Introduction
The South American fruit fly Anastrepha fraterculus (Wiedemann), formerly thought to be a highly polyphagous, wide-ranging species, is actually recognized as a complex of cryptic species composed of several different morphotypes (Stone, Reference Stone1942; Steck, Reference Steck1991; Steck & Sheppard, Reference Steck, Sheppard, Aluja and Liedo1993; Selivon et al., Reference Selivon, Perondini and Morgante1999; Smith-Caldas et al., Reference Smith-Caldas, McPheron, Silva and Zucchi2001; Hernández-Ortiz, Reference Hernández-Ortiz, Gómez-Anaya, Sánchez, McPheron and Aluja2004). Some of these morphotypes exhibit different host affiliations (Aluja et al., Reference Aluja, Perez-Staples, Macias-Ordoñez, Piñero, McPheron and Hernández-Ortiz2003), are genetically distinct (Morgante et al., Reference Morgante, Malavasi and Bush1980, Steck, Reference Steck1991; Steck & Sheppard, Reference Steck, Sheppard, Aluja and Liedo1993; Selivon et al., Reference Selivon, Perondini and Morgante1999, Reference Selivon, Perondini and Morgante2005; Smith-Caldas et al., Reference Smith-Caldas, McPheron, Silva and Zucchi2001) and exhibit pre and post zygotic partial reproductive isolation (Selivon et al., Reference Selivon, Perondini and Morgante1999; Vera et al., Reference Vera, Cáceres, Wornoayporn, Islam, Robinson, de la Vega, Hendrichs and Cayol2006; Cáceres et al., Reference Cáceres, Segura, Vera, Wornoayporn, Cladera, Teal, Sapountzis, Bourtzis, Zacharopoulou and Robinson2009). In some cases, these differences are so conspicuous that morphotypes should be considered as distinct species (Hernández-Ortiz et al., Reference Hernández-Ortiz, Gómez-Anaya, Sánchez, McPheron and Aluja2004).
Because of its economic importance, significant efforts are being made to develop a pest control strategy against A. fraterculus through area-wide application of the sterile insect technique (SIT) (Guillen & Sanchez, 2005), a method based on the release of sterile insects which are aimed at mating with wild fertile conspecifics to reduce population size through sterility induction. For this purpose, artificial rearing media have been developed (Jaldo, Reference Jaldo, Gramajo and Willink2001), effective radiation doses have been determined (Allinghi et al., Reference Allinghi, Gramajo, Willink and Vilardi2007), quality control parameters have been established (Vera et al., Reference Vera, Abraham, Oviedo and Willink2007) and methods to boost sterile male performance are being explored (e.g. Segura et al., Reference Segura, Utgés, Liendo, Rodríguez, Devescovi, Vera, Teal and Cladera2010).
Recent experience has shown that complete eradication of fruit fly pests cannot be fully attained based on SIT when sterile flies are released over areas that have no concise limits to pest population movement. Such a claim is rooted on the highly invasive ecology of these mobile insects (Thomas & Loera-Gallardo, Reference Thomas and Loera-Gallardo1998; De Longo et al., Reference De Longo, Colombo, Gómez-Riera, Bartolucci and Tan2000, Weldon & Meats, Reference Weldon and Meats2010). Therefore, SIT success is tied with an area-wide insect pest management scheme. Area-wide SIT refers to a coordinated, sustainable and preventive approach that targets pest populations in ample areas, including commercial and non-commercial orchards, urban settings and non-cultivated and wild host areas (Vreysen et al., Reference Vreysen, Robinson and Hendrichs2007), where eradication is not necessarily the main goal, and populations can be suppressed to levels below the economic thresholds. For A. fraterculus, the existence of a cryptic species complex poses important hurdles to area-wide SIT application, especially when dealing with reproductively isolated morphotypes. The situation is particularly complex in southern Brazil, where up to three morphotypes (A. fraterculus aff1, aff2 and aff3) are sympatric (Selivon et al., Reference Selivon, Perondini and Morgante2005), one of which (aff1) appears to extend to central Argentina. Under such a scenario, released sterile males of the ‘wrong’ morphotype will fail to induce sterility into the target pest population.
