Introduction
Nilaparvata lugens is an important rice migratory pest in Asia. It sucks rice phloem sap and also transmits damaging plant viruses, i.e. the rice ragged stunt virus and the rice grassy stunt virus (Hibino, Reference Hibino1996). Since the 1960s, following the introduction of fertilizer-tolerant varieties, high levels of nitrogen fertilization, close planting, and abuse of chemical pesticides, N. lugens has become the most serious pest of rice in Asia (Cheng et al., Reference Cheng, Wu and Ma2003). There have been successive outbreaks in China in recent years, resulting in serious damage to rice production (Cheng & Zhu, Reference Cheng and Zhu2006; Zhai & Cheng, Reference Zhai and Cheng2006). According to statistics from the Ministry of Agriculture of China, the loss of rice yield to N. lugens was about 1,880,000 tonnes in 2005 (Guo & Zhao, Reference Guo and Zhao2006). Nilaparvata lugens is perhaps the most problematic of the various insect pests threatening food security and ecosystem health in Asia (Cheng et al., Reference Cheng, Wu and Ma2003).
In 1967, Asahina & Tsuruoka (Reference Asahina and Tsuruoka1968) found many planthoppers migrating over a Pacific Ocean weather station (called ‘Tango’), about 500 km from the Southwest of Honshu, Japan. Based on the trapped insects on ships at ‘Tango’ and in the East China Sea, Kisimoto (Reference Kisimoto1971) concluded that planthoppers could undertake long-distance migrations. In the late 1970s, evidence for long-distance migration of N. lugens in Eastern China was obtained by a variety of methods, such as catches in interception traps on ships and airplanes and on mountain tops, catches in light traps, and mark-recapture (Cheng et al., Reference Cheng, Chen, Xi, Yang, Zhu, Wu, Qian and Yang1979; Deng, Reference Deng1981; National Cooperated Research Group of Brown Planthopper, 1981). The results showed that N. lugens could not overwinter in a temperate zone where there were no rice plants in winter time, but the region south of about 21°N provided stable overwintering areas for this species. It migrated northward or northeastward from tropical and subtropical areas annually in spring and summer, and undertook a series of southwestward ‘return’ migrations to overwintering areas in autumn.
Radar was introduced to monitor aerial migration of N. lugens by Britain's Natural Resources Institute (NRI) in the 1980s, because it offers the advantage of monitoring the whole process of migration, except in the presence of precipitation. Nilaparvata lugens would be detectable at a maximum range of only 300 m with a 3.2-cm wavelength conventional entomological radar (Riley et al., Reference Riley, Reynolds and Farrow1987), so an 8.8-mm wavelength scanning unit was developed. This could detect individual N. lugens to a range of about 1.3 km (Riley, Reference Riley1992). The migratory flight behaviour of N. lugens was investigated during the dry-season cropping period in the Philippines with the 8.8-mm radar unit (Riley et al., Reference Riley, Reynolds and Farrow1987), and the results showed that the average flight ceiling of 400 m was less than the 1000–2000 m height at which N. lugens had been trapped by aircraft over China (Deng, Reference Deng1981). In addition, N. lugens did not undertake long-distance migrations in the Philippines, they only moved distances of a few kilometres at dawn and dusk. From the late 1980s to the early 1990s, NRI cooperated with Nanjing Agricultural University to carry out radar observations on long-distance migration of N. lugens at Jiangpu, Jiangsu Province and Dongxiang, Jiangxi Province in Eastern China between late August and late September, obtaining estimates of key migration characters, such as take-off time, height of flight, flight duration time, and frequency of layer formation (Riley et al., Reference Riley, Chen, Zhang, Reynolds, Xu, Smith, Cheng, Bao and Zhai1991, Reference Riley, Reynolds, Smith, Rosenberg, Cheng, Zhang, Xu, Cheng, Bao, Zhai and Wang1994).
Southern China, being adjacent to the Indo-China Peninsula, is the first area within China affected by N. lugens each year, following immigration from Southeast Asia during April and May. Therefore, Southern China is the source area for the pest in Northern China (Cheng et al., Reference Cheng, Chen, Xi, Yang, Zhu, Wu, Qian and Yang1979; Guangxi Cooperated Research Group of Brown Planthopper, 1979). The average daily number of N. lugens captured in light traps during the last 20 days of May at Guilin, Guangxi Zhuang Autonomous Region was significantly positively correlated to the area and intensity of nationwide outbreaks of N. lugens (Hu, Reference Hu2010). However, there is a lack of data related to the seasonal migration of N. lugens in Southern China, as the NRI and Nanjing Agricultural University radar studies were restricted to Eastern China. A better understanding of the migration patterns and flight parameters of N. lugens in Southern China will be beneficial for the prediction and suppression of this pest in China and beyond.
