Introduction
Extreme abundance of flood-water mosquitoes (mainly Aedes sticticus) occurs after floods during May through August in the flood plains of the River Dalälven, central Sweden (Schäfer et al., Reference Schäfer, Lundström and Petersson2008). The massive nuisance caused by these mosquitoes after large floods may affect humans and other vertebrates up to a distance of 14 km from the larval production areas in the flood-prone inundation wetlands. As a response to decades of complaints from the locals, the Swedish Biological Mosquito Control Project (www.mygg.se) was initiated in 2000, and the first larval treatments were performed in 2002. Due to the potential vulnerability of the wetland environments (many of which are protected as national parks, nature reserves and Natura 2000 sites), Swedish national and county authorities demanded long-term follow up studies on the possible environmental impact caused by the biological control activities.
The mosquito larval control in central Sweden is based on aerial application of VectoBac G© granules (dried and minced culture of Bacillus thuringiensis var. israelensis (Bti), corn oil and pellets of corn cob, with protein crystals produced by Bti as the active ingredient) by helicopter. VectoBac G© and other Bti-based products are used in many countries worldwide to control the production of mosquitoes, to mitigate nuisance for neighbouring human populations and to counteract transmission of mosquito-borne infections (Becker et al., Reference Becker, Zgomba, Petric, Dahl, Boase, Lane and Kaiser2003). In Sweden, our aerial application of VectoBac G© at 13–15 kg ha−1 (0.4 kg Bti ha−1), using a helicopter sling system with a bucket spreader, gives close to 100% reduction of flood-water mosquito larvae and 90–95% reduction of adult mosquito abundance (Lundström & Schäfer, unpublished). To ensure geographic coverage and precision, the pilot receives digital map files with flight routes and reports upon landing by providing the helicopter log file showing all flight movements with open spreader. To ensure that the VectoBac G© dosing is in accordance with instructions, we provide the helicopter with the amount needed to treat each area, and on a few occasions we have used horizontal nets (size 0.5×0.5 m) within the treatment areas to verify that the VectoBac G© granules were distributed according to instructions.
Formulations of Bti used at the appropriate dosage for mosquito control do not have a significant impact on most other animals or plants (Boisvert & Boisvert, Reference Boisvert and Boisvert2000). In the cases when impact is reported, this is mainly on the family Chironomidae (Diptera: Nematocera) (Fillinger, Reference Fillinger1998; Hershey et al., Reference Hershey, Lima, Niemi and Regal1998; Pont et al., Reference Pont, Franquet and Tourenq1999). Such non-target effects are not welcomed as chironomids may have a major role in the aquatic ecosystems (Armitage et al., Reference Armitage, Cranston and Pinder1995).
Our studies on the potential environmental impact of Bti include a six-year monitoring of the adult insects produced in temporary flooded wet meadows and swamps, with a focus on the chironomids. About 650 species of chironomids are known from Sweden (Y. Brodin, unpublished data), which is more than 1% of all species of all organisms recorded in the country to date. Due to their high abundance, and the habit of forming large swarms, they are commonly used as food items by many bird and bat species (Laursen, Reference Laursen1978; de Jong & Ahlén, Reference De Jong and Ahlén1991; Vaughan, Reference Vaughan1997; Cox et al., Reference Cox, Hanson, Roy, Euliss, Johnson and Butler1998; Buchanan et al., Reference Buchanan, Grant, Sanderson and Pearce-Higgins2006; Encarnacao & Dietz, Reference Encarnacao and Dietz2006). As such, the chironomids form important constituents in the terrestrial food chain. The monitoring programme was designed to assess both possible short-term and long-term negative impacts on the production of chironomids caused by the Bti treatments for mosquito control. Only one other study on the impact of Bti on chironomids covering more than two years seems to have been published – a three-year study of wetlands in Minnesota in the USA (Hershey et al., Reference Hershey, Lima, Niemi and Regal1998).
In the present study, we elucidate the production of chironomids in the River Dalälven inundation wetlands over six consecutive years, and evaluate the possible impact of Bti used for mosquito larval control on the production of chironomids at family, subfamily and species level.
Material and methods
Study areas
The study was performed in six inundation wetlands in the River Dalälven flood plains, central Sweden (fig. 1) during the years 2002 up to and including 2007. Three wetlands treated with VectoBac G© were used as experimental wetlands, and three wetlands were untreated (reference wetlands). The variable water flow in the River Dalälven cause recurrent but irregular floods between April and December, and floods occurring during May to August induce hatching of eggs of Aedes sticticus and other flood-water mosquito species that cause massive nuisance. All six inundation wetlands can be completely aquatic during floods, or completely terrestrial in drier periods.