To overcome this problem, it is necessary to determine the exact limits of the distributional range of each A. fraterculus morphotype, as to be able to cope with it on a regional basis. A similar approach proved to be successful during the new world-screwworm, Cochliomyia hominivorax (Diptera: Calliphoridae) Coquerel, eradication in México and Central America (Richardson et al., Reference Richardson, Ellison and Averhoff1982). There is some evidence of genetic affinity between different Argentinean and southern Brazilian populations of A. fraterculus (Smith-Caldas et al., Reference Smith-Caldas, McPheron, Silva and Zucchi2001; Alberti et al., Reference Alberti, Rodriguero, Cendra, Saidman and Vilardi2002; Selivon et al., Reference Selivon, Perondini and Morgante2005). If such affinity translates into reproductive compatibility, it would allow grouping all these populations under the aff1 morphotype; and, in terms of pest management, this result would imply that SIT can be applied over a large area with important commercial production of A. fraterculus hosts such as apples, blue-berries, citrus, guavas, pears and peaches. Area-wide management of fruit flies of economic importance from northern Argentina is a logical extension of successful SIT application in semi-arid, irrigated fruit production areas in the western and Patagonian regions of the country (De Longo et al., Reference De Longo, Colombo, Gómez-Riera, Bartolucci and Tan2000; Guillén & Sánchez, Reference Guillén, Sánchez, Vreysen, Robinson and Hendrichs2005), and there is mounting interest in applying SIT for fruit fly management in Brazil (Malavasi & Nascimento, Reference Malavasi and Nascimento2003).
Concurrently, as an initial step for efficient SIT application in the region, we set out to establish the degree of pre- and post-zygotic compatibility among one Argentinean and two southern Brazilian populations of A. fraterculus. Our goal was to contribute in delimiting the extent of a potential area-wide SIT program in a region with ecological and climatic affinity and to initiate a comprehensive cryptic species distribution map that may also aid in understanding the speciation processes underlying the evolution of this complex and in facilitating its management.
Materials and methods
All experimental work was carried out at the FAO/IAEA Agriculture and Biotechnology Laboratories, Seibersdorf, Austria.
Biological material
Adult A. fraterculus from a northern Argentinean population (Tucumán) were obtained from a laboratory colony reared at the Estación Experimental Agroindustrial Obispo Colombres since 1997 following Jaldo et al. (Reference Jaldo, Gramajo and Willink2001) and Vera et al. (Reference Vera, Abraham, Oviedo and Willink2007). The laboratory strain was originally recovered from naturally infested guavas (Psidium guajava L.) collected at the vicinity of Tafí Viejo, Tucumán, Argentina (26°48′5″S; 65°9′50″W). Flies were transported as pupae to the Insect Pest Control, FAO/IAEA Agriculture and Biotechnology Laboratories and held under controlled conditions (T: 27°C; RH: 65%; Photoperiod: 12L:12D) until adult emergence. Brazilian populations were recovered from naturally infested guavas at the locality of Vacaria (28°27′52″S; 50°59′0″W) in April 2010 and from infested araça (Eugenia stipitata Mc. Vaugh) at the locality of Pelotas (29°28′19″S; 50°37′3″W) in May 2010. Vacaria and Pelotas wild pupae were transported or shipped as pupae to the FAO/IAEA Laboratories and reared for two and one generations, respectively, on an artificial carrot diet (Tanaka et al., Reference Tanaka, Okamoto and Chambers1970).