The present study used a millimetric scanning entomological radar (MSER) of the Chinese Academy of Agricultural Sciences (CAAS) and a searchlight trap, supplemented with capture of ascending N. lugens in field cages, dissections of females, and field surveys, to make direct observations of migratory flights of N. lugens in Southern China in the summer and autumn of 2007 and the autumn of 2009. Take-off and the duration and height of flight, are described in detail, and relationships between these flight behaviours and atmospheric factors are identified. The possible migration trajectories of immigrants and emigrants are simulated.
Materials and methods
The observations were conducted at the Plant Protection Station of Xing'an county (25°37′25.0″N, 110°40′36.9″E), 209 m above mean sea level (MSL) in the Northeastern Guangxi Zhuang Autonomous Region of Southern China. Xing'an county is located on the ‘Xiang-gui Corridor’, which is a narrow basin surrounded by the Yuecheng Range, Ocean Hill, Jiaqiao Range and Tianping Hill; it is the major pathway in China for the seasonal northward and return migrations of N. lugens (Cheng et al., Reference Cheng, Chen, Xi, Yang, Zhu, Wu, Qian and Yang1979). Rice grown in Xing'an is mainly a single-season ‘middle rice’, which constitutes about 70% of the crop and is harvested at the end of September. There is also some ‘early rice’ and ‘late rice’, each constituting about 15% and harvested at the end of July and at the end of October, respectively.
Observations were made with the MSER, which operates at K a band (8.0-mm) wavelength. The maximum range (R max) at which the target can be detected was calculated by the usual radar range equation,
 $$R_{{\rm max}} = \left[ {\displaystyle{{P_{\rm t} G^2 {\rm \lambda}^2 {\rm \sigma}} \over {(4{\rm \pi})^3 kT_0 BF({\rm SNR})_{{\rm min}}}}} \right]^{1/4}, \,({\rm Skolnik},2001),$$
$$R_{{\rm max}} = \left[ {\displaystyle{{P_{\rm t} G^2 {\rm \lambda}^2 {\rm \sigma}} \over {(4{\rm \pi})^3 kT_0 BF({\rm SNR})_{{\rm min}}}}} \right]^{1/4}, \,({\rm Skolnik},2001),$$
where P t is the transmitter power (W), G is the antenna gain (dB), λ is the radar wavelength (m), σ is the target radar cross-section (m2), k=1.38×10−23 W−1 K−1 (Boltzmann's constant), T 0=273 K, B (bandwidth)=1/τ (τ is the pulse length), F is the noise figure (dB), and (SNR)min is the radar threshold (dB).
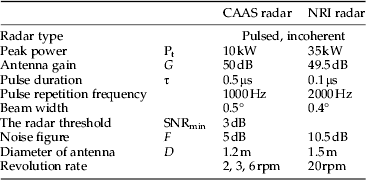
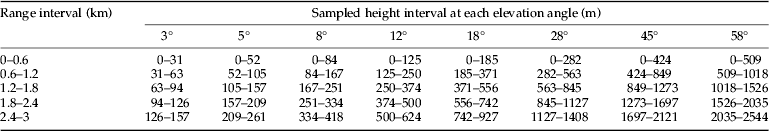
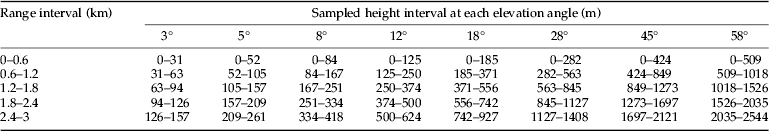
The CAAS radar differs from the NRI radar in many of its technical parameters (table 1). An average broadside radar cross-section for a female (the larger sex) N. lugens is about 5.5×10−2 cm2 at a wavelength of 8.8 mm (Riley, Reference Riley1992). Thus, the predicted maximum range detected by the CAAS radar for a broadside-on female N. lugens is about 2.5 km, in comparison with about 1.3 km for NRI unit. Observations were made on a plan position indicator (PPI) set to a maximum range of 3 km. The radar was mounted on a 5-m high pedestal. Observations were made from sunset until sunrise every night except when there was a storm or a power outage. In contrast to the method of recording PPI data on 16-mm film by means of a time-lapse cine-camera (Riley et al., Reference Riley, Chen, Zhang, Reynolds, Xu, Smith, Cheng, Bao and Zhai1991, Reference Riley, Reynolds, Smith, Rosenberg, Cheng, Zhang, Xu, Cheng, Bao, Zhai and Wang1994), the CAAS radar employed a digital data-acquisition system. When observations were made, the radar was operated with a routine sequence of elevation angles of 3°, 5°, 8°, 12°, 18°, 28°, 45°, and 58° as proposed by Drake (Reference Drake1981), so it can cover most of the flight altitudes of the insects. Real-time software in a microcomputer was used to control antenna elevation angle and capture digital images