were sliced with a circular saw to produce wood disks (70–80 mm in diametre, 2 cm thick) that were used as egg-laying substrate for females. Wood disks were frozen until use to prevent excessive drying. Cork layer was then detached from cambium in one piece with a penknife and was put back held in place with a rubber band. Decorking of disks greatly facilitated daily inspections and egg removal (see below). One wood disk bearing the female reference number was used per cage, being replaced by a new one if necessary. Tests were performed in a controlled environmental room at 25 ± 1°C, 60 ± 10% relative humidity and a L(14 + 2):D8 photoperiod, simulating typical summer conditions in the studied area (July). The first 14 photophase hours were at a 1000 lux luminosity and the last 2 h at 25 lux simulating dusk. The sexual activity of adults was continuously observed at dusk, observations being prolonged at night when necessary using a small red light lantern to avoid disturbing adults. Under these test conditions, adults fed, mated and oviposited normally.
Female tests
The following reproductive traits (and related biological variables) were recorded in an array of females (n = 45) or their offspring: female size (body length), fecundity (total eggs), daily fecundity, fertility (per cent of hatched eggs), longevity, preoviposition period (elapsed time between mating and first oviposition), oviposition period (time between first and last oviposition), postoviposition period (time between last oviposition and female death), mating number (polyandry), egg size, neonate size (head width) and incubation time (time elapsed between egg laying and eclosion).
The effect of female mating number (polyandry) on fecundity was explored by allowing randomly chosen virgin females to mate either singly or multiply (the range observed being 2–19 times). Two marked males were caged with each 4–5-day-old virgin female, 15–30 min before the onset of artificial dusk. If mating occurred, the unmated male was removed quickly to avoid male fights leaving the pair-bonded adults in the cage. The mated male was removed next morning when mating was completed. If mating did not occur within the dusk period, the two males were removed leaving the female isolated in her cage to prevent unobserved matings overnight. New males were routinely added/removed every 2–5 days in the multiple-mated group following the same protocol. Matings (both pair-bonding and male intromissions) were always verified at dusk (fig. 1).

Fig. 1. Mating at dusk of Cc on the cork oak disk used as egg-laying substrate.
Wood disks were inspected daily after removing the cork layer, and eggs were carefully detached and counted to assess daily fecundity. The small room between the cambium and cork layer was extremely attractive for ovipositing females as most eggs were found in this place (>95%). Eggs used to estimate hatching success and incubation time were transferred by date to individual 7.5 ml wells in 25-well, clear plastic tissue culture plates (Sterilin Ltd., Stone Staffordshire, UK). Egg-filled plates were incubated at 25°C in the environmental room, inspected daily, and the eclosion date recorded. Per cent fertility was assessed after hatching on each sample of eggs. Some unhatched eggs in which dead larvae were clearly visible inside the chorion were considered fertile (<5%). Eggs damaged when removed from wood disks were excluded from fertility estimates. Mated but unfertilized females (100% unhatched eggs) were excluded from data analysis.
Mean egg size was estimated for each female over 3–4 weeks from five eggs randomly chosen per oviposition day, or from all eggs if fewer eggs were available in a given day (total sample n = 841 eggs). Eggs were measured using a Nikon DS-U1 digital camera connected to a Leica S6D stereomicroscope. The resulting images were analysed with Eclipse Net 1.20 software to determine egg dimensions: length l, width w and thickness t. Egg volume (V, mm3) was calculated as an ellipsoid according to the formula V = π/6 (l · w · t). Egg volume and egg length were used as a proxy of egg size. Incubation time (the elapsed time between egg laying and eclosion) was determined in a random sample of 662 eggs obtained from 20 females over the first 3 weeks of the oviposition period. Correlations between neonate size (head width) and egg size, and between mandible size (length from condyle to apex) and head width were determined in a subset of 65 eggs from 18 females. We used as estimator of mandible size the mean length of both mandibles. Head width and mandible size of neonates were measured with the same device described for eggs.
Male tests
The reproductive traits (and related biological variables) recorded in males (n = 50) were: male size (body length), longevity and lifetime mating number (polygyny). These traits were obtained from the set of males that were allowed to mate with tested females above.
Data analysis
Linear regression analysis was used to test the correlation among reproductive variables in either females or males. Analysed variables were tested for departure from normality using probability plots prior to statistical test computation. Only per cent fertility was arcsine transformed. Nested and Model I analysis of variance (ANOVA) (either one- or multiple-way) were used for comparison of means and to explore the interaction between some of the studied traits. A nested ANOVA was computed to assess the effect of egg size (nested to individual female as random factor) on egg hatching. We used a subset of 20 females in which a sufficient sample of hatched (chorions) and unhatched eggs was available. We usually measured five eggs per egg-laying day, or all eggs if fewer eggs were available in a given day (n = 1181 eggs). The effects of female and oviposition week on incubation time were explored through a two-way ANOVA, in which both oviposition week (two classes: eggs laid either in the first week or in the second-to-third week) and female itself were considered fixed factors. A two-way ANOVA was used to examine the combined effects of female mating number (single vs. multiple mated females) and female size (large vs. small females, above and below mean female length, 44.1 mm; table 1) on fecundity. ANOVA was also used to compare body length and longevity between sexes. Statistical analyses were performed with Systat (2000) software in accordance with Sokal & Rohlf (Reference Sokal and Rohlf1995).
Table 1. Reproductive and associated biological traits of Cc females and males in laboratory-controlled conditions (25 ± 1°C, 60 ± 10% r.h.).
