Introduction
Cultivation of genetically modified (GM) crops has increased dramatically in the world since the first transgenic variety was commercially sown 17 years ago. In 2012, world area planted with GM crops reached 170 million ha (James, Reference James2012). Although the adoption rate of GM crops in the world has been particularly high, their deployment is controversial in some countries, particularly in Europe. One of the main concerns about GM crops is their potential impact on the environment, including biodiversity and ecological services provided by non-target organisms, among which arthropods (non-target arthropods, NTAs) are a key element. Biological control performed by insect natural enemies may be interfered if GM crops alter the abundance, diversity, or activity of arthropods such as herbivores and subsequent trophic levels, including insect predators and parasitoids. In addition, other arthropods inhabiting agricultural ecosystems that contribute to the decomposition of organic matter are important for building food webs and may play a major role in the biodiversity and abundance of other invertebrates and vertebrates in the landscape. Safety assessment of GM crops on NTAs has become mandatory in countries that seek to cultivate GM crops.
A sequential approach, from the laboratory to the field, to testing potential side effects of GM crops on NTAs has been proposed (Romeis et al., Reference Romeis, Bartch and Bigler2008). Proceeding to field testing transgenic traits or their products is challenging because of the difficulty in correctly interpreting the results in complex systems and because of its high cost. However, if early tier studies indicate potential risks, then field tests may be an appropriate way to check if negative impacts may occur in real conditions. Furthermore, for GM crops that do not express a toxin, field tests may be the only way to proceed because no specific hypotheses to be tested in the laboratory can be formulated or because no laboratory tests are available. Although many field trials for environmental risk assessment have been carried out in Europe (see a review in Ortego et al., Reference Ortego, Pons, Albajes, Castañera, Ferry and Gatehouse2009), several aspects of their design remain a subject of consideration. Whether it is necessary to sample a broad range of NTAs living in the crop or if it is preferable to select a few representative taxa that can serve as surrogates of major taxonomic or functional guilds is debatable. The representative biota that plays important roles in biological control functions may include non-target prey species, predators, and parasitoids.
A central concern in the design of field trials to assess non-target effects of a GM crop is whether field tests are capable of detecting the effects of treatments on NTAs when these actually exist. These effects are usually expressed as a percentage of the control mean density and are called detectable treatment effect (d c). The lower the d c, the higher the detection capability of the field test and taxon. Moreover, it is often considered that dc must be of at least 50% of the average value of the control or comparator (e.g., Perry et al., Reference Perry, Rothery, Clark, Heard and Hawes2003; Naranjo, Reference Naranjo2005; EFSA, 2010).
In most field tests, α [type I error, i.e., probability of rejecting the null hypothesis (H0) of no differences between a GM variety and a non-GM comparator or control when it is actually true] is usually set to 0.05 and β (type II error, i.e., probability of not rejecting H0 when it is not true) to less than 0.2. When the α and β values have been set, the value of d c depends mainly on the unknown experimental variability of the sampled taxa. This variability depends on taxon characteristics, making some taxa not suitable for being selected as representative taxa. Hence, the interest in database is large enough to enable to select the most appropriate taxa for field trials (e.g., Perry et al., Reference Perry, Rothery, Clark, Heard and Hawes2003; Prasifka et al., Reference Prasifka, Hellmich, Dively, Higins, Dixon and Duan2008).
Here, we aim to identify those NTA with higher capacity to detect the effects of GM maize on biological control functions. For this, we have examined several field trials conducted by authors from 2000 to 2010 in the northeast of the Iberian Peninsula to assess the potential risks of GM maize on NTAs and calculated the effect detection capacity of NTAs recorded.