Fig. 1. The six study wetlands adjacent to Lake Färnebofjärden in the flood plains of the River Dalälven, central Sweden. Chironomids were sampled continuously week 19 to 37 over six consecutive years to investigate production, and to study the effects on the non-target chironomid fauna of mosquito larviciding using Bti.
Nordmyra (experimental wetland)
During 2002 and 2003, this wet meadow was characterized by patches of Salix-bushes interspersed by wet grassland with a small-scale variation in topography due to the natural succession of grass, herb and bush species. However, in 2004 the bushes were removed and the small-scale topography eliminated by mechanical removal of roots, in an attempt to restore the historic use of the area for meadow grass production and harvest. Since then, the area has been used for meadow grass harvest using modern agricultural practise. The vegetation of a small lake, Nordmyrasjön, within the wetlands is dominated by reed and cattail. Based on the measured abundance of flood-water mosquito larvae, mosquito larvae control using VectoBac G© was performed twice in a 14-day period in July 2002, once in June 2005 and once in May 2006.
Laggarbo (experimental wetland)
This wet meadow is situated in the southern end of Lake Fängsjön and connected to Lake Färnebofjärden by the stream Laggarboån. The area is characterized by a reed belt in the more consistently wet parts, by Carex meadow in the drier parts and by extensive areas of Salix-bushes at the edges. Mosquito larval control was performed twice in a 14-day period in July 2002, once in May 2003, once in June 2005 and once in May 2006.
Valmbäcken (experimental wetland)
This is an alder swamp with rather dense vegetation and with a mixture of deciduous tree species in addition to the dominating Alnus glutinosa. The rivulet Valmbäcken in the central part of the swamp connects directly to Lake Färnebofjärden. Mosquito larval control was performed twice in a 14-day period in July 2002 and once in May 2006.
Lusmyren (reference wetland)
This is a wet meadow at the edge of Lake Färnebofjärden dominated by bushes of Myrica gale in the upper part and by Carex grassland in the lower part, a reed area between the meadow and the lake, and surrounded by a narrow stretch of Salix-bushes and pine woodland.
Fågle (reference wetland)
This is a wet meadow dominated by grass and with a few Salix-bushes and trees. The rivulet Fågleån flows through the meadow and connects directly to Lake Färnebofjärden.
Koversta (reference wetland)
This is an alder swamp area with rather dense vegetation and with a mixture of tree species in addition to the dominating Alnus glutinosa. A number of ditches in the central part of the swamp connect directly to Lake Färnebofjärden.
Insect sampling
Cone-formed emergence traps, modified Mundie′s trap (Service, Reference Service1993), were used to catch insects during both terrestrial and aquatic conditions in the temporary flooded wetland environments. The trap consists of three parts: a cone of white PVC plastic (height 0.6 m, bottom diameter 0.6 m, bottom area of 0.31 m2), a floating devise (air-filled bicycle inner tube with a 0.66 m diameter, and 0.06 m thickness) attached around the base of the cone, and a collecting jar (with ethylene glycol for killing and preserving the insects) at the top of the cone for collecting the emerging insects. These modified Mundie′s emergence traps float on the water during floods, and settle on the ground during periods without surface water. Traps were visited once a week and the collected insects were extracted from the ethylene glycol and preserved in 70% alcohol. The water depth under each trap was measured during each visit to provide information on whether aquatic or terrestrial conditions were prevailing. Mean water depth below 1 cm was considered to represent terrestrial conditions and 1 cm or more aquatic conditions.
Four traps were placed in each of the wetlands and the traps were distributed to cover the hydrological conditions, ranging from almost always aquatic to almost always terrestrial. Trapping of insects was performed over six consecutive years, 2002 up to and including 2007. The annual trapping period was from May (week 19) to September (week 37), and this five-month period provides a fairly accurate estimate of the annual production of emerging chironomids in the River Dalälven wetlands, judging from studies on chironomid productivity in similar climate conditions (Aagaard, Reference Aagaard1978; Paasivirta et al., Reference Paasivirta, Lahti and Perätie1988).