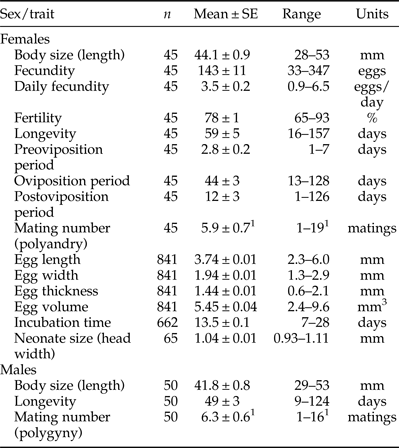
n, sample size; SE, Standard Error of the mean; Range, minimum-maximum values.
1 Experiments were not designed to define the maximum mating potential, so they could somewhat underestimate what occurs in the wild.
Results
Females
Sample sizes and estimates of the reproductive and biological traits of Cc females were recorded (table 1), and statistics from the regression analysis among traits provided (table 2). Mean fecundity was 143 eggs per female, but fecundity ranged widely from 33 to 347 eggs/female. Fecundity was positively correlated with female size (fig. 2a), with oviposition period (fig. 3a) and with longevity (fig. 3b). In the final regression model, the statistical fit improved substantially (R 2 = 0.42, F 1,42 = 30.30, P < 0.001) when the smallest tested female scored as an outlier was removed (Studentized residual = −4.14). Neither female longevity nor oviposition period were correlated with female size (table 2). As one would expect, oviposition period was positively related to longevity (R 2 = 0.52, F 1,43 = 46.25, P < 0.001), especially when the smallest female again scored as an outlier (Studentized residual = −7.59) was excluded (R 2 = 0.79, F 1,42 = 158.50, P < 0.001). Similarly, postoviposition period was closely related with longevity (R 2 = 0.58, F 1,43 = 59.45, P < 0.001). Fertility was not related to female size or lifetime fecundity (table 2). However, larger females tended to show shorter postoviposition periods and females with larger postoviposition periods tended to be less fertile (table 2).
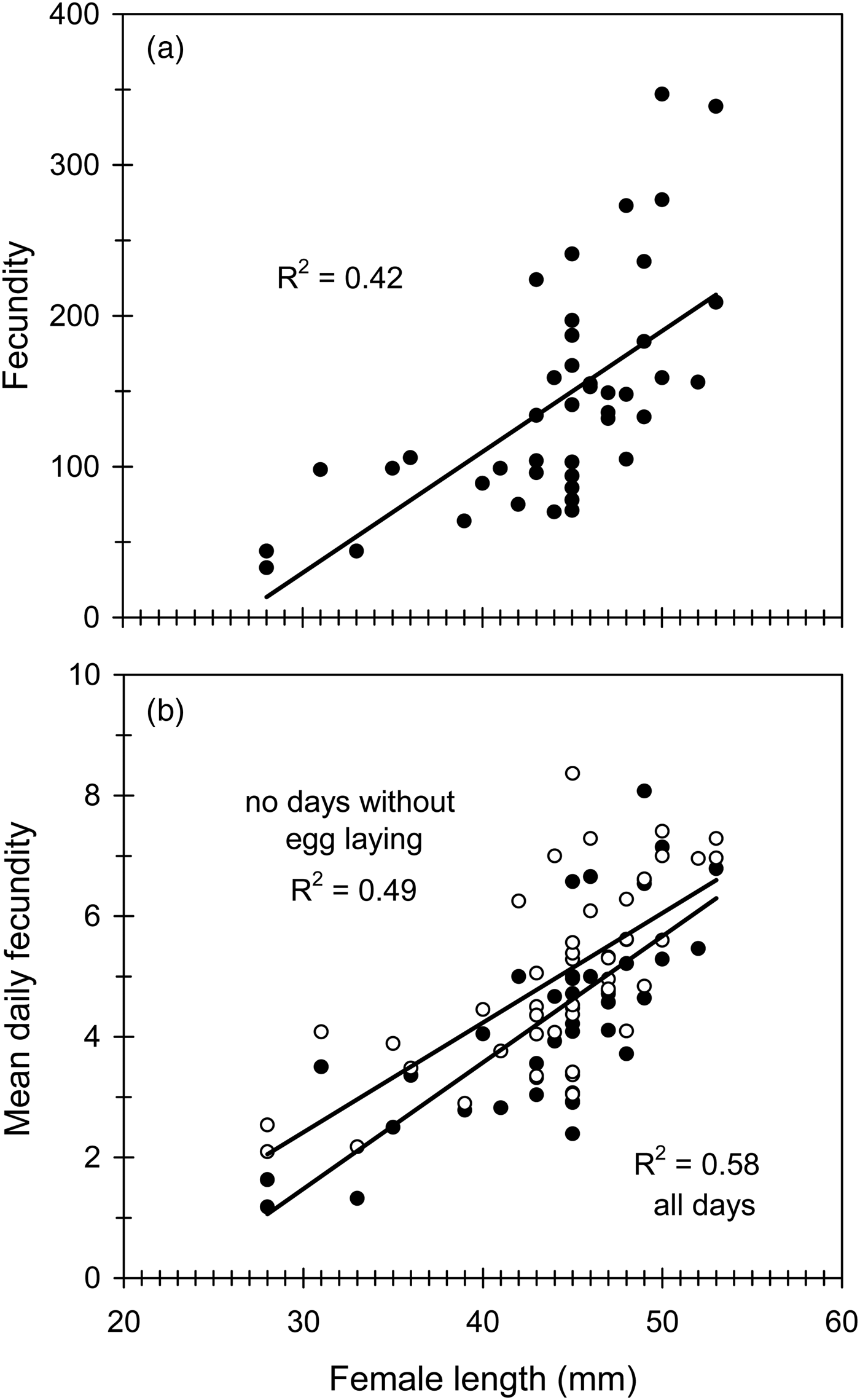
Fig. 2. The effect of female size (female length) on lifetime fecundity (a), and mean daily fecundity (b) in Cc. In the lower graph two regression lines are plotted, one including all egg-laying days (full circles) and the other excluding those days in which no eggs were laid (open circles). Regression equations and statistics were: (a) y = 8.02x – 211.00, F 1,43 = 30.60, P < 0.001; (b) y = 0.18x – 3.04, F 1,43 = 40.68, P < 0.001 (excluding dates with no eggs), and y = 0.21x – 4.81, F 1,43 = 58.12, P < 0.001 (all days).