Materials and methods
Field trials
The authors conducted 14 field trials to assess the effects of Bacillus thuringiensis (Bt) Berliner and herbicide-tolerant (HT) GM maize on NTAs. The 14 field trials covered a range of variable characteristics that are common in field testing of GM crops: different numbers of years (1, 2, or 3 years), sampling dates (4 to 10), numbers of treatments (2 to 10 with transgenic vs. near-isogenic varieties, pesticide vs. pesticide-free treatments, and a certain number of reference varieties), and different numbers of single Bt, or HT, or stacked traits (Table 1). All field trials were conducted from May to early October in the Lleida basin (NE Iberian Peninsula), an area where more than 70% of the maize grown in recent years has been Bt because of the high pressure of maize borer populations, Ostrinia nubilalis Hübner (Lepidoptera: Crambidae) and Sesamia nonagrioides Lefèbvre (Lepidoptera: Noctuidae). The area has a Mediterranean climate with high temperatures (annual maximum temperatures between 25 and 40 °C) and mean rainfall lower than 200 mm along the season and maize can be grown only with irrigation. The experimental maize plots were sized between 1000 and 5000 m2, with a shape close to a square, and they were always arranged in a randomized complete block design, with three or four blocks. NTA abundance was estimated by visual counting and capture in pitfall and yellow sticky traps, the most used sampling techniques in environmental risk assessment and post-market environmental monitoring studies. Abundance of crop plant-dwelling predators and herbivores was estimated by visually counting the number of individuals on a variable number of plants (from ten plants at mid maize growth stages to 25 plants at earlier and late growth stages) per plot. Three pitfall were arranged in each plot, regularly distributed along the plot length but at least 10 m from the field border, and left active for 5 days. Pitfall traps consisted of a glass jar of 9 cm diameter and 17 cm depth filled with water and detergent. Three yellow sticky traps per plot (21×31 cm, only one sticky side; Serbios, Badia Polesine, Italy in trials 2–9 and 28×22.8 cm, two sticky sides, Pherocon AM, Trece Inc, Salinas, CA, USA in trials 1 and 10–14) were put on a stake at canopy height (until V12) or at ear level (from V15 onward) and left active for 5 days. For more details of the sampling techniques, see Poza et al. (Reference Poza de la, Pons, Farinós, López, Ortego, Eizaguirre, Castañera and Albajes2005) and Albajes et al. (Reference Albajes, Lumbierres and Pons2009). Individuals were identified to different taxonomic levels. Vouchers are deposited in the Laboratory of Entomology at the University of Lleida.
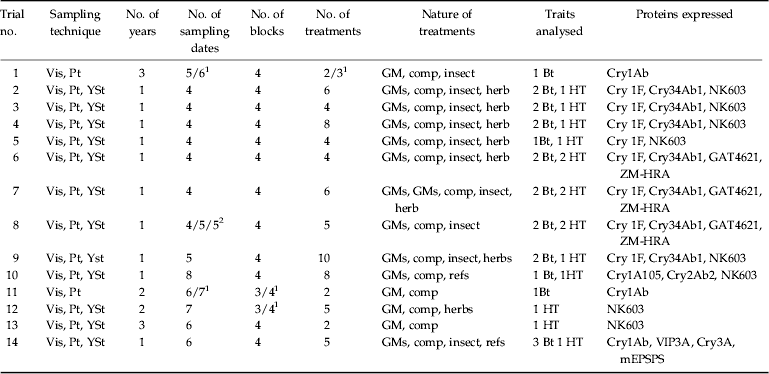
Table 1. Main characteristics of the field trials analysed. Sampling techniques were visual counting (Vis), pitfall traps (Pt), and yellow sticky traps (YSt). Trials were conducted along a different number of years (1–3) and plots were sampled at a different number of dates (4–10). The different kinds of treatments are shown in a Table footnote. Traits analysed were different Bacillus thuringiensis (Bt) and herbicide-tolerant (HT) traits expressing the proteins shown on the last column.

1 Variable with the year.
2 Number of sampling dates with Vis, Pt, and YSt, respectively.
Key of treatments. GM, transgenic trait; GMs, several or stacked traits; comp, comparator, near isogenic variety; insect, insecticide treatment; herb, broad spectrum herbicide; herbs, combination of broad spectrum and conventional herbicide; refs, reference non-transgenic varieties.
