Introduction
Storage mites are a primary cause of loss of quality in cereals and cereal-based products in temperate climates (Anon, 2003). In addition, they contaminate the commodities with potent allergens, which can be hazardous to both operators and consumers, causing a range of medical conditions that include farmer's lung, rhinitis, urticaria, asthma and dermatitis (Cuthbert et al., Reference Cuthbert, Brostoff, Wraith and Brighton1979; Van Hage-Hamsten et al., Reference Van Hage-Hamsten, Johansson, Hoglunds, Tull, Wiren and Zetterstrom1985; Stengard Hansen et al., Reference Stengard Hansen, Herling and Danielson1996). There have also been several reported cases of anaphylaxis and anaphylactoid reactions following the ingestion of mite contaminated food (Guerra Bernd et al., Reference Guerra Bernd, Arruda and Barros Antunes2001; Matsumoto et al., Reference Matsumoto, Hisano, Hamaguchi and Miike1996; Sanchez-Borges et al., Reference Sanchez-Borges, Capriles-Hulett, Fernanndez-Caldas, Suarez-Chacon, Caballero, Castillo and Sotillo1997).
The key to successful integrated pest management (IPM) for storage mites is early detection and identification, as it allows for timely implementation of appropriate control strategies before threshold levels are exceeded. Thus, IPM can reduce costs, damage and pesticide usage. Moreover, in so doing, occupational exposure to both pesticides and to mite allergens can be reduced.
To date, the most commonly used method to detect mites in the field is sieving followed by visual inspection. Other detection methods include flotation (Thind, Reference Thind2000), the BT Mite Trap (Thind, Reference Thind2005) and measurement of guanine content (Bischoff et al., Reference Bischoff, Fischer, Liebenberg, Bieva, Courtois and Govaerts1989; Ransom et al., Reference Ransom, Leonard and Wasserstein1991). Sieving is unreliable (Lynch & Thind, Reference Lynch and Thind1985), subjective and potentially hazardous through exposure to airborne allergens; the flotation technique is time-consuming and technically exacting because it requires an expert acarologist to complete identification; the guanine assay is relatively non-specific as substances other than mites can confound the interpretation of results (Hallas et al., Reference Hallas, Xue and Schou1993); and the BT Mite Traps, although easy to use, are unable to provide rapid results, as they require four days to lure mites into the traps. The absence of a rapid, robust and sensitive detection system by which to help avoid the build up of infestation may be contributing to the apparent increase in the contamination by mites of cereal-based food products in retail outlets (Anon, 1996; Thind & Clarke, Reference Thind and Clarke2001).
Immunoassays are a well-established detection technique based on the detection of specific antigens by antibodies. They are reliable, rapid, do not require expert knowledge and can be developed into user-friendly, low-cost field kits, such as lateral flow devices (Danks & Barker, Reference Danks and Barker2000). Species-specific immunoassays have been developed for the detection of storage pests, such as the grain weevil, Sitophilus granarius (L.), in wheat (Chen & Kitto, Reference Chen and Kitto1993), the khapra beetle, Trogoderma granarium (Everts) (Stuart et al., Reference Stuart, Barak and Burkholder1994), and a wide variety of common insect pests in both grain and flour (Brader et al., Reference Brader, Lee, Plarre, Burkholder, Kitto, Kao, Polsten, Dorneanu, Szabo, Mead, Rouse, Sullins and Denning2002). For mites, there is an ELISA method for the detection of the house dust mite, Dermatophagoides pteronyssinus (Trouessart) (Luczynska et al., Reference Luczynska, Li, Chapman and Platts-Mills1989), but there has been little investigation for such an approach for the detection of storage mites. However, Härfast et al. (Reference Härfast, Johansson, Johansson and van Hage-Hamsten1996) used a monoclonal antibody to detect and quantify storage mite allergens from Lepidoglyphus destructor (Schrank) in dust samples collected from barns; and recently, Kudlíková et al (Reference Kudlíková, Stejskal, Hỳblová, Chalupníková and Hubert2004) raised polyclonal antibodies to detect Acarus siro (L.). Härfast's monoclonal, although proved specific to two Glycyphagid species of mite, but cross-reacted with Aleuroglyphus ovatus (Troupeau, 1878), it lacked sensitivity in the presence of grain (Chambers et al., Reference Chambers, Dunn and Thind1999a,Reference Chambers, Thind, Dunn and Pearsonb; Dunn et al., Reference Dunn, Thind, Banks and Chambers2002) and Kudlíková's polyclonals have yet to be validated using grain samples.