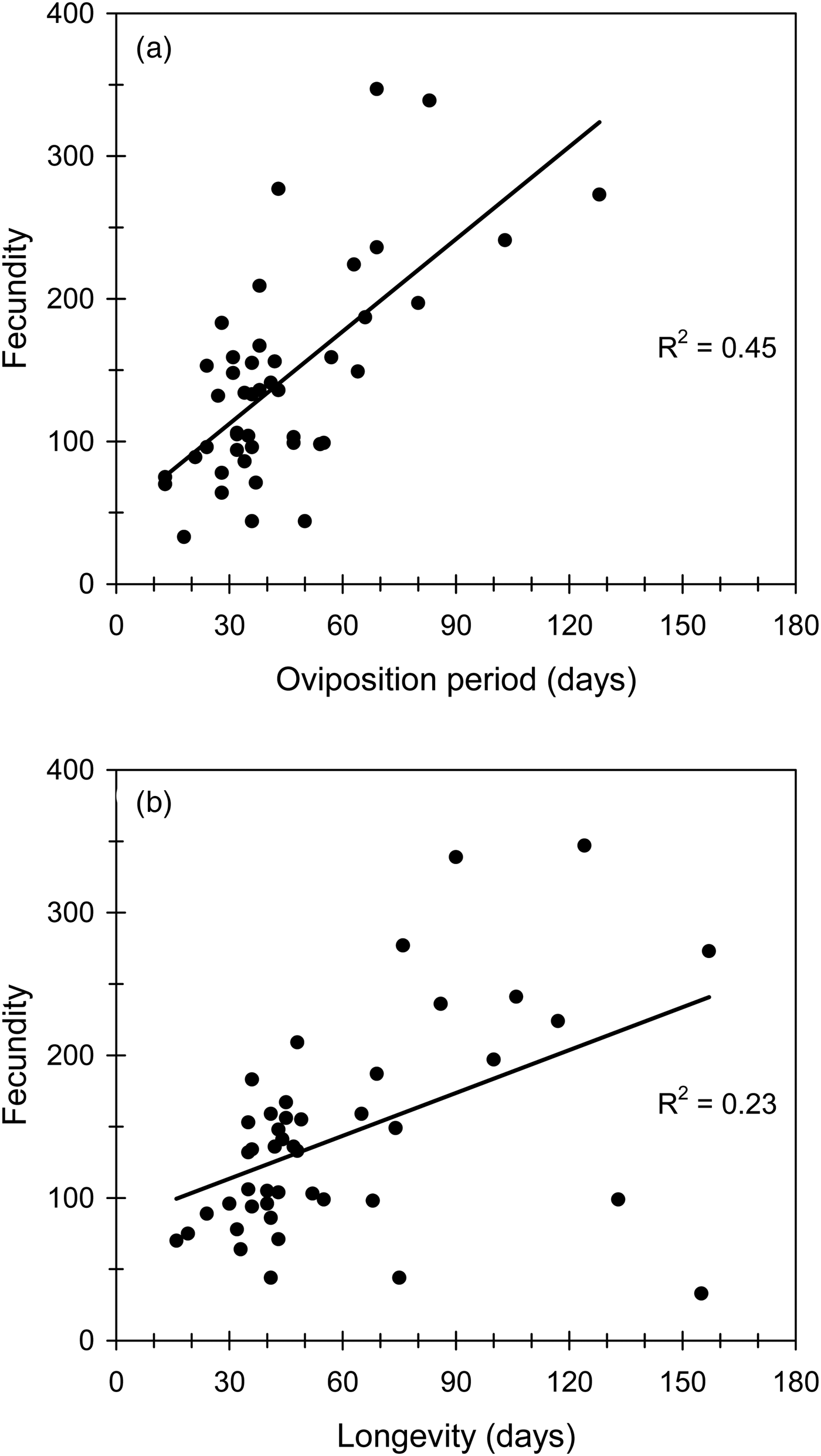
Fig. 3. The effect of either oviposition period (a) or longevity (b) on lifetime fecundity in Cc. Regression equations and statistics were: (a) y = 2.16x + 47.70, F 1,43 = 35.34, P < 0.001; (b) y = 1.00x + 83.33, F 1,43 = 12.50, P < 0.001.
Table 2. Linear regression analyses (Pearson's R correlation matrix) among the reproductive and associated biological traits of Cc females in laboratory-controlled conditions (25 ± 1°C, 60 ± 10% r.h.).
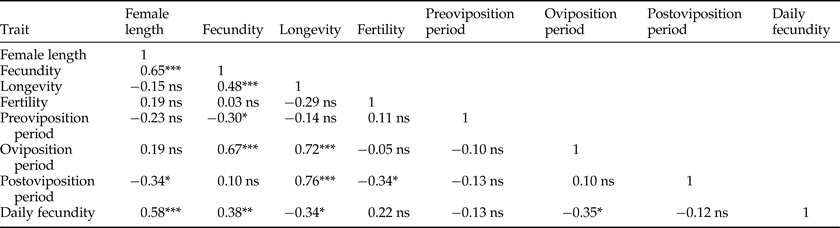
Signification levels: ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Daily fecundity averaged 3.5 eggs/day, but, there was a notable fluctuation over the oviposition period (from 0.9 to 6.5 eggs/day) even between consecutive days (table 1; fig. 4a). Despite such a fluctuation, daily and lifetime fecundity were significantly correlated (table 2). As expected, daily fecundity was negatively correlated with both oviposition period and female longevity (table 2). Mean daily fecundity also experienced a slight but significant decrease over the female's lifetime (fig. 4a). Mean daily fecundity was positively correlated with female size, with the results being very similar when days with no eggs laid were excluded (fig. 2b). Consequently, large females had a higher reproductive output than small females over the whole oviposition period (fig. 4a). Maximum daily fecundity ranged widely, depending on egg-laying day and female size from about 5 to 15–20 eggs/day, with some large females laying up to 30 eggs in a single day (fig. 4b).