Prezygotic isolation tests
Prezygotic isolation tests followed the standard procedures to evaluate mating compatibility, as proposed in the FAO/IAEA/USDA (2003) product quality control manual. Two to three days before adult emergence, pupae from all three populations were placed in 15 cm diameter×45 cm high cylindrical Plexiglass cages. Cages were covered at one end with a tight mesh and at the other by a long sleeve, also made with mesh that could be tied and untied in a knot to facilitate fly transfer to and from the cage. At emergence, adults were sorted by sex and placed in similar cages with ad libitum access to water and food (consisting in wheat germ, hydrolyzed yeast and sugar at a 1:1:3 ratio). One to two days before reaching sexual maturity (10–18 days depending on the strain) males and females of each population were marked on the notothorax with a small dot of distinctive acrylic paint, a procedure that does not affect sexual performance of A. fraterculus (Petit-Marty et al., Reference Petit-Marty, Vera, Calcagno, Cladera, Segura, Allinghi, Rodriguero, Gómez Cendra, Viscarret and Vilardi2004a). Twenty-five marked males and 25 marked females of each population were placed in smaller 11×11×17 cm square cages with water and food. The following day at 8:00 am (the hour at which the lights were turned on in the room where the flies had been kept since emergence) marked flies (25 individuals of each sex) of two different populations were released inside a 2.0×1.6×1.9 m cage. In each cage, one potted Citrus sinensis Osbeck (Rutaceae) (L.) tree (2 m high with a canopy of about 1.1 m in diameter) provided the flies an arena for resting and mating activity. Cages were installed inside a greenhouse where temperature and light could be manipulated. On cool mornings, the greenhouse was heated and flies were released once temperature reached at least 23°C. Simultaneous releases were done in four adjacent field cages. One observer in each cage recovered mating couples from the tree and cage walls and ceiling, recording each time: colour (origin) of male and female, time at which copulation initiated and mating location. To record mating location, the cage was divided in four quadrants according to cardinal points estimated by looking at the position of the rising sun (East). The height at which mating couples were detected was also noted as upper, middle and low. We also recorded whether matings occurred over or under the leaves. Mating location was recorded in order to detect a potential spatial partition of mating arenas among populations. Shortly after the detection of a mating pair, the couple was gently captured in a small (3.7 cm in diameter, 4 cm high) plastic cup, which was capped and placed over a plastic tray to record the time at which copulations ended. To record latency to mate, because not all replicates began at exactly the same time, for each replicate, the hour of copulation was subtracted from the beginning hour of the first copulation in the cage (which invariably occurred immediately after females were released). Flies were observed for ca. three hours, a time lapse that guarantees covering the period of sexual activity for populations from Argentina and southern Brazil (De Lima et al., Reference De Lima, Howse and Salles1994; Petit-Marty et al., Reference Petit-Marty, Vera, Calcagno, Cladera, Segura, Allinghi, Rodriguero, Gómez Cendra, Viscarret and Vilardi2004a; Vera et al., Reference Vera, Cáceres, Wornoayporn, Islam, Robinson, de la Vega, Hendrichs and Cayol2006), after which mated couples and remaining unmated adults were taken to the laboratory.
Postzygotic isolation tests
Ten mated couples from each possible combination were transferred to 45×15 cm previously described Plexiglass cages. To recover eggs, the bottom of a Petri dish (13.9 cm in diameter) was removed and replaced with a piece of cloth previously lined with a fine layer of black silicone (Sanitarsiliko, Murexin, AG). The oviposition device was placed over the top of the cylindrical Plexiglass cage and filled with tap water. With the aid of a Pasteur pipette, eggs were recovered every other day and placed over a piece of black filter paper. The filter paper was placed in a Petri dish that contained a piece of moistened thin sponge at the bottom. The Petri dish was then closed and incubated at 27°C, 65% RH for 48 h. When eggs began hatching, the black filter paper was gently transferred over a Petri dish (9 cm in diameter) filled with carrot diet (Tanaka, 1970). After an additional 48 h (seeding eggs into diet right after collection resulted in no hatch), the number of hatched eggs was counted and recorded, and the filter paper was removed from the diet to prevent fungal growth. Each Petri dish was then capped, placed in a 250 ml cylindrical container with a mesh covered cap and a thin layer of vermiculite as pupation substrate. Plastic containers with Petri dishes were kept under a dark cloth at 27°C, 65% RH and, after three days, the top of the Petri dishes were removed. When larvae completed development (attempting to leave diet to pupate), diet was examined and pupae and late instar larvae were counted and placed over the vermiculite. Pupae were incubated at 27°C, 65% RH for ca. 8–10 days when adults began to emerge. At emergence, the number and sex of adults was recorded, and F1 adults were transferred to cylindrical Plexiglass cages with water and food. F1 adults were left in cages for 15 days and when couples began mating; an oviposition device (as described above) was placed on top of cages; eggs were recovered; and F2 egg hatch was recorded, following the procedures described for F1 egg hatch estimation.