Insect sorting and chironomid species identification
Adult chironomids were picked out from the insect material and identified to species level when possible. Males could be identified on morphological characters based on literature information. Although most females could not be identified on morphological characters alone, species identification was generally possible by associating females with safely identified males occurring simultaneously in the same trap.
Literature information on ash-free weight of individual species of chironomids, particularly Potter & Learner (Reference Potter and Learner1974), Paasivirta (Reference Paasivirta1975) and Paasivirta et al. (Reference Paasivirta, Lahti and Perätie1988), formed the bases for the calculations of annual biomass production of chironomids in the River Dalälven wetlands.
Statistical analysis
The number of chironomids per trap and week was not normally distributed, and thus all abundance data was log-transformed before analysis. All calculations were done using SAS statistical software, version 9.1 (SAS Institute, 2004). Analysis of variance (ANOVA, using PROC GLM) was used for evaluating production (measured as abundance) differences coupled to year, week, area, floods and treatments. The ANOVAs were run for the family Chironomidae, for the subfamilies Chironominae, Orthocladiinae and Tanypodinae, and for the 25 most abundant species. The temporal aspects studied were short-term view (two weeks before and two weeks after Bti-treatment) and long-term view (one to six years after Bti-treatment). Time of year (week) was included either as covariate or factor. On average, chironomid production was negatively correlated with water depth (r=−0.35467, P<0.001, df=113, effect of week partial out). Therefore, water depth was mostly included as covariate, except for estimation of variance components where water depth was grouped into five categories.
Variance component analysis was used to study the relative explanatory importance of each measured variable with respect to the total observed variation in the weekly abundance of chironomids in emergence traps. The variance components were estimated in PROC VARCOMP using restricted maximum likelihood.
Carry over effects were studied on a year to year basis using t-test with levels of significance adjusted for multiple comparisons by the SIMULATE adjustment provided by SAS software. This procedure computes adjusted P-values from the simulated distribution of the maximum or maximum absolute value of a multivariate t random vector.
Results
Taxonomic composition and temporal abundance
The insect material sampled in 24 emergence traps operated over six summer seasons in temporary flooded wetlands provided at total of 21,394 chironomids. A total of 135 species in four subfamilies were identified: Orthocladiinae (58 species, 17,442 individuals), Chironominae (57 species, 2920 individuals), Tanypodinae (19 species, 1031 individuals) and Podonominae (1 species, 1 individual). One species new to science, Tavastia yggdrasilia, was identified (Brodin et al., Reference Brodin, Lundström and Paasivirta2008). The emergence period for 25 of the most commonly occurring species was quite extensive (fig. 2). Emergence of adult chironomids was recorded during 15–19 weeks for 68% of the species, and 8–13 weeks for 32% of the species.

Fig. 2. Emergence period for 25 commonly occurring Chironomidae species in temporary flooded wetlands of the River Dalälven flood plains, central Sweden, based on emergence data from early May to middle of September during six years.
The temporal abundance of chironomids produced in all six wetlands and all traps varied greatly, both within and between years (fig. 3). The total number of chironomids sampled in all wetlands varied from 2162 to 5342 per year, with an average of 3566 individuals per year. The within-year variation, based on weekly catches, showed that the annual maximum chironomid abundance occurred at almost any week during the vegetation season (week 20 to week 30) and with no distinct seasonal pattern in abundance. The abundance of the subfamilies showed similar complex patterns, both within and between years.

Fig. 3. Temporal patterns of chironomid abundance by week and year in relation to mosquito larviciding using Bti in temporary flooded wetlands of the River Dalälven flood plains, central Sweden. Each weekly figure is based on total catch in 12 emergence traps operated in three temporary flooded experimental wetlands (treated with Bti), and 12 traps in three reference wetlands (without treatment). This figure presents the raw data, but analyses were done on values corrected for water levels and/or time of the year (cf. table 1) (![]() , reference; —, experimental).
, reference; —, experimental).
The variation in chironomid abundance from one year to the next was tested for each wetland (table 1). A significant decrease in chironomid production was observed for the experimental wetland Nordmyra between 2002 and 2003, as well as for the reference wetland Koversta, and a significant increase for all the experimental wetlands between 2004 and 2005. Finally, an increase was noted for the reference wetland Koversta and the experimental wetland Valmbäcken between 2005 and 2006.
Table 1. Year by year changes in chironomid production in temporary flooded wetlands of the River Dalälven flood plains, central Sweden. Mosquito larval control using Bti was performed in experimental wetlands (Exp), while the reference (Ref) wetlands were untreated.