This paper reports the development and preliminary validation of species and genera-specific ELISAs for the detection of stored product mites: the flour mite (A. siro), the cosmopolitan food mite (L. destructor), the grocers' itch mite (Glycyphagus domesticus (DeGeer)), the grainstack mite (Tyrophagus longior (Gervais)), mites of the Tyrophagus genus, mites of the Glycyphagus genus, and all storage mites. Tests were performed using different qualities of grain and various grain storage pests.
Materials and methods
Mites
The mite species used were: three strains of A. siro and one strain of Acarus farris (Oudemans), Acarus immobilis (Griffiths) and Acarus gracilis (Hughes); two strains of T. longior and Tyrophagus putrescentiae (Schrank) and one strain of Tyrophagus palmarum (Oudemans), Tyrophagus brevicrinatus (Robertson), Tyrophagus neiswanderi (Johnston and Bruce) and Tyrophagus perniciosus (Zachvatkin); and two strains of L. destructor and G. domesticus, and one strain of Lepidoglyphus michaeli (Oudemans), Aleuroglyphus ovatus (Troupeau), Caloglyphus berlesei (Michael), Carpoglyphus lactis (L.), Tyrolichus casei (Oudemans), Cheyletus eruditus (Schrank), C. malaccensis (Oudemans) and Ctenoglyphus plumiger (Koch).
Cultures of these mite strains were reared in the dark at 20°C and 80% RH in 50 ml conical flasks, on a finely ground, sterilised and conditioned mite diet consisting of flour and dried yeast (1:3 w/w) with the exception of C. eruditus, C. malaccensis and C. plumiger. The predatory mites (C. eruditus and C. malaccensis) were bred in tubs (14 cm×14 cm×14 cm) at 25°C and 75% RH and fed a mixture of storage mite species (A. siro, T. putrescentiae and T. longior). C. plumiger was bred in 50 ml flasks, at 20°C and 80% RH on a diet of mouldy yeast and flour, supplemented with tropical fish flakes (Aquarian).
Insects
Insect species tested were Sitophilus granarius (L.), Ahasverus advena (Watl), Oryzaephilus surinamensis (L.), Cryptolestes ferrugineus (Stephens) and Liposcelis bostrychopila (Badonnel).
Insect species were reared in the dark at 25°C and 70% RH in 0.75-l Kilner jars. O. surinamensis was maintained on a diet of wheatfeed, rolled oats and yeast (5:5:1 w/w), S. granarius was reared on wheat only and L. bostrychopila on skimmed milk powder, wheatfeed, yeast and wholemeal flour (1:1:1:1 w/w).
Fungi
The fungal species tested were: Penicillium verrucosum (Dierckx), Alternaria alternata ((Fr.) Keissl), Aspergillus ochraceus (G. Wilh), Eurotium amstelodami ((Talice & J.A. Mackinnon) Kozak.) and Cladosporium cladosporioides ((Fresen.) G.A. de Vries). All fungal species were cultured at 25°C on czapek yeast agar in the dark.
Antigen extraction
As a preliminary to extracting antigen for immunisation purposes, mites were thoroughly washed with phosphate buffered saline (PBS) to remove any adhering materials. The mites were then soaked in 0.15 M sodium chloride overnight at 33°C, and the suspension was then centrifuged at 18,000 gX force for 5 min. The resultant supernatant was then used as mite antigen.