Potential distribution of A. sp. aff1 fraterculus
The potential distribution map of the aff1 morphotype was generated by plotting locations for all populations with published records of reproductive compatibility (Petit-Marty et al., Reference Petit-Marty, Vera, Calcagno, Cladera, Segura, Allinghi, Rodriguero, Gómez Cendra, Viscarret and Vilardi2004a; Selivon et al., Reference Selivon, Perondini and Morgante2005; Vera et al., Reference Vera, Cáceres, Wornoayporn, Islam, Robinson, de la Vega, Hendrichs and Cayol2006), genetic affinity (Smith-Caldas et al., 2001; Alberti et al., Reference Alberti, Rodriguero, Cendra, Saidman and Vilardi2002), karyotypic similarity (Basso et al., Reference Basso, Sonvico, Quesada-Allue and Manso2003) and morphological similarity (Hernández-Ortiz et al., Reference Hernández-Ortiz, Gómez-Anaya, Sánchez, McPheron and Aluja2004), as well as the populations analysed in this study (Pelotas, Tucumán and Vacaria; see table 1) using Google Earth®.
Table 1. Published records of Argentinean and Brazilian populations of A. fraterculus showing affinity according to different criteria.

Data analysis
Prezygotic isolation between population pairs was assessed by calculating the index of sexual isolation (ISI), the male relative performance index (MRPI) and the female relative performance index (FRPI) following Cayol et al. (Reference Cayol, Vilardi, Rial and Vera1999). For ISI, values close to zero indicate random mating; values close to 1 indicate assortative mating (i.e. sexual isolation) and values close to –1 complete outbreeding. For MRPI and FRPI, values close to zero indicate equal participation from males (MRPI) or females (FRPI) of the two populations. In all, the joint analysis of ISI, MRPI and FRPI provides a complete and reliable picture of the sexual compatibility between populations (Cayol et al., Reference Cayol, Vilardi, Rial and Vera1999). Departures from random mating were assessed by estimating confidence intervals at 95% to see if zero was included in the interval.
Within each population combination, frequencies of different mating combinations (A♂A♀, B♂A♀, A♂B♀, B♂B♀) among population pairs for each replicate were log(x+1) transformed, subjected to a Cochran test to verify homogeneity, and compared with a one-way ANOVA followed by Tukey comparison of means.
Latency to first mating and mating duration were compared among mating combinations by means of a one-way ANOVA followed by Tukey comparison of means. Kruskal-Wallis tests were applied for data sets failing to fit the normal distribution (after Shaphiro-Wilks test). Mating position for each possible male/female mating combination was compared to a uniform distribution of matings according to height and cardinal point by means of Chi-square test of homgeneity.
A Kruskal-Wallis test was used to compare F1 egg hatch for all possible mating combinations within each pair-wise population combination and F2 egg hatch among self crosses of F1 adults. Only egg collection dates yielding more than ten eggs were considered in the analyses. All analyses were performed using Statistica 7 software (Statsoft, Inc., Tulsa, OK, USA).
Results
Prezygotic isolation
Percentage of flies involved in matings and indices of mating compatibility and performance are presented in table 2. In general, populations were mating compatible (95% confidence intervals included zero for the case of ISI). While geographic origin had no effect on male performance (MRPI), females from Tucumán displayed greater mating propensity than females from both Brazilian populations; and, in the case of the Vacaria-Pelotas combination, Pelotas females mated in lower frequencies than Vacaria females, perhaps due to differences in maturation rates (see FRPI). Nevertheless, such a tendency did not result in reproductive isolation, since females did not discriminate among males of different origin (mated at different rates with males of any origin).
Table 2. Mean±SE percent of mating couples and mean±se sexual isolation and mating performance indexes (and 95% confidence intervals) for three inter population mating combinations of Anastrepha fraterculus.