of the PPI screen. Radar echoes in the PPI images were coloured according to their reflectivity factor. Echoes of 0–5 dBz, which are interpreted as mainly being due to insects, were coloured light blue. The height, distance, and position of any detected radar echo were obtained directly from the captured images using non-real-time software during analysis. Photoshop CS5 Extended (Adobe Company, USA) was used to determine the total numbers of pixels of echoes, which were light blue on the PPI images. Because echoes from insects in the radar below 200 m above ground level (AGL) overlapped fully, we only counted the radar echoes between 0–5 dBz above 200 m AGL, and excluded some permanent echoes from mountains that were of the similar reflectivity factor. The pixel counts were made for five range intervals at each elevation angle (table 2). In a preliminary process, the pixel count for a single radar echo had been estimated, for each range interval, as the average numbers of pixels for 20 individual echoes. The numbers of insect echoes were then calculated, for each range interval, as the total numbers of pixels of radar echoes divided by the numbers of pixels for a single radar echo at that range. The sampling volume between ranges r 1 and r 2 from which a target of the nominal cross-section will return an above-threshold signal during a full 360° scan was calculated according to Drake (Reference Drake1981). So, the effective sampling volume for N. lugens at elevation angle ε was 5.02×107 cos (ε) m3 for the 600–1200 m range interval. The volume density was calculated as the number of radar echoes divided by the sampling volume for each range interval. Insect density profiles were estimated using a variant of the method of Feng et al. (Reference Feng, Wu, Cheng and Guo2003). As the sampling height intervals overlapped (table 2), the volume density for each 100-m profile interval was obtained by averaging all measured volume density values that extended over that height range. For example, the volume density between 500 and 600 m AGL was obtained by averaging seven volume densities: those for the range interval of 0.6–1.2 km at 28°, 45°, and 58°, those for 1.2–1.8 km at 18°, 28°, that for 1.8–2.4 km at 18°, and that for 2.4–3 km at 12°. The area density was calculated by summing the sequence of products of the volume density and the height interval for which each volume density was estimated (Drake, Reference Drake1981; Feng et al., Reference Feng, Wu, Cheng and Guo2004a ).
Table 1. Technical parameters for MSERs of CAAS and NRI.
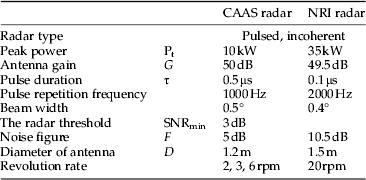
Table 2. Range intervals and sampled height intervals between each range ring at each elevation angle.
To identify the targets producing the radar echoes, a searchlight trap was set up near the radar to provide samples of high-flying insects (Feng et al., Reference Feng, Wu, Cheng and Guo2003). The searchlight trap was equipped with a 1-kW metal-halide lamp, which incorporated a parabolic reflector that generated a narrow vertical beam of light. It can sample insects flying up to or above 500 m AGL. Below the lamp was an inverted metal cone which collected the insects and directed them into a plastic basin containing 1% detergent solution to kill and retain them. The light was turned on after sunset every evening, and turned off after sunrise. The trap was emptied and the insects counted every morning.
During the peak period of insect activity, three cages were used for trapping macropterous N. lugens emigrating from nearby fields. The cylinder cages, 1.2 m in diameter and 2.0 m high, were made of fine nylon netting on aluminium alloy frames. They were set up in late afternoon and examined approximately half-hourly until late evening. Any macropterous N. lugens on the cage walls were counted and removed by suction tube.