More detailed information of some of the trials can be found in the following references. Trial 1 (Poza et al., Reference Poza de la, Pons, Farinós, López, Ortego, Eizaguirre, Castañera and Albajes2005); Trial 11 (Albajes et al., Reference Albajes, Farinós, Pérez-Hedo, Poza de la, Lumbierres, Ortego, Pons and Castañera2012); Trial 13 (Albajes et al., Reference Albajes, Lumbierres and Pons2009, Reference Albajes, Lumbierres and Pons2011).
Computing d c
Field test detection capacity (d c) was computed using equation (1) derived from the two independent samples classical difference t test:
where s c is NTA density or abundance relative experimental variability of sampled data. i.e., the ratio between sampled NTA experimental variability (s) and the comparator's average (m c), t (1−α/2) and t β the (1−α/2) and β the centiles of the t distribution with n degrees of freedom and N the number of replicates of the treatments. The probability of types I and II errors (α and β) were fixed at 0.05 and 0.2, respectively. In equation (1), d c is expressed as a percentage of the mean value of the controls (comparators). Percentages of 50 or 25% were considered acceptably low to detect medium or low effects in accordance with many authors in the literature of environmental risk assessment (e.g., Perry et al., Reference Perry, Rothery, Clark, Heard and Hawes2003; Naranjo, Reference Naranjo2005; EFSA, 2010).
Before analysis, NTA density or abundance data (x) were transformed using log10 (x+1) rather than the SQRT (x+0.5) used in previous field studies published by the authors, because the former gave a better normalization and homogeneity of variances in most cases and it is the transformation recommended by EFSA (2010).
Sampled NTA experimental variability (s) was obtained from the square root of mean squares of the residual error term from the analysis of variance (ANOVA) analysis of the model described in (2) including all treatments, not just the transgenic treatment and corresponding near-isogenic variety plots.
As interactions between treatment and sampling dates were seldom significant (P>0.05) and the main focus of the present analysis was not population dynamics, NTA abundance and captures were expressed as mean season values, i.e., all sampling dates were pooled. However, because the values of NTAs recorded in visual sampling before growth stage V6 (plants with six fully developed leaves) were essentially zero, these data were not taken into account in the calculation of seasonal averages. Moreover, since no significant interactions were detected between year, block, and treatments (P>0.05), these interactions were not taken into account in equation (2). In each field trial all treatments (GM, near isogenic, and reference varieties) were considered in the analysis. All factors except blocks were considered fixed.
We take the abundance of Orius spp. in visual counting in trial # 1 (Table 2) as an example to compute field test detection capacity. This trial was repeated 3 years. It consisted of two treatments (GM vs. near-isogenic variety), randomly allocated to four blocks. Therefore, the total number of plots were 24 (3 years×4 blocks×2 treatments) and consequently, the number of replicates of each treatment (N) were 12 (3 years×4 blocks). In total, nine sampling campaigns were performed each year; however the first sampling in the season was not considered in the calculations because densities were very low in all treatments. According to equation (2), the number of degrees of freedom of s was 17, (24 plots-1) – (3 years-1) – (4 blocks-1) – (2 treatments-1). Consequently given that α and β, was set to 0.05 and 0.2, respectively, the values of t (1−α/2) and t β were 2.11 and −0.86, respectively. In terms of t values, the minimum treatment increase (or decrease) of the mean of the control (μ0) that the field test was able to detect (δμ) can be computed as t (1−α/2)−t β, with our data δμ took the value 2.97.