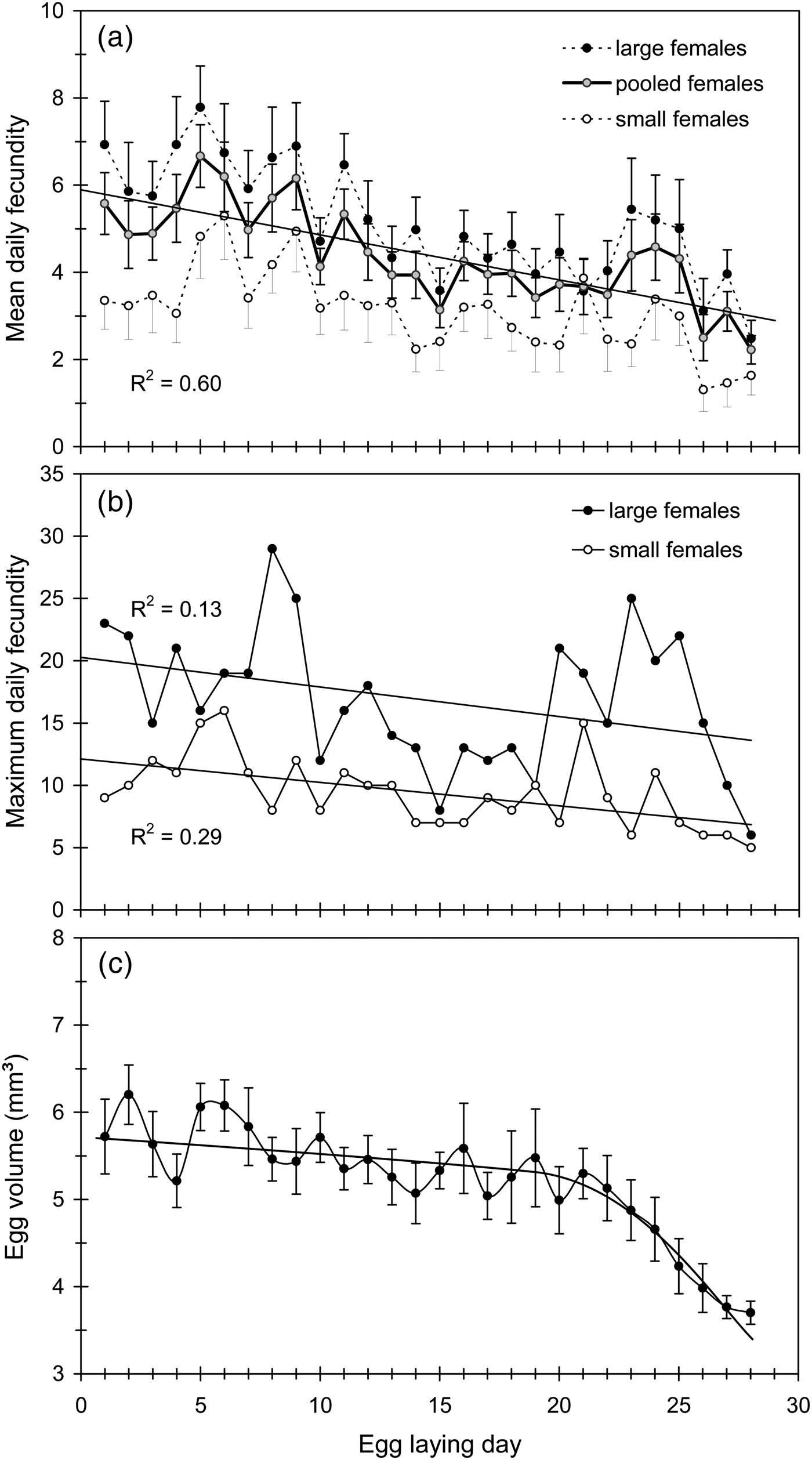
Fig. 4. (a) Mean daily fecundity (by female size and pooled), (b) maximum daily fecundity (by female size) and (c) egg size (egg volume) variation over the oviposition period in Cc. In the upper graphs, females were scored in two body size classes (large and small, above and below mean female length: 44.1 mm, see table 1). Vertical lines represent the SE of the mean.
Cc eggs were relatively large (table 1). Egg size showed a slight decreasing trend during the first three oviposition weeks and then experienced a sudden drop over the 4th week (fig. 4c). Egg size (volume) was strongly correlated with female size (fig. 5a). However, the relative size of eggs (the ratio egg length/female length) was negatively correlated with female size, so that smaller females produced proportionally larger eggs (fig. 5b). The nested ANOVA computed to assess the effect of egg size (nested to female as random factor) on egg hatching confirmed that egg size largely varied among females (F 19,20 = 10.38, P < 0.001) and also showed that hatched eggs tended to be larger than unhatched ones (F 20,1148 = 1.70, P < 0.05) indicating that hatchability was dependant on egg size.