Female N. lugens were dissected to assess the breeding development stage of the population. 20–30 N. lugens females caught with the searchlight trap and a similar number from nearby fields were dissected under a microscope and graded on an I–V scale of ovarian development (Chen et al., Reference Chen, Cheng, Yang and Yin1979).
Profiles of the local wind were obtained at about 20.00 h every evening by tracking a balloon with a theodolite. High-altitude atmospheric data were obtained from the United States National Weather Service's National Center for Environmental Prediction (http://dss.ucar.edu/datasets/ds083.2), the Final Operational Global Analysis data for the 1.0°×1.0° cell covering the radar site being downloaded every 6 h. The Hybrid Single Particle Lagrangian Integrated Trajectory (HYSPLIT) Model platform developed by the United States National Oceanic and Atmospheric Administration Air Resources Laboratory (see http://ready.arl.noaa.gov/HYSPLIT.php) was used for trajectory analysis. Backward trajectories were generated for immigrants and forward trajectories for emigrants. The starting time was assumed to be the dusk take-off time, and the start or end location was taken to be the radar site. Nilaparvata lugens were assumed to move with the same velocity as the wind. The height of flight was taken to be that at which the radar observations showed the maximum insect density. Captures in airplane-borne nets and radar observations have shown that the aerial density of N. lugens is lower by day than by night (Deng, Reference Deng1981; Riley et al., Reference Riley, Chen, Zhang, Reynolds, Xu, Smith, Cheng, Bao and Zhai1991); therefore, in this paper only trajectories commencing at dusk have been considered.
Local times in this paper are Beijing Time (UTC+8 h).
Results
Identification of radar targets
More than 200 insect species in 10 orders and 48 families were caught by the searchlight trap (Yang, Reference Yang2008). The percentage of N. lugens for a night was the number of N. lugens caught that night divided by the total numbers of all species in the catches. There were four principal migration periods of N. lugens, as indicated by peak catches in this trap in 2007 and 2009 (fig. 1):
-
i 22–26 July 2007;
-
ii 13–20 August 2007 (except 16 August);
-
iii 26–27 September and 4–6 October 2007; and
-
iv 30 September to 3 October 2009.
By 22–26 July 2007, early rice was nearly harvested and its nutritional condition was not suitable for N. lugens propagation. Ovarian development was mainly in the early stages in the fields, and the searchlight trap samples were dominated by stage II with a lesser percentage of stage I (fig. 2a). It is inferred that a large number of the N. lugens were locally bred and were emigrating. The numbers of N. lugens caught in the searchlight trap were the highest during this period, with this species constituting 60% or more of the catch (83.3% on 22 July) (fig. 1). Therefore, it seems certain that the radar echoes in this period were principally due to N. lugens.

Fig. 1. Number of N. lugens caught in a searchlight trap, and proportion of the total insect catch, in 2007 and 2009.

Fig. 2. Ovarian development stage of N. lugens caught in the fields and in the searchlight trap on (a) 22–26 July, (b) 13–20 August, and (c) 26 September–6 October 2007.
During 13–20 August 2007, the local middle rice was at the tillering and booting stages, which provided suitable nutrition for N. lugens. Ovarian development was mainly in late stages in the fields, while females in the searchlight trap samples were predominantly at stage II (fig. 2b), suggesting that these were mainly immigrants and overflying migrants. The percentage of N. lugens caught in the searchlight trap exceeded 30% in this period (fig. 1). Also, the numbers of Cyrtorhinus lividipennis (Reuter), a mirid bug that is an important predator of N. lugens, were sometimes higher than those of N. lugens. The total percentage of N. lugen and C. lividipennis in the searchlight trap exceeded 60% (91.3% on 13 August). Therefore, it seems certain that the radar echoes during this period were principally due to N. lugens and C. lividipennis.
During late September and early October, the local middle rice was nearly harvested; ovarian development was mainly in the early stages both in the fields and in the searchlight trap (fig. 2c), and so it is inferred that the N. lugens in the searchlight trap samples were mainly emigrants, with also some overflying migrants. The percentage of N. lugens in the searchlight trap catches exceeded 40%, which was the highest proportion of all species caught (fig. 1). Therefore, it seems certain that the radar echoes in the autumn were principally due to N. lugens.