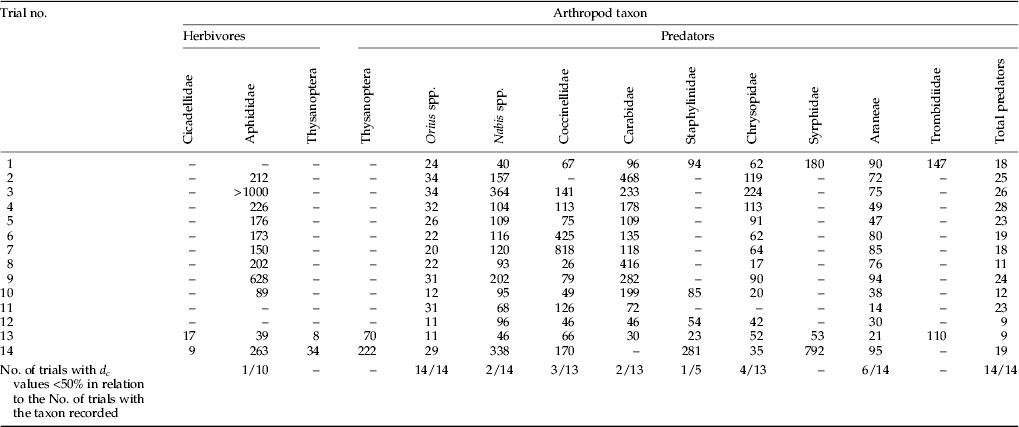
Table 2. Detectable treatment effects (d c) of visual sampling, expressed as a percentage of the seasonal average value of the comparator variety (α=0.05, 1−β=0.8). Both adults and juveniles are included.

Before performing the analysis, NTA abundance obtained in the field (x) were transformed by log10 (x+1). The value assigned to each plot was the mean of the eight sampling campaigns. The mean of the control (m c) was 0.238. The experimental variability of NTA (s) was obtained from the square root of mean squares of the residual error term from the ANOVA analysis of equation (2); the value obtained was 0.047. The value of the detection capacity of the field test (d) was 0.057, given by ![]() $s \times \sqrt {2/N} \times {\rm (}t_{{\rm (}1 - \alpha /2{\rm )}} - t_{\rm \beta )}$, i.e., 0.047×0.408×2.97. Finally, the detection capacity relative to the mean of the control (d c), expressed as percentage, was 24%, i.e., 0.057/0.238×102 (equation 1).
$s \times \sqrt {2/N} \times {\rm (}t_{{\rm (}1 - \alpha /2{\rm )}} - t_{\rm \beta )}$, i.e., 0.047×0.408×2.97. Finally, the detection capacity relative to the mean of the control (d c), expressed as percentage, was 24%, i.e., 0.057/0.238×102 (equation 1).
All statistical analyses were performed using the R statistical package (R Development Core team, 2008).
Results
A total of 25 different arthropod taxa classified as herbivores, predators, parasitoids, and decomposers were recorded with the three sampling techniques in most of the field trials conducted; seven with visual counting, six with pitfall traps and 12 with yellow sticky traps. Some other arthropods were also recorded but only occasionally in a few of the trials. The value of d c of NTA density and capture differences between GM varieties and non-GM comparators varied widely according to the sampling technique, the organism recorded, and the trial.
Visual counting
Table 2 shows the d c of organisms recorded by visual counting. In general, d c were fairly high, rarely dropping below 50% of the comparator mean value, indicating that nearly all taxa did not meet the acceptable d c criteria of 50% or below. For aphids, the only herbivores regularly recorded in visual counting, the d c was above 100% and only in one trial it was below 50%. Among predators, the d c for Orius spp. was always below 50% and in 7 out 14 trials it was below 25%. Spiders (Araneae) had the next lowest d c with a d c below 50% only in six cases, but in four cases the d c was between 50 and 80% and never was it above 100%. Chrysopidae showed d c below 50% in less than half of the trials. Coccinellidae, Carabidae, and Nabidae gave even poorer results. The sparse values calculated for other predators were quite variable. However, the total number of predators showed a d c far lower than 50% in all trials.