Values within parentheses are level of significances. Bold, significant change; –, decrease; all others are increases. The analysis in this table is based on least square means for production adjusted for time of the year and water levels. As the production is highly correlated with water level, the pattern here might differ from that given by the raw data in figs 3 and 4.
Production of chironomids
The production of chironomids per m2 varied between years and between the six wetlands (fig. 4). The annual production of adult chironomids ranged from 264 to 6777 individuals per m2 and year, with an average of 1917 individuals per m2 and year. The production differences between the wetlands were large, with the annual highest production in the experimental wetland Laggarbo in 2002, in the experimental wetland Nordmyra in 2003, in the experimental wetland Valmbäcken in 2004, in the reference wetland Koversta in 2005 and 2006, and in the reference wetland Lusmyren in 2007. Thus, only the reference wetland Fågle out of the six wetlands was not the most productive during any of the six years studied.

Fig. 4. Annual production of chironomids by wetland and year in the temporary flooded wetlands of the River Dalälven flood plains, central Sweden. For each group of bars, the first three illustrate annual production in experimental wetlands (treated with Bti against mosquito larvae), and the next three illustrate production in three reference wetlands (without treatment). This figure presents the raw data, but analyses were done on values corrected for water levels and/or time of the year (cf. table 1) (□, Nordmyra exp; ![]() , Valmbäcken exp; ▪, Laggarbo exp;
, Valmbäcken exp; ▪, Laggarbo exp; ![]() , Lusmyren ref;
, Lusmyren ref; ![]() , Koversta ref;
, Koversta ref; ![]() , Fågle ref).
, Fågle ref).
The annual chironomid biomass production, calculated for the six wetlands together, was 37, 49, 63, 29, 48 and 20 g ash-free dry weight per m2 for the years 2002, 2003, 2004, 2005, 2006 and 2007, respectively, and averaged 41 g ash-free dry weight per m2. The lowest annual biomass (20 g ash-free dry weight per m2) was produced in 2007, the driest year, when aquatic conditions prevailed under the traps at only 8% of all sampling occasions. However, the highest annual biomass (63 g ash-free dry weight per m2) was also produced in a relatively dry year, 2004, when aquatic conditions prevailed under the traps at 19% of all sampling occasions.
Chironomids and Bti-based mosquito larval control
The possible effect of Bti treatment on production of the family Chironomidae, and the subfamilies Chironominae, Orthocladiinae and Tanypodinae was evaluated for the log-transformed individual trap data by week over the full six year period (table 2). No significant effects were found for the family and the subfamilies, neither in the short-term view (two weeks before and two weeks after Bti treatment) nor in the long-term view (between years).
Table 2. Analysis using ANOVA of the effects of mosquito larval control with Bti on the abundance of chironomids at family and subfamily levels in temporary flooded wetlands of the River Dalälven flood plains, central Sweden. The short-term analysis was based on weekly samples two weeks before and two weeks after Bti application. The long-term analysis was based on weekly samples during weeks 19 to 37 in three experimental wetlands (treated with Bti) and three reference wetlands (without treatment).
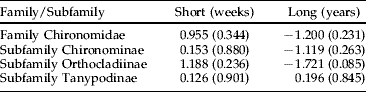
The figures are t-values and values within parentheses are level of significances. Bold, significant change; –, decrease; all others are increases.
The possible effect of Bti treatment on Chironomid species was evaluated for the log-transformed individual trap data over the six year study period (table 3). In the short-term view, the ANOVA showed significant differences between Bti-treated and untreated wetlands for one out of 12 species. Tanytarsus curticornis (Chironominae) had significantly higher production in experimental wetlands than in reference wetlands. In the long-term view, the ANOVA showed significant differences between Bti-treated and untreated wetlands for five out of 23 species. Four species, Tanytarsus medius (Chironominae), Pseudosmittia angusta (Orthocladinae), Ablabesmyia longistyla (Tanypodinae) and Paramerina cingulata (Tanypodinae), were significantly more numerous in experimental wetlands compared to reference wetlands in a long-term view. One species, Telmatopelopia nemorum (Tanypodinae), was significantly less numerous in experimental than in reference wetlands in long-term view. However, the majority of the 135 Chironomid species were either not sufficiently abundant or had too patchy distribution for analysis at species level.