Variations of area density during the evening
The field-cage observations of 22 and 24 July 2007 indicated that N. lugens took off at dusk reaching a maximum value of 8–9/1.1 m−2 (amount to 7.27–8.18 m−2) with a peak period from 19.30 to 20.00 h (fig. 3). The peak time at dusk observed with the radar coincided with that of upward flight in the cages. The dusk take-off started 1 h before sunset (sunset time, 19.27 h) and reached its peak 0.5 h after sunset. After the peak, area density generally decreased quickly to a low level which then persisted through the rest of the night, and reached a minimum at 03.45 h (fig. 4a). Migration of N. lugens therefore continued for up to about 9 h. The radar detected a second period of mass take-off at dawn (fig. 4a). This started at 05.00 h (sunrise was at 06.00 h) and reached its peak at 06.30 h. The density of take-off at dawn was less than that at dusk. The maximum area density was 40,000 km−2, on 22 July 2007. There were breaks in radar monitoring due to rain showers at about 22.00 h on both 22 and 23 July. The insect density had not decreased greatly after the rain, which apparently had little effect on the migration.

Fig. 3. Variation with time of N. lugens catches in the field cages during the period around dusk, on the nights of 22 and 24 July 2007.

Fig. 4. Variation of area density of insects during the evening for selected nights during the peak migration periods for N. lugens in (a) July, (b) August, (c) September–October 2007, and (d) October 2009. The solid triangle symbol indicates rain which persisted for 1 h.
Because rain fell after midnight, radar observations were terminated early on 13, 14, 18, and 19 August (fig. 4b). On these nights, the number of insects detected by the radar continued to increase after the dusk take-off peak, until the observations were terminated. Therefore, it seemed likely that the insects observed with the radar before mid-night were due to a relatively small local take-off and many overflying migrants from not too far away. Catches in the searchlight trap showed that more N. lugens and C. lividipennis were trapped after midnight than before. These N. lugens and C. lividipennis may have been forced to land by the rain.
In autumn, insects showed a pattern of dusk and dawn take-offs (fig. 4c, d) that was similar to those observed in July, although sometimes there was only a dusk take-off (e.g. 4–5 October 2007 and 30 September–1 October 2009). Typical dusk take-off of N. lugens was observed between 30 September and 2 October 2009, when sunset was at about 18.25 h; the take-off started at sunset and reached its peak after 1–1.5 h. The dawn take-off reached its peak at sunrise (sunrise about 06.27 h). The maximum area density was 44,000 km−2 on 30 September 2009 (fig. 4d). The peaks between dusk and dawn take-offs may have been due to overflights of migrants that had taken off from places progressively further away. From the times of take-off (18.25 h) and minimum density (04.20 h), it is inferred that the maximum duration of flight at night was about 10 h in autumn.
Variations in the vertical profile of insect density during an evening
In summer, planthopper-size targets generally flew below 1800 m AGL, although some insects reached 2000 m AGL (fig. 5a, c). Multiple layers were common (fig. 6), with layers often occurring at altitudes of 400–500 m AGL, 600–1000 m AGL, and 1400–1800 m AGL, with more than one layer frequently present. Almost every night, layers at 400–500 m AGL and 600–1000 m AGL were observed. The lowest layer, at 400–500 m AGL (fig. 5a), was stable and simple, but the second (600–1000 m AGL) and the third (1400–1800 m AGL) were deeper and had higher densities and more complicated forms (fig. 5c). The highest layer top recorded was 1800 m AGL and the maximum density was 48,000 km−3, at 19.38 h on 13 August 2007.

Fig. 5. Profiles of the volume density of insects, the wind direction, and the wind speed during the evenings of (a, b) 13 and (c, d) 14 August 2007.

Fig. 6. Image of PPI at 21.37 h with radar beam at 45° elevation angle on 14 August 2007, showing layering. The outer range ring is at 3 km.
The vertical distribution of insects was generally similar in autumn, although planthopper-size targets flew lower than in summer – usually below 1100 m AGL, although with some reaching 1700 m AGL (fig. 7a). As during the summer migration, multiple layers were common, often occurring at altitudes of 300–500 m, 600–700 m, and 900–1100 m AGL. The layer thicknesses were less than those of the summer layers.

Fig. 7. Profiles of the volume density of insects, the wind direction, and the wind speed during the night of 1–2 October 2009.