Pitfall traps
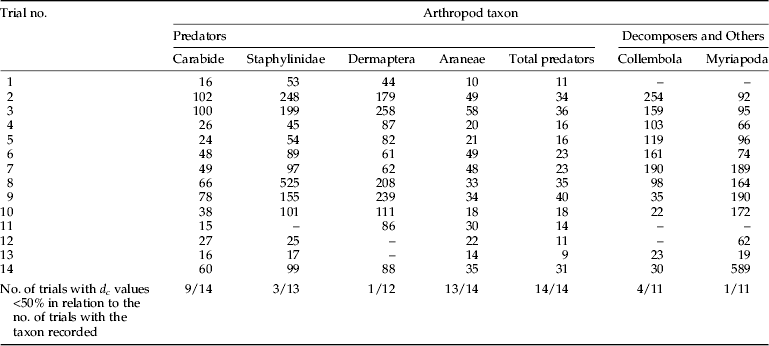
Table 3 shows the d c of organisms recorded in pitfall traps. Among predators, the Araneae taxon showed d c equal to or lower than 50% in all trials but one and in six trials the values were even lower than 25%. Carabidae followed Araneae with the next lowest d c, which were under 50% in most trials (nine out of 14). Dermaptera and Staphylinidae had d c below 50% only in a few trials. The heterogeneous feeding group composed of Myriapoda (centipedes and millipedes) very rarely reached d c values below 50%. Decomposers represented by collembolans showed values below 50% only in four out of the 11 trials.
Table 3. Detectable treatment effects (d c) of pitfall trap sampling, expressed as a percentage of the seasonal average value of the comparator variety (α=0.05, 1–β=0.8). In predators, only adults were counted.

Yellow sticky traps
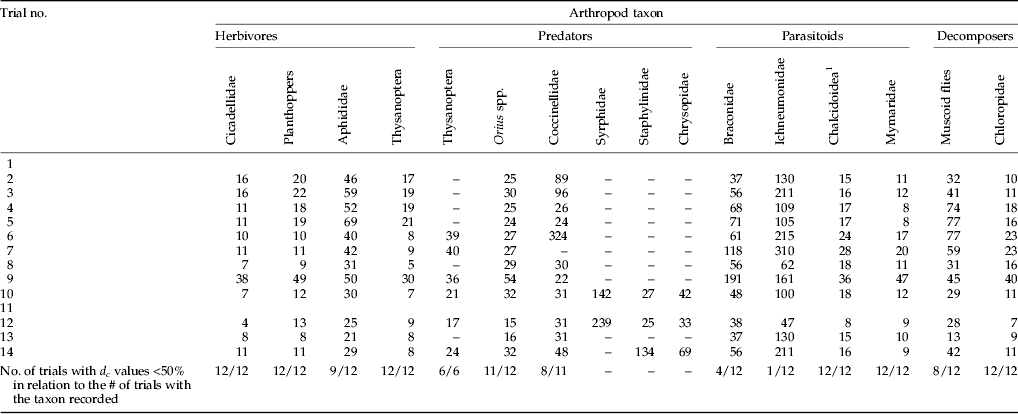
Several arthropods caught in yellow sticky traps showed excellent d c (Table 4). Herbivores, leafhoppers, planthoppers, and thrips, had d c below 50% in all trials, as did aphids in most trials (nine out of 12). This was also the case with the predators regularly recorded in most trials, such as the genus Orius and the family Coccinellidae. Moreover, d c values for Orius were below 25% in five cases. Predatory thrips were counted in six out of the 14 trials, and always showed d c values below 50%. Parasitoids had d c values below 50% only in the case of Chalcidoidea, and particularly the family Mymaridae, whereas Braconidae and Ichneumonidae only showed values below that threshold in four and one trials, respectively, of the 12 conducted with yellow sticky traps. The two taxa representing decomposers showed d c values below 50% in all cases for chloropids and in eight cases for muscoid flies.
Table 4. Detectable treatment effects (d c) of yellow sticky trap sampling, expressed as a percentage of the seasonal average value of the comparator variety (α=0.05, 1−β=0.8). Only adults and alate morphs were trapped.

1 Mymaridae excluded.
Discussion
In order to keep the amount of work for environmental risk assessment and post-market environmental monitoring feasible, it may be necessary to reduce the number of sampled arthropod taxa inhabiting agricultural ecosystems to detect effects of GM crops on NTAs. Selection of representative taxa is driven by several criteria among which is the statistical quality and reliability of the data derived from using such taxon in terms of its capacity to detect small treatment differences between GM and non-GM crops. To discuss the suitability of the taxa recorded in the 14 trials as representative taxa, they are grouped by their ecological function as herbivores, predators, parasitoids, and decomposers.