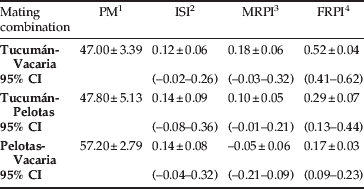
1 Percentage of mating=number couples obtained/number potential couples×100
2 Index of Sexual Isolation=[(AA+BB)–(AB+BA)]/N
3 Male Relative Performance Index=[(AB+AA)–(BA+BB)]/N
4 Female Relative Performance Index=[(BA+AA)–(AB+BB)]/N
AA, the number of couples involving males and females from the first population mentioned; AB, the number of couples involving males of the first population mentioned and females from the second population and so on; N, the total number of matings achieved.
Each mating combination was replicated eight times.
In the case of the Tucumán-Vacaria combination, a one-way ANOVA revealed significant differences in mating frequencies among mating combinations (F3,28=20.44, P<0.001). Irrespective of male origin, Vacaria females mated less frequently than Tucumán females (fig. 1a). A similar tendency was observed for the Tucumán-Pelotas combination (F3,28=4.86, P=0.007) (fig. 1b). In the case of the Vacaria-Pelotas, Vacaria males and Pelotas females mated less frequently than all other mating combinations (F3,28=5.72, P=0.003) (fig. 1c), perhaps because Pelotas females, which mature later than males, took longer to become fully receptive than Vacaria females.

Fig. 1. Mean (±SD) mating frequency per replicate (N=8) for different mating combinations (A♂A♀, B♂A♀, A♂B♀,B♂B♀) among three different population pairs (a) Tucumán-Vacaria; (b) Tucumán-Pelotas; (c) Vacaria-Pelotas of Argentinean and Brazilian Anastrepha fraterculus. Columns with different letters are statistically different at the 0.05 level.
There were no statistical differences in latency to mate among different mating combinations for Tucumán-Vacaria (F3,154=2.41; P=0.068), Tucumán-Pelotas (F3,128=2.16; P=0.096) or Vacaria-Pelotas (F3,200=1.45; P=0.227). Mating duration was also similar for all mating combinations within the three pair-wise populations combinations evaluated (H3,184=7.24; P=0.064 for Tucumán-Vacaria; F3,131=1.11; P=0.348 for Tucumán-Pelotas; H3,204=0.42; P=0.936 for Vacaria-Pelotas; table 3). Irrespective of fly origin, most matings occurred on the tree (72.16%); and, of those, the vast majority occurred on the underside of leaves (96.78%). There was a strong tendency for matings to occur in the upper part of the tree canopy (69.00%), and this occurred for the three populations, among which there were no significant differences (Chi6=5.85; P=0.440 for Tucumán-Vacaria; Chi6=2.71; P=0.843 for Tucumán-Pelotas; Chi6=7.60; P=0.268 for Vacaria-Pelotas). There was no clear pattern in mating location according to quadrant for any of the three populations. Matings tended to occur in quadrants with most intense light (East and North) and to become evenly distributed as the sun position began to rise. This resulted in no significant differences in mating location for Tucumán-Vacaria (Chi6=16.59; P=0.053) and Tucumán-Pelotas (Chi6=8.26; P=0.500). By contrast, for Vacaria-Pelotas mating combinations, couples occupied particular quadrants (Chi6=17.70; P=0.038) and Vacaria male-Pelotas female matings tended to occur in the South side of the tree canopy.
Table 3. Latency to mate and copula duration (mean±SE (N)) for heterotypic and homotypic crosses of three different populations of Anastrepha fraterculus.

Postzygotic isolation
There were no significant differences in fertility of F1 eggs among different crosses (H3,20=3.90; P=0.271 for Tucumán-Vacaria; H3,22=2.27; P=0.518 for Tucumán-Pelotas; H3,23=5.38; P=0.145 for Vacaria-Pelotas; table 4). F1 eggs seeded in artificial diet yielded F1 adults in all cases, and there were no significant differences in F1 adult sex ratio between the three possible pair-wise population comparisons (H3,20=3.31; P=0.345 for Tucumán-Vacaria; H3,19=2.57; P=0.46 for Tucumán-Pelotas; and H3,19=4.44; P=0.216 for Vacaria-Pelotas). There were no differences in F1 adult fertility (F2 egg hatch) among the four crosses within any mating combination (H3,18=6.91; P=0.074 for Tucumán-Vacaria; H3,15=5.15; P=0.160 for Tucumán-Pelotas; and H3,20=6.20; P=0.102 for Vacaria-Pelotas; table 4).