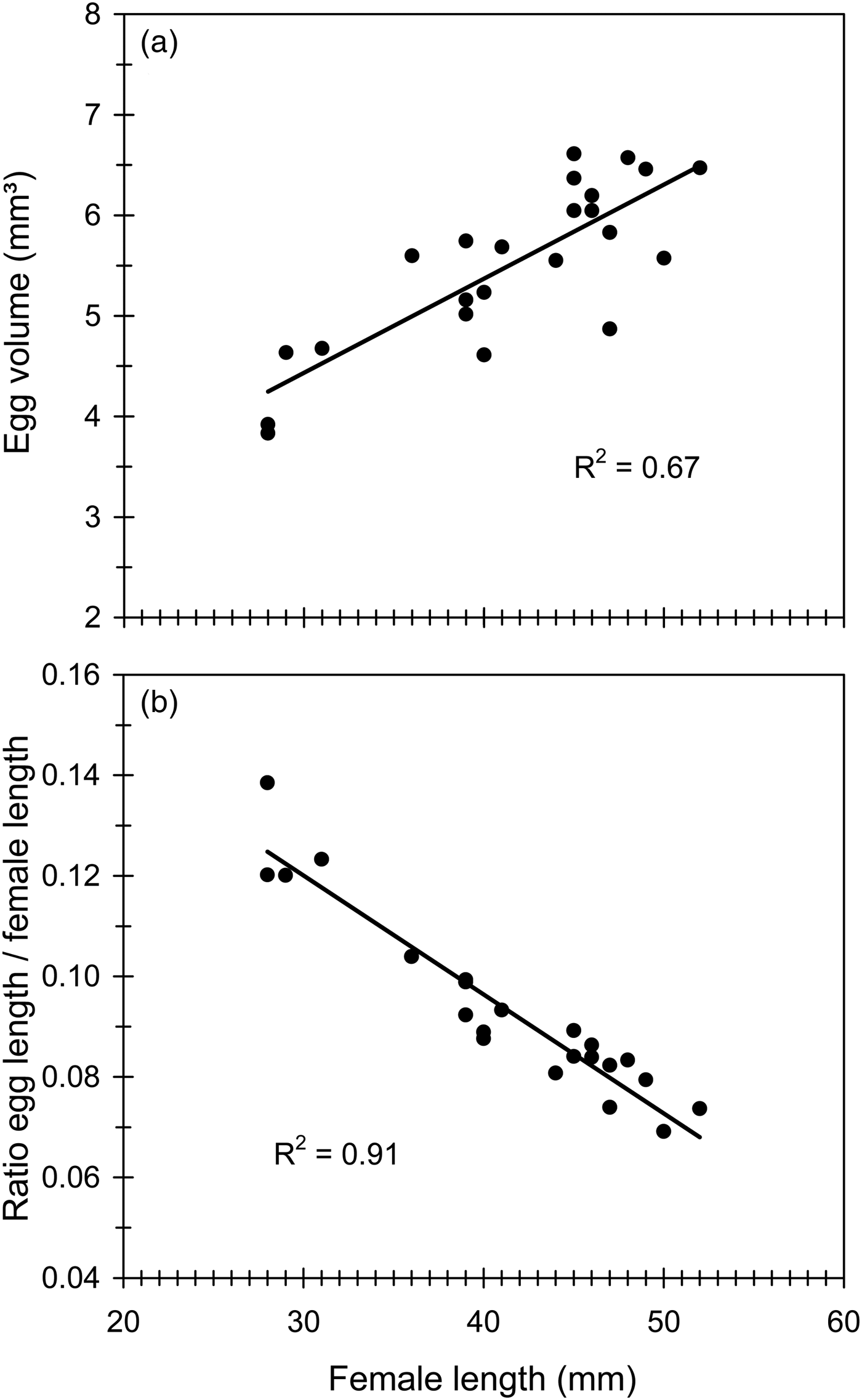
Fig. 5. The effect of female size (female length) on egg size (egg volume) (a), and the effect of female length on the ratio egg length/female length (b) in Cc. Regression equations and statistics were: (a) y = 0.094x + 1.63, F 1,21 = 41.52, P < 0.001; (b) y = – 0.003x + 0.19, F 1,21 = 202.09, P < 0.001.
Incubation time averaged almost 2 weeks (table 1) and was unrelated to female size (F 1,18 = 0.13, P = 0.73, n = 20). Incubation time was quite variable, not only among females, but, also among eggs laid by the same female. Eggs laid by a single female in the same day very often did not hatch synchronously, a variation of up to 2 weeks being recorded in some cases. A two-way ANOVA showed that incubation time was significantly affected by individual female (F 19,622 = 10.74, P < 0.001) and that it was unaffected by oviposition week, a proxy measure of female age (F 1,622 = 0.06, P = 0.81). However, there was an interaction between the two factors (F 19,622 = 3.10, P < 0.001) making interpretation difficult. Successive one-way ANOVAs for each female showed that the interaction arose because, the effect of oviposition week on incubation time was female-dependent. In 15 females (75%), incubation time did not change with female age, in three females (15%) incubation time decreased with female age, and in two females (10%) incubation time increased with female age. Neonate size (head width) was positively correlated with egg volume (R 2 = 0.19, F 1,63 = 14.43, P < 0.001), but, was unrelated to egg length (R 2 < 0.001, F 1,63 = 0.10, P = 0.76). Unexpectedly, mandible size was not significantly correlated with head width in neonates (R 2 = 0.01, F 1,63 = 0.63, P = 0.43).
Cc females were polyandrous and some of them mated up to 19 times during their lifetime (table 1). The two-way ANOVA showed that fecundity did not depend on mating number (F 1,41 = 1.98, P = 0.17) and confirmed that fecundity increased with female size (F 1,41 = 8.19, P < 0.01), with no interaction between the two factors (F 1,41 = 0.002, P = 0.97). Linear regression showed that polyandry did not increase egg fertility (R 2 = 0.02, F 1,43 = 0.91, P = 0.35). Polyandry was positively correlated with oviposition period (R 2 = 0.18, F 1,43 = 9.13, P < 0.01) and also marginally with longevity (R 2 = 0.10, F 1,43 = 4.28, P = 0.045). Finally, we observed that larger females experienced a slight trend to mate multiply (R 2 = 0.30, F 1,43 = 4.32, P = 0.044).