Herbivores
Effects on non-target herbivores may be important not only because they may indicate direct interference of GM crops with biological control functions, but also because their abundance may indirectly affect the abundance of selected representative taxa among predators or parasitoids through the food web (Lundgren et al., Reference Lundgren, Gassmann, Bernal, Duan and Ruberson2009). Among the herbivores recorded, homopterans were those that showed better detection capacity, particularly in yellow sticky traps. In the study area, homopterans include leafhoppers, planthoppers, and aphids (Albajes et al., Reference Albajes, López and Pons2003, Reference Albajes, Lumbierres and Pons2009; Pons et al., Reference Pons, Lumbierres, López and Albajes2005). Leafhopper populations are composed mainly of Zyginidia scutellaris (Herrich-Schäffer), a species that colonizes maize early in the season and has been reported to be the main species responsible for building the maize food web of aerial species (Albajes et al., Reference Albajes, Lumbierres and Pons2011); it is therefore a key herbivore for measuring the impact of GM maize on NTAs. In the present study leafhoppers were always present and in yellow sticky traps rendered d c that were almost always below 25%, and even below 12% in most cases. Leafhoppers sampled with yellow sticky traps are easy to identify (Le Quesne & Payne, Reference Le Quesne and Payne1981) and may therefore be a valuable representative taxon. Other homopteran taxa in maize agrosystems, such as planthoppers and aphids, are less suitable as representative taxa. Another representative of herbivory in maize includes phytophagous thrips, which are easily distinguishable from common predatory thrips, and are a common prey of generalist predators such as Orius spp. (Lewis, Reference Lewis1973). Their abundance on sticky traps associated with low d c make them valuable as a representative taxon.
Predators
According to the results of the d c analysis performed, Orius spp., Araneae and Carabidae are the most suitable representative taxa to measure potential impacts of GM maize on predators in Mediterranean conditions. The genus Orius and the group of Araneae were the most common taxa recorded on plants in the present study similarly to that reported by Albajes et al. (Reference Albajes, López and Pons2003, Reference Albajes, Lumbierres and Pons2009) and Poza et al. (Reference Poza de la, Pons, Farinós, López, Ortego, Eizaguirre, Castañera and Albajes2005) in Mediterranean maize ecosystems.
Carabidae and Araneae were the prevalent predatory taxa recorded in pitfall traps in agreement with the findings of other studies conducted in the region (Asín & Pons, Reference Asín, Pons, Nieto Nafria and Dixon1998; Albajes et al., Reference Albajes, López and Pons2003, Reference Albajes, Lumbierres and Pons2009; Poza et al., Reference Poza de la, Pons, Farinós, López, Ortego, Eizaguirre, Castañera and Albajes2005). Orius spp. was also captured extensively by yellow sticky traps with d c lower than 50%; however, relationships between the number of Orius spp. recorded on plants and those caught on yellow sticky traps should be investigated in order to use only one of the two techniques to monitor this taxon. Orius sp. (mainly O. majusculus Reuter and O. niger Wolff) have been considered to play a major role in preventing homopterans from reaching economic thresholds in European maize (Albajes et al., Reference Albajes, Lumbierres and Pons2011). Thrips, which also output a good d c, are also heavily predated by Orius sp. Therefore, a bottom-up effect of GM maize through the food web considering these herbivores and Orius sp. may occur as noted previously by authors (Albajes et al., Reference Albajes, Lumbierres and Pons2011). All these elements reinforce the interest of Orius sp. as a representative taxon.