Mite antigen was extracted from infested grain samples by shaking grain in 0.15 M sodium chloride solution (1 ml g−1 of grain) in a 30-ml plastic container containing five stainless steel ball bearings (5 mm dia.) for 1 min. The protein concentrations of the extracts were determined, where applicable, using standard Biorad methodology (Bradford, Reference Bradford1976) and bovine serum albumin (Sigma-Aldrich, derivatization grade) as the standard protein. Solutions were diluted to the appropriate concentrations using PBS.
Antibodies
Monoclonal antibodies (Mab) were raised against A. siro, L. destructor, G. domesticus, T. longior and T. putrescentiae according to standard procedures (Harlow & Lane, Reference Harlow and Lane1988).
For initial antibody screening of supernatants from fusions, 96-well micro titre plates (Nunc Immunoplate, maxi-sorp) were coated overnight with 100 μl per well of 5 μg ml−1 antigen in coating buffer. Supernatants were screened by an indirect, plate trapped antigen (PTA) ELISA, using rabbit anti-mouse IgG labelled alkaline phosphatase (Sigma) as the second antibody. An optical density difference of at least 3:1 in the recognition of the target antigen compared to control wells (blanks) was chosen as a discriminatory threshold for potential diagnostic antibodies. Cell lines were cloned twice by limiting dilution.
Once the chosen cell lines had produced a sufficient volume of tissue culture supernatant (ca. 500 ml), the antibodies were isotyped (Immune Systems) and then purified according to standard methods using HiTrap Protein G affinity columns (Pharmacia, Biotech).
Indirect, TAS and competitive ELISAs
Indirect PTA, triple antibody sandwich (TAS) and competitive ELISAs were performed in accordance with standard procedures (Wilson & Goulding, Reference Wilson and Goulding1986; Harlow & Lane, Reference Harlow and Lane1988). Optimised TAS ELISAs were used with the A. siro Mab, and optimised competitive ELISAs with all the other antibodies raised.
Comparison of antigen obtained from two strains of each of the four primary pest species by Multiphor electrophoresis
Mite antigen supernatants of two strains of L. destructor, G. domesticus, T. longior and T. putrescentiae were each diluted in PBS to a concentration of 0.1 mg ml−1. These antigens plus two markers (BioRad High and Low) were then denatured before running the samples on an ExcelGel (SDS Homogenous 12.5) according to the manufacturer's instructions (Multiphor II Electrophoresis Systems, Pharmacia Biotech). The gel was stained using a freshly prepared sensitive silver stain according to Multiphor II manufacturer's instructions.
Reactivity with other species of mites including predatory, insects, wheat and fungi
To determine the cross-reactivity of the potential diagnostic antibodies with other potential contaminants, two-fold serial dilutions were made of each respective antigen. These were then used to assess the specificity of the ELISAs described above.
Reactivity with laboratory infested and ‘clean’ grain samples
Ten 10 g samples of wheat were seeded with mites (0, 2, 4, 8, 16, 32, 64, 128, 256, 512 and 1000 adult A. siro). ELISAs were then used to compare seeded grain samples with controls (10 g of unseeded, ‘clean’ grain) and mites only (no grain).
Evaluation of assay by comparison with flotation technique using both laboratory and field samples
Two batches of wheat samples, each weighing 25 g, were seeded with equal numbers of adult mites (0, 10, 20, 40, 60, 80, 100, 150 and 250 mites) using a light microscope and a single-haired paint brush. One batch was analysed using the ELISA and the other batch by flotation technique (Thind, Reference Thind2000) with a minor modification to its pre-extraction stage. This modification entailed subjecting the wheat grain, when suspended in the aqueous phase, to ultrasonication for ten minutes in an ultrasonic bath. This procedure increased the recovery of mites from grain and speeded-up the extraction process. Previous experiments (unpublished) have demonstrated that this additional step enhances the extraction of mites from within grain kernels.