Insect layers in relation to atmospheric structure
Comparison of the vertical density distribution with the wind profile did not reveal any close relationship between the wind and the first or the third layer, but in summer the second layer at 600–1000 m AGL was frequently observed to coincide closely with a zone in which there was strong shear in wind direction and a maximum of wind speed. For example, on 13 August 2007 (fig. 5a, b) the maximum insect density occurred at 750 m AGL. The wind profile measured before rain fell at 23.00 h showed that there was a zone of wind shear with a sharp change of wind direction of more than 80° at about 750 m AGL. The wind direction was easterly (94°) at 750 m AGL, but almost northerly (11°) at 800 m AGL. Another example occurred the following night (fig. 5c, d) when a high-density layer formed at 750–1000 m AGL with a density maximum at 950 m AGL. At the same height, there was a strong wind shear of nearly 70°. In both cases, the zone of directional wind shear coincided with the local maximum wind speed. The wind speed exceeded 11 m s−1 at 950 m AGL on 14 August 2007. This coincidence of high-density insect layers and wind maxima indicated that insects were taking advantage of fast airflows during their long-distance migrations. A regression analysis for the period of balloon ascent on 13 August 2007 showed that, over the height range 350–1550 m AGL, there was a significant linear relationship between the insect density D h at height h to the wind speed V h at the same height, described by D h =61.2V h +172 (P<0.01). On 1 October 2009, there were high-density insect layers at 400 and 700 m AGL, and wind speed maxima that exceeded 10 m s−1 at these altitudes, but there was no shear of the wind direction (fig. 7a, b). So, as in summer the insect layers were coincident with maxima in the wind speed profile.
The maximum height of N. lugens-type insect echoes was 1800 m AGL in summer, and 1100 m AGL in autumn. The elevation of Xing'an is about 200 m above MSL, so temperature data were obtained for the 800 hPa pressure level (about 1920 m above MSL) in summer and for 850 hPa (about 1470 m above MSL) in autumn, to coincide with the insects’ flight ceiling. It was found that air temperature at 800 hPa at 20.00 h was between 15.6 and 18.2 °C during the migration periods of summer 2007. The air temperature at 850 hPa at 20.00 h was in the range of 17.9–20.1 °C in autumn 2007, and 15.3–18.1 °C in autumn 2009. The relative humidity for the range of flying heights of the insects was 60–95% in summer, 70–90% in autumn 2007, and 50–90% in autumn 2009.
Sex ratio
In summer, the proportion of female N. lugens in the searchlight trap catches exceeded 50% except on 23 July (fig. 8), whereas in autumn this proportion was less than 50%. Thus females outnumbered males in the summer migrations, and the males were more numerous in autumn.

Fig. 8. Percentage of female N. lugens caught in a searchlight trap during peak migration periods in the summer of 2007 and the autumn of 2009. Both * and ** indicate significantly different from 50%, at P=0.05 and 0.01 levels, respectively.
Possible migration trajectories
Based on the results of our radar observations, trajectories were calculated for flight at the height of the maximum volume density of insects. The flight duration of migration in summer was assumed to be 9 h. Zhai (Reference Zhai1992) has shown that a small proportion of N. lugens individuals has strong flight capability and can fly for 24–26 h. To determine whether N. lugens could reach overwintering areas, the flight duration in autumn was assumed to be 26 h. During 22–26 July 2007, the HYSPLIT trajectories indicated that N. lugens emigrating from the radar site and carried by the southwesterly wind would have travelled northeastwards for 260–470 km, and would have reached Northern Hunan Province (fig. 9a). In autumn, N. lugens carried on the prevailing northeasterly wind and emigrating from the radar site would have arrived in the Southern Guangxi Zhuang Autonomous Region and Northern Vietnam (fig. 9a).

Fig. 9. Examples of (a) 9-h forward trajectories for N. lugens emigrating from the radar site at 19.00 h on 22–26 July 2007 (dashed lines) and 26-h forward trajectories on 5 October 2007 and 30 September–3 October 2009 (solid lines); and (b) of 9-h back trajectories ending at the radar site at 04.00 h on 13, 14, 18, 19, and 20 August 2007. The star indicates the radar station at Xing’ an.
On 18–19 August 2007, under the influence of typhoon ‘Sepat’, Hunan, Guangdong, and Jiangxi Provinces and Guangxi Zhuang Autonomous Region were experiencing strong continuous northerly or northeasterly winds. Captures in the searchlight trap showed that N. lugens predominated after midnight. These N. lugens, which rain forced to land near the radar site, would have come from an area extending up to 310 km to the northeast, in the central part of Hunan Province (fig. 9b).