Males
Estimates of the reproductive and biological traits of Cc males were calculated (table 1). As in females, male longevity was not correlated with body size (R 2 ~ 0.00, F 1,48 = 0.001, P = 0.97). Results showed that Cc males were polygynous and some of them mated up to 16 times during their lifetime (table 1). As with females, tests were not designed to ascertain the maximum mating potential of males, and so mating number may be higher in the wild. Fights for mates, female harassment, punishing of resisting females and mate guarding (pair-bonding) were commonly observed male behaviours during tests. Mating number was unrelated to male size (R 2 ~ 0.00, F 1,48 = 0.07, P = 0.79) and mating history had no detectable detrimental effect on male longevity. Long-lived males were able to mate more times during their lifetime because they had more mating chances (R 2 = 0.31, F 1,48 = 21.97, P < 0.001). Finally, a comparison between sexes (ANOVA) showed that females were larger (F 1,93 = 4.10, P < 0.05) and may live longer than males, although this observation was determined at the limit of the significance (F 1,93 = 3.49, P = 0.06 ~ 0.05).
Discussion
As in most animals, reproductive output in insects is directly related with (although not equivalent to) adult fitness, so its estimation is essential to optimize protection guidelines in endangered species such as Cc. Reproductive output assessment is also relevant to improving short-term management tactics when Cc populations attain a harmful or pest status derived from natural causes or anthropogenic interference. Our results showed that reproductive output of Cc females was quite similar to the average value for the Cerambycidae family (see Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016 for a mini review). Mean Cc fecundity reported in this study was slightly higher (almost 10%) than in the similar-sized congeneric species Cw in the same experimental conditions (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016). By contrast, fecundity obtained in this study was more than twice that reported by El Antry (Reference El Antry1999) perhaps due to between-population differences in female size or experimental conditions.
Female size had a central effect on reproductive output. Larger Cc females showed higher lifetime fecundity and daily fecundity, and also produced larger eggs and neonates. A positive correlation between fecundity and female size is widespread in insects (Honěk, Reference Honěk1993), including longhorn beetles, both Cerambycinae (Iwabuchi, Reference Iwabuchi1988; Matsumoto & Irianto, Reference Matsumoto and Irianto1998; Wang et al., Reference Wang, Shi and Davis1998, Reference Wang, Shi, Song, Rogers, Davis and Chen2002; Kato et al., Reference Kato, Yamada and Shibata2000; Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016) and Lamiinae (Lawrence, Reference Lawrence1990; Togashi, Reference Togashi1997, Reference Togashi2007; Keena, Reference Keena2002; Togashi et al., Reference Togashi, Appleby, Oloumi-Sadeghi and Malek2009). Moreover, a large variation in adult size is often reported within cerambycids (especially in large-sized species) and other wood-boring insects, depending on an array of factors including inter-annual temperature differences, multi-seasonal larval development, host tree species, host tree age and, especially, host wood quality as larvae cannot move between trees to improve their nutritional status if host quality is poor (Starzyk & Strojny, Reference Starzyk and Strojny1985; Hanks et al., Reference Hanks, Paine and Millar2005; Walczyńska et al., Reference Walczyńska, Dańko and Kozlowski2010; Michalcewicz & Ciach, Reference Michalcewicz and Ciach2012; Lupi et al., Reference Lupi, Jucker, Rocco, Harrison and Colombo2015; Torres-Vila et al., Reference Torres-Vila, Zugasti, De-Juan, Oliva, Montero, Mendiola, Conejo, Sánchez, Fernández, Ponce and Espárrago2015). In addition, wood-borers often produce smaller adults when adverse environmental conditions arise rather than fail to attain adulthood (Andersen & Nilssen, Reference Andersen and Nilssen1983), a plastic response with evident adaptive significance.
Cc adults were long-lived (about 2 months on average), even if longevity was seen to be extremely variable among individuals in both sexes (from 2–3 weeks to >5 months). These longevity records are in agreement with values reported by Duffy (Reference Duffy1953), and with maximum life spans recorded in a recent mark-recapture study in the same area that varied between 39 and 56 days depending on sex (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz and Sánchez-González2017). Longevity of Cc was also consistent with the longevity of Cw observed under the same experimental conditions (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016). However, Cc longevity estimates were substantially higher than those reported by El Antry (Reference El Antry1999), who recorded mean longevity of 8–11 days (perhaps due to the use of uncontrolled-age adults or suboptimal rearing conditions). In this regard, it has been reported that Cw longevity may be drastically reduced in experimental arenas in which target adults are continuously grouped, attributed to the high stress derived from the intense interaction, competition, harassment and fights among conspecifics (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016). It follows that if Cc longevity is shorter in the wild than in the laboratory, then reproductive output will also be proportionally reduced.
Fecundity was positively related to longevity, as observed in other longhorn species (Iwabuchi, Reference Iwabuchi1988; Wang et al., Reference Wang, Shi and Davis1998; Togashi et al., Reference Togashi, Appleby, Oloumi-Sadeghi and Malek2009; Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016). Long-lived Cc females were more fecund in part because they also exhibited extended oviposition periods. Oviposition period averaged 1.5 months in Cc, but was extremely variable among females (from 13 to 128 days), as was reported in Cw (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016). The postoviposition period was also quite irregular and unexpectedly long in some individuals. Many females did not oviposit for a long time after reproducing (up to 126 days), while their fecundity was not significantly reduced. This somewhat remarkable and apparently non-adaptive situation has also been described in Cw and tentatively attributed to an imbalance between the reproductive and somatic reserves derived from good adult feeding in laboratory conditions (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016). The large unexplained variation among females in the postoviposition period contributed to the lack of correlation between longevity and female size as reported in other cerambycids (Koutroumpa et al., Reference Koutroumpa, Vincent, Roux-Morabito, Martín and Lieutier2008; Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016). As in females, male longevity was unaffected by male size.