For the ELISA, a standard curve was constructed from a different set of samples seeded with a known number of mites. This standard curve was then used to deduce the number of A. siro mites in the grain samples analysed using the ELISA method. The results from the flotation test and the ELISA were then correlated using linear regression analysis.
A further batch of grain samples, each weighing 10 g, was seeded in the laboratory with numbers of mites unknown to the operator. Each sample was tested in triplicate. The calibration curves described above were then used to assess the mite numbers. This procedure was replicated nine times with the A. siro ELISA and five times with the Tyrophagus genus ELISA.
Five field samples of wheat with unknown levels of mite contamination were also tested. Using a sample divider, three 50 g sub-samples were taken from each sample and analysed by flotation analysis. Similarly, three 10 g sub-samples were taken from each field sample and tested in triplicate using the A. siro and Tyrophagus genus ELISAs.
Optimisation of A. siro ELISA by confirming applicability to a wide variety of sample types
Ten gram samples of two cultivars of wheat, Hereward and Consort; two cultivars of oilseed rape Royal (low erucic acid var.), HEAR with high erucic content; one cultivar of barley (Fighter); and one cultivar of oats (Gerald) were seeded with 0, 2, 4, 8, 16, 32, 64, 128, 256, 512 and 1000 adult A. siro mites. Each sample was tested five times in a TAS ELISA.
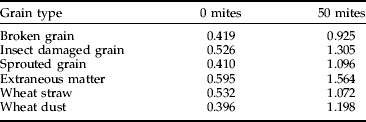
The ELISA's validation was completed using batches of 10 g grain samples. Each batch contained either 10% of broken grains, insect damaged grain, sprouted grain, wheat straw, wheat dust or extraneous matter (fungal contamination with P. verrucosum). Each batch (n=5) was then seeded with 50 mites and tested in a TAS ELISA.
Results
Comparison of antigen obtained from two strains of each of the four primary pest species (L. destructor, G. domesticus, T. longior and T. putrescentiae) by Multiphor electrophoresis showed species-specific but not strain-specific banding.
Reactivity of A. siro Mab to other species of mite including predatory mites, insects, wheat and fungi
A. siro assay
Specificity tests were conducted with adults of nine species of storage mite, five species of storage insects, two species of predatory mite, a detritus feeding mite and five species of fungi. The results (table 1) show that the A. siro assay was species specific (P<0.001).
Table 1. Specificity for A. siro with the A. siro Mab against nine other species of storage mite, five species of storage insect, two species of predatory mite, one fungivorous mite and five species of fungi (n=5).

* Reading not significantly different from background.
** Reading significantly different from background (P<0.001).
Tests were undertaken to confirm that the selected antibodies did not cross-react with wheat when analysing mite-infested grain samples. Figure 1 shows that the selected antibodies fulfilled our test criteria and that the assay's performance was not confounded by the presence of wheat co-extractants.

Fig. 1. Quantitative detection of A. siro with A. siro Mab with and without the presence of 10 g samples of wheat (n=5). (—◆—, mites+wheat;– – ![]() – – , mites only;
– – , mites only;![]() , wheat only.)
, wheat only.)
Panel of antibodies
The results (table 2) show that the antibodies produced for T. longior, L. destructor, G. domesticus the Tyrophagus and Glycyphagus genera and for all storage mites were specific to their target antigen(s).
Table 2. Reactivity of the six monoclonal antibodies against different mite species, mite diet (yeast and flour), wheat, predatory mites and insects (n=3).

* Reading not significantly different from background.
** Reading significantly different from background (P<0.001).
Reactivity of A. siro Mab with laboratory infested and ‘clean’ grain samples
Tests, with larger quantities of grain (25 g and 50 g) showed the antigen extraction process and the assay were still able to detect and quantify A. siro in these volumes without any detrimental affect on performance (fig. 2).