Table 4. F1 fertility (mean±SE), F1 total number of emerged adults and average sex ratio and F2 egg hatch (fertility) for all possible mating combinations among three Anastrepha fraterculus populations.

Distribution
The potential distribution of the A. fraterculus aff1 morphotype encompasses an area going from Castelar (Buenos Aires Province, Argentina) to the South to Sete Lagoas (State of Minas Gerais) to the North (fig. 2).

Fig. 2. Distribution of populations from Argentina and southern Brazil compatible with A. fraterculus aff1. The black line represents the potential range of A. fraterculus aff1.Blue dots represent two sympatric incompatible populations at the putative limit of the range.
Discussion
The present study analysed mating compatibility among Argentinean and southern Brazilian populations of A. fraterculus as a prerequisite to develop an area-wide approach using the sterile insect technique against this pest. We found no evidence of reproductive isolation among the three populations evaluated in this study. Sexually mature adults of all populations mated randomly among themselves, mating duration was not affected by fly geographic origin, and there was no clear evidence of spatial partition of mating location. In the laboratory, homotypic and heterotypic crosses displayed similar levels of fertility and yielded F1 adults without distortion of the sex ratio. Finally, F1 adults produced equally viable F2 eggs after self crosses. Such results suggest that these entities belong to a single wide-ranging population that can be targeted in an area-wide SIT regional eradication or suppression programme using sterilized flies from a single mass-reared strain.
Earlier studies on mating compatibility among four geographically distant Argentinean populations of A. fraterculus revealed that north-western and north-eastern Argentinean populations belong to a single biological entity (Petit-Marty et al., Reference Petit-Marty, Vera, Calcagno, Cladera, Segura, Allinghi, Rodriguero, Gómez Cendra, Viscarret and Vilardi2004a). Further cross mating studies, including one population from each region, also showed a lack of postzygotic isolation (Petit-Marty et al., Reference Petit-Marty, Vera, Calcagno, Cladera, Vilardi and Barnes2004b). The Argentinean population included in our study (Tucumán) was also evaluated by these authors. Using a molecular approach (allelic variation of citochrome oxidase I) Smith-Caldas et al. (Reference Smith-Caldas, McPheron, Silva and Zucchi2001) compared genetic affinity among several species and populations in the fraterculus species group. Such study clustered a northern Argentinean population (Tucumán), with four southern Brazilian populations of A. fraterculus among which a population from Vacaria was included. Similarly, Alberti et al. (Reference Alberti, Rodriguero, Cendra, Saidman and Vilardi2002) found close genetic affinity (isoenzymes and mitochondrial rDNA) among several Argentinean populations (including Tucumán) and the southern Brazilian population of Pelotas, which was also included in our study. Along these lines, Basso et al. (Reference Basso, Sonvico, Quesada-Allue and Manso2003) concluded that Argentinean populations and a population from Pelotas share the same karyotype. Finally, Hernández-Ortiz et al. (Reference Hernández-Ortiz, Gómez-Anaya, Sánchez, McPheron and Aluja2004), using a morphometric approach, clustered two southern Brazilian and the Tucumán population together. Not surprisingly, populations with close genetic affinity and morphologically similar (e.g. Pelotas, Tucumán and Vacaria) were shown to be reproductively compatible. If genetic and morphological similarities also represent reproductive compatibility among other populations from Argentina and Brazil, the geographical range of the A. fraterculus aff1 morphotype could be extended as far north as Monte Alegre do Sul and as far south as Buenos Aires (Castelar).