Cc females exhibited a typical synovigenic egg-laying pattern, as they produced, matured and laid eggs at a moderate rate throughout an extended oviposition period. There was a slight decreasing trend over time in daily fecundity (both mean and maximum values), with oviposition rates being always higher in larger than smaller females. Therefore, daily fecundity was positively related with female size, but, negatively related with female age, as reported in other cerambycids (Lawrence, Reference Lawrence1990; Keena, Reference Keena2002; Smith et al., Reference Smith, Bancroft and Tropp2002), including Cw (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016). Synovigeny is characteristic of long-lived income-breeder species that rely on feeding in adult stage to gather vital energy resources for reproduction and somatic maintenance (Stearns, Reference Stearns1977). Synovigeny appears to be widespread in cerambycids (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016 and references therein), although a few short-lived species producing egg clusters show proovigeny like those in the genus Xystrocera (Matsumoto et al., Reference Matsumoto, Santosa, Nazmulah and Irianto1996; Matsumoto & Irianto, Reference Matsumoto and Irianto1998). From an ecological perspective, synovigenic patterns may entail both fitness advantages and constraints in a species such as Cc. Thus, females may benefit from a reduced daily egg-laying rate because, more time devoted to the right location of each single egg in the tree bark could enhance female fitness if predation and parasitism rates are minimized in better hidden eggs. By contrast, this scenario could be reversed if early female predation occurs. A high fluctuation in maximum daily fecundity occurred among and within Cc females, with values fluctuating from 0 to 30 eggs. Irregular oviposition patterns are common in cerambycids (Shibata, Reference Shibata1987; Jikumaru et al., Reference Jikumaru, Togashi, Taketsune and Takahashi1994; Keena, Reference Keena2002; Koutroumpa et al., Reference Koutroumpa, Vincent, Roux-Morabito, Martín and Lieutier2008; Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016), and tentatively attributed to intraspecific differences in female size, oogenesis rate or ovariole number (Togashi et al., Reference Togashi, Appleby, Oloumi-Sadeghi and Malek2009). It has been also suggested that due to an extremely long mating duration, females may be constrained by males on mating days to lay eggs (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016) even if pair-bonded females are able to oviposit between intromissions.
Cc eggs were relatively large (length × width: 3.7 × 1.9 mm2) with mean dimensions slightly longer than those previously reported by other authors: 3.3 × 1.6 mm2 (Marovic, Reference Marovic1973), 2.5–3 × 1 mm2 (El Antry, Reference El Antry1999) and 3.1 × 1.5 mm2 (Vitali, Reference Vitali2001). Mean egg size in Cc remained relatively constant throughout most of the oviposition period and only decreased rapidly in the 4th week, probably due to a depletion of energy resources not compensated for by adult feeding (Torres-Vila & Rodríguez-Molina, Reference Torres-Vila and Rodríguez-Molina2002). As in most insects, larger longhorn females produce larger eggs (Togashi et al., Reference Togashi, Akita, Nakane, Shibata and Nakai1997; Kato et al., Reference Kato, Yamada and Shibata2000; Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016), resulting from the allometric scaling between female size and egg size. This applies even if in relative terms (in relation to female size) eggs of Cerambycinae are smaller than those of Lepturinae and Lamiinae (Hernández, Reference Hernández1991), suggesting in turn an underlying phylogenetic background regulating egg size. We discovered that Cc females laid more eggs than Cw females and those eggs were significantly smaller in Cc (about 67% in volume) than in Cw (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016) when controlling for body size. From the perspective of the reproductive energetic balance (with the female's reproductive effort fixed), smaller egg size is consistent with higher fecundity (Torres-Vila et al., Reference Torres-Vila, Cruces Caldera, Rodríguez-Molina and Cauterruccio2012b and references therein). However, from an evolutionary perspective, such a variation in egg size is remarkable between two sympatric and congeneric species with similar size and analogous behaviour exploiting the same oak forest habitat. Large Cc eggs hatched better than small ones, whereas in Cw hatching success did not depend on egg size (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016).
The potential adaptive significance of the interspecific variation in egg size between Cc and Cw is unknown and only speculation is possible. One factor could be the distinct selective force exerted by egg parasitoids of the genus Oobius. Preliminary results with sentinel eggs show that the parasitism rate exercised by Oobius is higher in Cc than Cw, and so a smaller egg size in Cc could be a selective response being driven by these parasitoids. Another factor involved could be a species-specific trade-off between egg size and larval performance. Our results show a positive correlation between neonate size (head width) and egg size (volume), as reported in others cerambycids (Kato et al., Reference Kato, Yamada and Shibata2000; Walczyńska, Reference Walczyńska2008). However, we also found unexpected differences between Cc (this study) and Cw (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016) regarding egg (and neonate) allometry, and have no explanation. For instance, in Cc, neonate size (head width) was correlated with egg volume but not with egg length, while the opposite situation was found in Cw. Moreover, mandible size of neonates was not correlated with head width in Cc, although it was in Cw. Such differences could arise if mandible size in Cc has reached a minimum selective value (so that current response to selection is non-existent), whereas neonate size could still be under negative selection. In this regard, it should be noted that mean neonate size (head width) found in this study (1.04 mm) was larger than the 0.87 mm reported in Moroccan populations (El Antry, Reference El Antry1999). A more closely defined set of experiments is required to explore whether such allometric relationships have an underlying adaptive significance.