Fig. 2. Detection of A. siro with A. siro Mab seeded in 0 g, 10 g, 25 g and 50 g of wheat (n=5). (–![]() –, 0 g;–
–, 0 g;–![]() –, 10 g;
–, 10 g;![]() , 25 g; - -×- -, 50 g.)
, 25 g; - -×- -, 50 g.)
Evaluation of A. siro and Tyrophagus genera assays
The results from the laboratory-seeded samples tested using both the ELISA and flotation methods correlated well with a linear regression of r 2=0.9091 (y=0.9563×+5.0059) for A. siro and r 2=0.9895 (y=1.0145×−7.1755) for the Tyrophagus genera.
Calibration curves were then set up (using standards) for the ELISA. Results from the ‘blind’ laboratory samples (tables 3 and 4) and samples received from the field (tables 5 and 6) show that when these samples were run alongside the standards, mite levels were successfully determined, and these results were comparable to those produced by flotation.
Table 3. Mean number of A. siro mites detected from samples seeded ‘blind’ in 10 g wheat (nine replicates at each mite density and each replicate tested in triplicate on ELISA plate and LFD).

Table 4. Mean number of Tyrophagus mites seeded ‘blind’ (five replicates at each mite density) detected in 10 g of wheat.

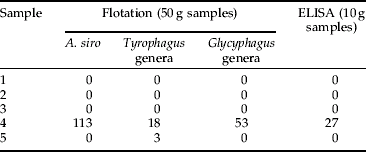
Table 5. Mean number (n=3) of A. siro mites detected in five field samples (wheat) by flotation and ELISA.
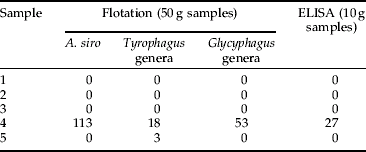
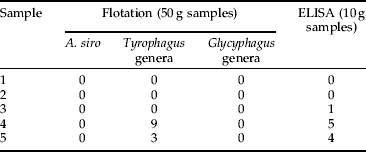
Table 6. Mean number (n=3) of Tyrophagus mites detected in five field samples (wheat) by flotation and ELISA.

Optimisation of A. siro ELISA by confirming applicability to a wide variety of sample types
The results show that with the A. siro ELISA there was a general and regular increase in response with increase in infestation level and this was similar for all the different types of cereal and oilseed tested (fig 3). Furthermore, despite a high background reading with zero mites due to contaminants within the samples, there was a significant difference (t-test, P<0.001) in response with the samples seeded with mites (table 7).

Fig. 3. Detection of A. siro with A. siro Mab seeded in two cultivars of OSR (Royal and HEAR), three cultivars of wheat (Hereward, Consort and Mercia) and A. siro alone (n=5). (![]() , OSR – Royal;
, OSR – Royal;![]() , OSR-HEAR; - - -▲- - -, Hereward;
, OSR-HEAR; - - -▲- - -, Hereward;![]() , consort;
, consort;![]() , Mercia;
, Mercia;![]() , mites only.)
, mites only.)
Table 7. Optical density values (405 nm) for admixture and screening.
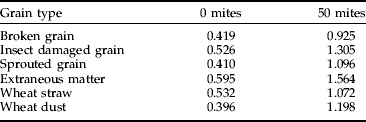
Discussion
Effective control of mite pests requires an efficient detection method that provides early warning of an infestation. Results from this study show that ELISAs can feasibly fulfil this need. For example, the Mabs could detect extremely low levels of mites and measure their increase in numbers in a wide variety of cereal types and qualities (fig 4, table 7) and were specific to their target antigen(s) with no cross-reactivity to other storage pests, thus minimising the potential for false positives (tables 1 and 2).