Notwithstanding the above, Vera et al. (Reference Vera, Cáceres, Wornoayporn, Islam, Robinson, de la Vega, Hendrichs and Cayol2006) found evidence of prezygotic isolation between a southern Brazilian and southern Argentinean population of A. fraterculus (Tucumán-Piracicaba). The Piracicaba population, originally thought to be aff1, as it was obtained from guavas, is geographically close to Santa Isabel, where at least two morphotypes or putative species of the A. fraterculus cryptic species complex coexist in sympatry (aff1 and aff2: Selivon et al., Reference Selivon, Perondini and Morgante2005). Consequently, further studies on the Piracicaba population need to be carried out before it can be assigned to a specific morphotype. These findings are consistent with those of earlier studies by Selivon et al. (Reference Selivon, Perondini and Morgante2005) and suggest that the area could be considered as the northern limit of the aff1 morphotype where it overlaps with aff2. Despite sympatry and partial reproductive compatibility (Selivon et al., Reference Selivon, Perondini and Morgante2005), both morphotypes maintain their genetic integrity.
Considering the diverse repertoire of chemical, visual and vibrational cues that males display during courtship, it would be interesting to compare pheromone and cuticular hydrocarbon composition, as well as several behavioural parameters of male courtship between these and other A. fraterculus morphotypes. Along these lines, differences in male sexual pheromone composition have been reported between Peruvian and Argentinean A. fraterculus morphotypes (Cáceres et al., 2009), and such differences can act as reproductive barriers causing the rapid evolution of reproductive isolation (Segura et al., Reference Segura, Vera, Rull, Wornoayporn, Islam and Robinson2011). These findings suggest that such a mechanism can aid in explaining divergence of the whole A. fraterculus cryptic species complex, and perhaps of complexes in other genera of tropical fruit flies such as the Bactrocera dorsalis complex (Clarke et al., Reference Clarke, Armstrong, Carmichael, Milne, Raghu, Roderick and Yeates2004).
Additionally, because sympatric morphotypes are still partially compatible (Selivon et al., Reference Selivon, Perondini and Morgante2005), it would be interesting from a basic perspective to examine the evolution of remating rate and cross response to male accessory gland products under selection against maladaptive hybridization.
Results of the present work constitute an important contribution to establishing the distributional range of the aff1 morphotype and a potential area-wide SIT region. Nevertheless, there is still little information on the status of A. fraterculus in Bolivia, Paraguay and Uruguay. Because of the climatic affinity among some regions of these countries and northern Argentina (Sánchez-Santillán & Garduño, Reference Sánchez-Santillán and Garduño2008), the range of aff1 could extend to such areas. A viable approach to gain insight on this hypothesis would be to use published records of aff1 distribution (e.g. Oroño et al., Reference Oroño, Albornoz-Medina, Núñez-Campero, Van Nieuwenhove, Bezdjian, Martin, Schliserman and Ovruski2008) and simulate the potential range of this morphotype according to microclimatic requirements using GARP and/or CLIMEX. Once the putative range of the aff1 is projected, some populations from the range limits could be collected and tested for compatibility against known populations of aff1 (e.g. Tucumán) using an approach that comprises morphological, genetic and behavioural studies, including mating and remating behaviour, as well as sexual pheromone analysis, methods that have proven to be efficient in differentiating entities within this cryptic species complex.
For area-wide SIT application, a laboratory strain with proven mass rearing qualities such as Tucumán could be hybridized with feral males from different populations to yield large numbers of competitive sterile males to suppress pest populations in areas of commercial production of tephritid host fruit.
Acknowledgements
We are grateful to Sohel Ahmad and Marc Schutze for assistance during field cage trials, to Andrew Jessup, Marc Vreysen, Jorge Hendrichs, Jesús Reyes and Rui Pereira for their support during the entire experimental process at the FAO/IAEA Agriculture and Biotechnology Laboratories in Seibersdorf Austria. To Magali Evrard, Adrene Despars and Anne Lorenz for helping with permits, transfers, paperwork and supplies. Funding was provided through a research contract (16038) as part of the FAO/IAEA Coordinated Research Project on Resolution of cryptic species complexes of Tephritid pests to overcome constraints to SIT and international trade as well as a FAO/IAEA consultancy to JR and a sabbatical leave fellowship provided by EMBRAPA to AK.