The adaptive significance of egg size in insects is rather controversial, as there is no generalizable relationship between egg/neonate size and larval performance. In some species, however, large neonates have an adaptive advantage over small ones in adverse environments (Torres-Vila & Rodríguez-Molina, Reference Torres-Vila and Rodríguez-Molina2002; Torres-Vila et al., Reference Torres-Vila, Cruces Caldera, Rodríguez-Molina and Cauterruccio2012b). The adaptive advantage of larger progeny size could be important in cerambycids developing in hardwood trees (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016). A better performance of larger neonates could result from their advantage in initial host perforation or making larvae more skilled to offset the array of physical and biochemical countermeasures that host tree displays in response to borer injury (Hanks, Reference Hanks1999; Kato et al., Reference Kato, Yamada and Shibata2000; see Sallé et al., Reference Sallé, Nageleisen and Lieutier2014 for a review). However, available evidence in cerambycids (as in most insects) supporting a higher performance in larger than smaller larvae is scarce (but see Togashi et al., Reference Togashi, Akita, Nakane, Shibata and Nakai1997; Kato et al., Reference Kato, Yamada and Shibata2000). The adaptive significance of neonate size could also be related with the fact that small females laid proportionally larger eggs than large females when controlling for body size (Togashi et al., Reference Togashi, Akita, Nakane, Shibata and Nakai1997; Yumino & Togashi, Reference Yumino and Togashi2015; Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016; this study). The impact of neonate size on early survival and larval performance merits further research in Cerambyx species and other wood-borer insects given its potential adaptive significance.
Mean incubation time of Cc eggs was 13.5 days, more than twice the 6.5 days reported by El Antry (Reference El Antry1999) under the same temperature regime (25°C). This noteworthy difference between Spanish and Moroccan populations suggests heritable variation in the trait. The huge variation recorded in incubation time among females in our study (7–28 days) support that suggestion. It is worth noting that incubation time is likely to be heritable in longhorns because it depends on phylogeny and varies among subfamilies (Hanks, Reference Hanks1999). As reported for Cw (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016), we observed that some Cc larvae, already formed inside the chorion, delayed the expected eclosion date and thereby contributed to a lengthened incubation time. A notable variation in incubation time within cerambycid species has been reported (Morgan, Reference Morgan1960) and recently revisited in Psacothea hilaris Pascoe (Yumino & Togashi, Reference Yumino and Togashi2015) and Cw (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016). It has been also suggested that a strong variation in incubation time within species (and within a single female's progeny) could arise from a risk-spreading (bet-hedging) strategy if females undergo significant fitness gains when at least a fraction of their progeny manages to avoid unfavourable and unpredictable environmental conditions (Hopper, Reference Hopper1999; Yumino & Togashi, Reference Yumino and Togashi2015).
Both sexes of Cc mated multiply during their lifetime; up to 16 and 19 matings in males and females, respectively. Mating number could be higher in the wild as our experiments were not designed to ascertain the maximum mating potential. Polyandry did not increase fecundity in Cc as also observed in Cw (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016). Polyandry was positively correlated with oviposition period and longevity, but our experimental design did not allow us to establish a causal relationship supporting male nuptial gifts increasing female lifespan, as long-lived females also had more mating opportunities. Polygyny in turn was not detrimental to male longevity; in fact, long-lived males mated more times because they also had more mating chances. Our results suggest that the adaptive significance of high polygyny and polyandry in Cc would be more likely to promote cryptic female choice and sperm competition than exploit male-derived nutrients to improve fecundity (e.g. Arnqvist & Nilsson, Reference Arnqvist and Nilsson2000; Gillot, Reference Gillot2003; Torres-Vila et al., Reference Torres-Vila, Rodríguez-Molina and Jennions2004; Torres-Vila, Reference Torres-Vila2013). However, it has been suggested that a reduced effect of male-transferred substances on female fecundity could become significant if females are suffering a feeding deficit in the wild (Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016); an idea that needs further research. The impact of multiple mating on female reproductive output in cerambycids is still poorly understood. Cerambycids are a very diverse group, and so reproductive (and behavioural) traits largely differ between and within subfamilies (Linsley, Reference Linsley1959; Hanks, Reference Hanks1999; Allison et al., Reference Allison, Borden and Seybold2004; Torres-Vila et al., Reference Torres-Vila, Mendiola-Diaz, Conejo-Rodríguez and Sánchez-González2016), with environmental conditions also being extremely important (Hanks, Reference Hanks1999; Keena, Reference Keena2002, Reference Keena2006; Smith et al., Reference Smith, Bancroft and Tropp2002; Wang et al., Reference Wang, Shi, Song, Rogers, Davis and Chen2002; Millar et al., Reference Millar, Paine, Joyce and Hanks2003; Bybee et al., Reference Bybee, Millar, Paine, Campbell and Hanlon2004).
Conclusions
We conclude that the information provided in this study on the basic aspects of the reproductive biology of Cc may be useful for developing species-specific conservation guidelines and management strategies depending on the ecological prevalence of this charismatic longhorn in each particular oak forest context.
Acknowledgements
The author is very grateful to the other members of the Integrated Protection team, Y. Conejo Rodríguez, F.J. Mendiola Diaz, Á. Sánchez González, F. Ponce Escudero, R. López Calvo, F. Fernández Moreno, E. Cruces Caldera and L. López Pajares for their technical assistance. The author is indebted to the owners of the dehesas, Juande del Pozo Quintanilla (dehesa La Jara) and Juan Gragera Facundo (dehesa La Serrana) for their kind permission to investigate and the logistic support provided. The author acknowledges the valuable comments and suggestions provided by two anonymous reviewers and the journal editor. This research was supported by the Servicio de Sanidad Vegetal (SSV, Junta de Extremadura) and benefited from ERDF/FEDER funding to the GR15112 research group.