The group of Carabidae also showed good detection capacity in pitfall traps. They have been used as indicators of non-target effects of Bt maize (Lopez et al., Reference Lopez, Prasifka, Bruck and Lewis2005). Although this family includes carnivores, seed predators, and omnivores (Luff, Reference Luff2007; Kotze et al., Reference Kotze, Brandmayr and Casale2011), and this can hinder the use of this group as an indicator of the possible effect of GM crops on biological control functions, 95% of the total individuals caught in traps belonged to the following species: Pseudophonus rufipes De Geer, Poecilus cupreus L., Agonum dorsale Pontoppidan and Bembidion spp. All these species have carnivorous feeding habits even though P. rufipes tends to prefer feeding on seeds (Toft & Bilde, Reference Toft, Bilde and Holland2002) and all of them may be indicators for measuring impacts on biological control functions. On the other hand, some carabid species are sensitive to changes in surrounding margins of field crops, in cover crops (Sotherton, Reference Sotherton1985; Carmona & Landis, Reference Carmona and Landis1999; Holland, Reference Holland and Holland2002), and in field coverage by weeds (Albajes et al., Reference Albajes, Lumbierres and Pons2009), two features that may be modified by changing weed management practices as a consequence of deployment of GM HT crops and make carabids good indicators for potential impacts of GM HT crops.
Araneae have been used to evaluate the impact of GM crops on non-targets (Peterson et al., Reference Peterson, Lundgren and Harwood2011) and our results show that, as a group, they may be considered a good candidate to be used as a representative taxon for measuring impacts of GM maize on both plant and soil-dwelling predators, as also reported by other authors (Meissle & Lang, Reference Meissle and Lang2005; Prasifka et al., Reference Prasifka, Hellmich, Dively, Higins, Dixon and Duan2008). Araneae are generalist predators that have been reported to successfully suppress pests when they act as multispecies assemblages (Riechert, Reference Riechert1999). They are quite sensitive to changes in agricultural systems (Sunderland & Samu, Reference Sunderland and Samu2000) and therefore to potential impacts caused by GM maize. Moreover, web-weaving spiders are able to feed on maize pollen trapped on the web and may be exposed to the Bt protein when expressed in the pollen, as in the case of some Bt maize events. However, no hazard from Bt maize pollen to spiders has been reported yet (Ludy & Lang, Reference Ludy and Lang2006; Meissle & Romeis, Reference Meissle and Romeis2009; Peterson et al. Reference Peterson, Romero and Harwood2010). Araneae, however, is a large and diverse group and, although many of the species found in our conditions are generalist predators, a greater taxonomic resolution may be required to determine more precisely which representative taxa may be more useful to measure the potential impact of GM maize on Araneae (Peterson et al., Reference Peterson, Lundgren and Harwood2011). Staphylinids and Dermapterans, which were also recorded, cannot be recommended as representative taxa because of their poor d c, which is presumably due to their high variability between years and plots in the first case and their low numbers in the second case.
Parasitoids
Fewer studies than those devoted to predators have dealt with parasitoids when measuring effects of GM crops on biological control functions, despite the fact that parasitoids may be affected by Bt toxins when they feed on host gut, or by a lower quality of the host exposed to Bt toxins or through food web. Most of the published studies dealing with GM crops and their effects on parasitoids refer to Bt maize or cotton and lepidopteran parasitoids. In the present study, the group that showed the most valuable d c (<25% in most cases) were mymarids and non-mymarid chalcidoids while braconids and ichneumonids only gave d c below 50% in a few of the trials. Most mymarids are egg parasitoids of Homoptera Auchenorrhyncha (Gauld & Bolton, Reference Gauld and Bolton1988) and most of the captures were presumably parasitoids of leafhoppers and planthoppers, two abundant groups recorded on the same yellow sticky traps. When leafhoppers and planthoppers are used as indicators of herbivory, as proposed in this study and by Rauschen et al. (Reference Rauschen, Eckert, Frank Schaarschmidt, Schuphan and Gathmann2008), the use of mymarids may provide complementary information about impacts caused by GM maize on the biological control functions performed by parasitoids. On the other hand, the utility of non-mymarid chalcidoids may be compromised because they include a great variety of parasitoid families with a broad range of insect hosts (Gauld & Bolton, Reference Gauld and Bolton1988).