Discussion
The estimates of aerial density presented here make it clear that populations of N. lugens in Xing'an were lower by roughly an order of magnitude than those reported from Eastern China in the 1980s (Riley et al., Reference Riley, Chen, Zhang, Reynolds, Xu, Smith, Cheng, Bao and Zhai1991, Reference Riley, Reynolds, Smith, Rosenberg, Cheng, Zhang, Xu, Cheng, Bao, Zhai and Wang1994). Unlike during the autumn of 1988 in Eastern China, there were no reports of outbreak populations of N. lugens in Southern China during 2007 and 2009. The results of ground sampling were lower than in Eastern China. In autumn, some characteristics of N. lugens migration in Southern China were similar to those reported from Eastern China (Riley et al., Reference Riley, Chen, Zhang, Reynolds, Xu, Smith, Cheng, Bao and Zhai1991, Reference Riley, Reynolds, Smith, Rosenberg, Cheng, Zhang, Xu, Cheng, Bao, Zhai and Wang1994). In both regions, there was a mass take-off of N. lugens at dusk, and sometimes there was another, less strong at dawn. The flight duration of N. lugens in Southern China was approximately 10 h, compared with 12 h in Eastern China. Most individuals in Southern China flew at heights between 300 and 1100 m AGL in autumn, a few to 1700 m AGL; these heights were slightly greater than the 300–1000 m observed with radar in Eastern China in the same season (Riley et al., Reference Riley, Chen, Zhang, Reynolds, Xu, Smith, Cheng, Bao and Zhai1991, Reference Riley, Reynolds, Smith, Rosenberg, Cheng, Zhang, Xu, Cheng, Bao, Zhai and Wang1994). Deng (Reference Deng1981) in Central China caught most N. lugens between 500 and 1000 m AGL (maximum height 1500 m AGL) in autumn, and between 1500 and 2000 m AGL (maximum height 2500 m AGL) in summer. We found that most individuals in Southern China flew at heights between about 400 and 1800 m AGL with the maximum height 2000 m AGL in summer. Regional differences in height of flight (e.g. between coastal and interior areas) may similarly be accounted for by the temperature–height profile (Tu, Reference Tu, Argosino, Iargrove and Mendoza1980). There are some high mountains in Southern China, whereas Eastern China and Central China comprise a broad plain. The seasonal differences of flight height were probably caused by variation of temperature (Deng, Reference Deng1981).
The concentration of insects in layers at altitudes of several hundred meters has often been detected by radar, and appears to be a very common feature of long-range migration (Drake, Reference Drake1984). In Eastern China, the migrants flew often in a dense layer (Riley et al., Reference Riley, Chen, Zhang, Reynolds, Xu, Smith, Cheng, Bao and Zhai1991, Reference Riley, Reynolds, Smith, Rosenberg, Cheng, Zhang, Xu, Cheng, Bao, Zhai and Wang1994). However, multiple layers were observed almost every night in Southern China. Sometimes complex layer structures with two peaks were observed, as reported also during radar observations of the pyralid moth Loxostege sticticalis (Feng et al., Reference Feng, Wu, Cheng and Guo2004b ). We considered two hypotheses, both supported by some evidence, for the mechanism by which insect migrants select and maintain their preferred altitudes. One is that the insects seek the warmest air (Reynolds & Riley, Reference Reynolds and Riley1997) and the other is that they accumulate where there is a maximum in the wind speed or shear in the wind direction (Domino et al., Reference Domino, Showers, Taylor and Shaw1983; Drake, Reference Drake1985; Wolf et al., Reference Wolf, Westbrook, Sparks and Sparks1986; Beerwinkle et al., Reference Beerwinkle, Lopez, Witz, Schleider, Eyster and Lingren1994; Riley et al., Reference Riley, Reynolds, Smith, Edwards, Zhang, Cheng, Wang, Cheng and Zhai1995). In Xing'an, air temperature at ceiling height in summer 2007 (between 15.6 and 18.2 °C) and in autumn 2009 (between 15.3 and 18.1 °C) were similar to that observed at Nanjing (between 15.2 and 17.0 °C) (Riley et al., Reference Riley, Chen, Zhang, Reynolds, Xu, Smith, Cheng, Bao and Zhai1991, Reference Riley, Reynolds, Smith, Rosenberg, Cheng, Zhang, Xu, Cheng, Bao, Zhai and Wang1994). Ceiling air temperatures of between 17.9 and 20.1 °C in autumn 2007 were higher those than observed at other times, but the height of migration did not increase. Deng (Reference Deng1981) found that the air temperature thresholds for flight were 12 °C (low) and 30 °C (high). There remains the possibility that the height of flight was mainly influenced by other factors when air temperature was suitable for flight. In our study, in summer, some layers occurred at heights where both a wind direction shift and a wind speed maximum were located. In autumn, well-defined strong insect layers were located at the heights of maximum wind speed. It appears N. lugens can locate the zone of maximum wind speed and exploit this to migrate long distances with the assistance of air currents. The more complicated phenomenon of multiple layering requires further study.