Fig. 4. Detection of A. siro with A. siro Mab seeded in four different types of grain (n=5). (![]() , mites+wheat;
, mites+wheat;![]() , mites+barley; - - -△- - -, mites+oats; - - -×- - -, mites+OSR;
, mites+barley; - - -△- - -, mites+oats; - - -×- - -, mites+OSR;![]() , mites only.)
, mites only.)
The immunoassay's success was aided by the development of an innovative antigen extraction procedure. For this, antigen was extracted using a novel ‘rinsing’ method with an extraction buffer that had sufficient molarity to remove sufficient proteins from the mites. This method avoided the physical homogenisation of the wheat and its associated problems (Chambers et al., Reference Chambers, Dunn and Thind1999a) and will be rapid and easy for the user.
The successful development of the A. siro ELISA provided the basis for the study's second phase – the raising of further Mabs that could detect other important and prevalent UK storage mite pests. Prior to the raising of these Mabs, the antigens were assessed to determine whether there were differences in the protein profiles between strains of the same species, as this could potentially affect the sensitivity of the ELISA with field populations. No differences in the banding patterns were found using Multiphor electrophoresis between strains, but distinct differences were observed between species.
The species-specific Mabs produced for the detection of T. longior, L. destructor and G. domesticus, showed high specificity with no cross-reactivity to other species of mites or insects tested, nor to mite food or wheat (table 2). Similarly, the Tyrophagus and Glycyphagus genus-specific Mabs reacted strongly with only those mites belonging to their respective genus, and the all storage mite Mab reacted with all the storage mite species tested with the exception of predatory mites and storage insects (table 2). The successful development of an all storage mite assay has many benefits. For example, it would provide the user with a cost-effective means to confirm freedom from mites throughout the food supply chain.
Once the Mabs were raised, ELISA methodology was developed. However, preliminary validation was performed with just two, namely the A. siro and the Tyrophagus genera assays. These two Mabs were selected as mites of these genera are considered as being the most important mite pests within the UK cereal and allied industry (Thind & Ford, Reference Thind and Ford2004; Thind, Reference Thind2005). Both the A. siro and Tyrophagus genera ELISAs showed good correlation with the flotation technique (correlation coefficient of 0.91 for A. siro and 0.99 for Tyrophagus) and were able to accurately indicate levels of mites infesting grain in both laboratory and field samples (tables 3–6). Furthermore, in one sample, the Tyrophagus genera ELISA was able to detect mites where the flotation technique failed (table 6, sample 3). In addition, the ELISA accurately indicated the presence or absence of mites in all the sub-samples and provided quantitative data.
The development and application of immunoassays for the detection of storage mites in cereals is a major advance from previous works and has wide implications for farmers, grain store keepers, millers and the whole food supply chain management, in as much that the assays can provide a non-subjective, unambiguous result on which to base contractual agreements. Secondly, once fully developed and validated, immunoassays can provide a cost-effective means for quality assurance by establishing its storage-mite free status; and, thirdly, if a mite infestation were to develop, the assays would provide accurate data, allowing for the most appropriate pest control strategy(s) to be applied.
The success of this ELISA-based approach for the detection of mites in grain means that it is now feasible to extend the application of this laboratory-based immunoassay into a storage mite diagnostic kit suitable for use in the field by adapting the assays into lateral flow devices (LFDs). LFDS have several advantages, of which its main attributes are that it can be used on site by end users without the need of any specific expertise to provide almost instantaneous results. Preliminary investigations (unpublished) with prototype LFDs showed promising results with quality assurance personnel detecting low levels of mites in a variety of cereals at their respective premises. In addition, our preliminary results (unpublished) indicate that these prototype LFDs can also detect mites in processed cereals (e.g. flour, dry pet food and animal feed).
Acknowledgements
The authors would like to thank the UK Home-Grown Cereals Authority for funding the work, Gaynor Johnson for culturing the cell lines, Sioban Ostoja-Starzewska for assistance with the gels and Dr Richard Watkins for advice and review of the manuscript. We would also like to thank various cereal processors for use of their premises.