Decomposers
Chloropidae is a family of flies whose adults are mostly decomposers consuming plant juices, although the larvae may be phytophagous or carnivorous (Prasifka et al., Reference Prasifka, Lopez, Hellmich, Lewis and Dively2007). Although their biological control functions are not particularly important, Chloropidae may be considered as a taxon representing decomposers. Values of d c of collembolans, muscoid flies and Myriapoda were too poor for considering those groups as suitable representative taxa. However, it should be taken into account that pitfall traps are not the best technique for sampling the soil for collembolans and estimations of their abundance/activity may lack the required precision in some circumstances (Prasifka et al., Reference Prasifka, Lopez, Hellmich, Lewis and Dively2007). The interest of Collembola lies in their role as alternative food for soil-dwelling generalist predators when herbivores are not abundant (Bilden et al., Reference Bilden, Axelsen and Toft2000). On the other hand, impacts of HT crops on collembolans were recorded in farm-scale evaluations carried out in the UK by Brooks et al. (Reference Brooks, Bohan and Champion2003), who noted that an increase in the abundance of decomposer Collembola could lead to a significant early-season elevation of predator abundance and subsequent pest control. It would be necessary to further investigate the d c of Collembola, and therefore their utility as a representative taxon, when alternative sampling techniques such as litter bags are used.
In general, for all groups, as noted by Naranjo (Reference Naranjo2005) and Duan et al. (Reference Duan, Jiang, Head, Bhatti, Ward, Levine, Nickson and Nemeth2006), NTA abundance/activity could have a primordial influence in determining d c; NTAs with the highest densities/activities were those with the greatest power and, consequently, the lowest d c. Abundance of a taxon is not only a function of the number of individuals of each species but also the degree to which species are grouped into a higher taxonomic level, although for the amalgamated group to be an effective representative taxon for measuring the change all of its components must respond similarly to the treatment (Storkey et al., Reference Storkey, Bohan, Haughton, Champion, Perry, Poppy and Woiwood2008). The influence of taxonomic level recorded and some other factors such as the number of treatments, years, and replicated blocks, which are potentially involved in determining d c, did not seem to be so influential although there was a tendency to improve d c as those increased. A systematic study of the influence of the trial characteristics and experimental design used to analyse the power (EFSA, 2010) or the power-increasing effects of a meta-analysis approaches (Marvier et al., Reference Marvier, McCreedy, Regetz and Kareiva2007; Wolfenbarger et al., Reference Wolfenbarger, Naranjo, Lundgren, Bitzer and Watrud2008; Shelton et al., Reference Shelton, Naranjo and Romeis2009) is being conducted with the results of the 14 field trials to contribute to design field studies on the effects of GM crops on NTAs. Results of these studies will be reported in a future article.
In conclusion, the statistical analysis performed here with the data of 14 field trials and three sampling techniques in a randomized complete block design indicates that there are several useful biota for measuring effects of GM maize on ecological functions, including representatives of herbivores, predatory arthropods, parasitoids, and decomposers. Homopterans – particularly leafhoppers – and phytophagous thrips may be suitable representative taxa of herbivory. For these species it is recommended to use sticky yellow traps because the insects are rapidly identified with this technique but less easily detected by visual sampling. Among predators, Orius spp. and the whole group of Araneae in visual sampling, Carabidae and again the group of Araneae in pitfall traps, and Orius spp. on yellow sticky traps are the most efficient taxa to be recorded for environmental risk assessment purposes. Recommended indicator parasitoids for GM maize include the family Mymaridae if impacts on leafhoppers are expected and the superfamily Chalcidoidea without mymarids for a broader impact range, in both cases collected on yellow sticky traps. Chloropidae caught in yellow sticky traps are suitable representative of decomposers. It must be noted that the utility of arthropods organisms as representative taxa has been ranked according to their suitability to detect effects of treatments but other criteria must be used for a final selection of indicator groups; inversely, in any selection based on other criteria, the d c of organisms selected must be checked.
Acknowledgments
The results reported herein were obtained in field trials that were funded by the following public agencies and private companies: the Ministerio de Ciencia y Tecnología (AGF99-0782, AGL2002-00204, AGL2005-06485, AGL2011-23996), INIA & the Ministerio de Medio Ambiente, Medio Rural y Marino, and Pioneer Genetique, Monsanto and Syngenta Seeds companies.