As in Eastern China, collective orientation by N. lugens was not observed.
Deng (Reference Deng1981) found that N. lugens tended to fly at relative humidities of 75–85% in summer and 40–50% in autumn. Ohkubo (Reference Ohkubo1981) found that flights in the laboratory were longer under high humidity (85%). We found that the relative humidity during flight was 60–95% in summer and 50–90% in autumn. As in Riley et al.(Reference Riley, Chen, Zhang, Reynolds, Xu, Smith, Cheng, Bao and Zhai1991) study, there was no obvious relationship between the insect layers and relative humidity.
Cyrtorhinus lividipennis was common in the searchlight trap in summer, sometimes more numerous than N. lugens. Catches on the East China Sea (Kisimoto, Reference Kisimoto1979; Liu et al., Reference Liu, Liu and Zhu1983), in airplane traps over central China (Deng, Reference Deng1981), and with kite-borne nets in Eastern Central China (Riley et al., Reference Riley, Chen, Zhang, Reynolds, Xu, Smith, Cheng, Bao and Zhai1991, Reference Riley, Reynolds, Smith, Rosenberg, Cheng, Zhang, Xu, Cheng, Bao, Zhai and Wang1994), have demonstrated that this species is a long-distance accompanying migrant in Temperate Eastern Asia. In the present study, the migratory activity of C. lividipennis was similar to that of N. lugens.
Evidence about the sex-ratio of migrating N. lugens is ambiguous. Kisimoto (Reference Kisimoto1987) found that female N. lugens tended to be under-represented (38–54%) in catches from migrations over the East China Sea. Observations in Eastern China showed that equal numbers of both sexes took off at dusk, but there was a tendency for males to continue their migratory flight longer than females (Riley et al., Reference Riley, Chen, Zhang, Reynolds, Xu, Smith, Cheng, Bao and Zhai1991). Laboratory experiments indicated that female N. lugens fly longer than males, although the difference was not significant (Feng et al., Reference Feng, Zhai and Zhang2001). Catches in airplane-borne traps over four Chinese provinces showed that females were more numerous in the air than males, without seasonal variation (Deng, Reference Deng1981). However, males outnumbered females in catches in a mountain-top net on Huang Mountain in the south of Anhui Province (Chen et al., Reference Chen, Wang and Zou1978). In our study, females slightly outnumbered males in summer migration, whereas the opposite was the case in autumn. The sex-ratio of N. lugens is influenced by many factors, and requires further study.
In autumn, N. lugens arrived in the central part of the Guangxi Zhuang Autonomous Region after a 9-h flight. Late rice was then at the milk stage, and therefore the offspring of these immigrants would have been unable to complete their development. However, with a flight of 26 h, as recorded for a small proportion of N. lugens by Zhai (Reference Zhai1992), areas in Northern Vietnam (south of 21° N), where overwintering is possible in all years (Cheng et al., Reference Cheng, Chen, Xi, Yang, Zhu, Wu, Qian and Yang1979), would be reached. Therefore, we suspect that the migration trajectory of N. lugens forms a closed circuit in Asia, and the species re-migrates northward the following spring. Further observations are required to discover what percentages of N. lugens in Southern China in autumn reach the overwintering areas.
Overall, radar is widely used to study the behaviour and spatiotemporal distribution of migratory pests, with significant benefits for prediction and prevention of those pests. A network of entomological radars for monitoring insect pest migration in China is being considered. These first MSER observations of the seasonal migrations of N. lugens in Southern China demonstrate the potential of radar observation and thus provide support for the construction of such a network.
Acknowledgements
This study was supported by the National ‘973’ Programme (grant no. 2010CB126200) and the National Natural Science Foundation of China (grant no. 31101431). We are grateful to an anonymous referee for valuable suggestions and language editing.