Table 3. Analysis using ANOVA of the effects of mosquito larval control with Bti on the abundance of chironomid species in wetlands of the River Dalälven flood plains, central Sweden. The short-term analysis was based on weekly samples two weeks before and two weeks after Bti application. The long-term analysis was based on weekly samples over six consecutive years (2002 up to and including 2007).
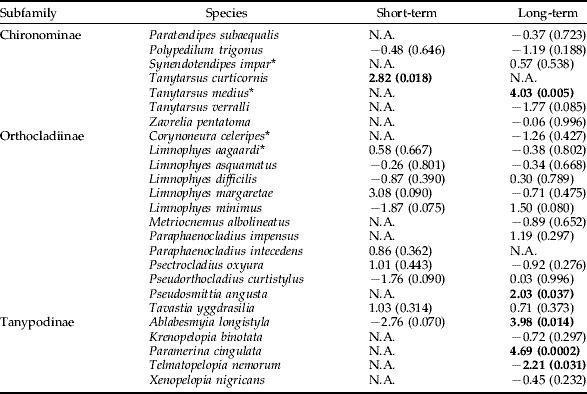
The figures are t-values, values within parentheses are level of significance, and NA (not applicable) denotes that either temporal or spatial abundance made data unsuitable for statistical analysis. Bold, significant change; –, decrease; all others are increases.
* species where most females are tentatively associated to the species in question.
Environmental factors contributing to the observed variation
The numbers of chironomids trapped in each trap over all six years were log transformed and the influence of the different environmental factors to the temporal and spatial variation in the numbers were analysed by variance component analysis. In the short-term view, water level was most important but explaining only 7.4% of the total variation. Year (0.82%), week (0.10%), locality (0.14%) and Bti treatment (0.59%) all explained very small amounts of the variation in the numbers. Less than 10% of the variation was explained by the factors we studied. In the long-term view, water level (0.71%), year (1.20%), week (1.20%), locality (0.66%) and Bti treatment (0.20%) together explained only about 4% of the variation.
From figs 3 and 4, it is obvious that the total variation was very large. The remaining variation, not explained by the environmental factors mentioned above, amounts to 90% and 96% for short-term and long-term effects, respectively. Thus, most of the variation is stochastic or depends on other factors. Observe the small contribution of the Bti treatments to the variation (see above).
Discussion
Monitoring the chironomid production for six consecutive years in wetlands with and without Bti-based mosquito control, revealed no signs of reduced production attributable to the Bti treatment at neither family, nor subfamily level. These results are in agreement with the results of most previous short-term studies of chironomid abundance in relation to Bti-based mosquito control (Boisvert & Boisvert, Reference Boisvert and Boisvert2000), but stands in sharp contrast to the results reported from a long-term study performed in Minnesota wetlands (Hershey et al., Reference Hershey, Lima, Niemi and Regal1998). The Minnesota study was performed over three years, and they reported increasing annual negative effects on the family Chironomidae, on the subfamilies Chironominae, Orthocladiinae and Tanypodinae, and also on many other taxa of non-target insects with approximately 60–80% reduction in numbers the third and last year (Hershey et al., Reference Hershey, Lima, Niemi and Regal1998). However, these results were not possible to repeat when the study (after criticism from other researchers) was continued for the 7th and 8th year (Read et al., Reference Read, Balcer, Schmude and Lima1999). The only negative effect the continued Minnesota study found on Chironomidae was a slight reduction in the abundance of tribe Chironomini within subfamily Chironominae. Positive effects of increases were observed in other chironomid subfamilies (Read et al., Reference Read, Balcer, Schmude and Lima1999). In addition, there was no reduced production observed in the other five dipteran families or other insect families investigated.
The unusual results of the first three years of the Minnesota study (Hershey et al., Reference Hershey, Lima, Niemi and Regal1998) may depend on the VectoBac G© dosing, since chironomids are susceptible to Bti, albeit at much higher concentrations than used for mosquito larval control. This could be exemplified by a recent attempt to control chironomids by application of Bti; a high dosage (7.2×1010 ITU ha−1) and specialized equipment for distribution near the bottom of the lake was needed to achieve a 35% reduction in abundance (Vaughan et al., Reference Vaughan, Newberry, Hall, Liggett and Ormerod2008). In our experiment, we used 3.0×109 ITU ha−1 and achieved near 100% reduction of flood-water mosquito larvae. Thus, a 24 times higher dosage was needed to achieve a 35% reduction in chironomid numbers, than to achieve a close to 100% reduction in flood-water mosquito abundance. In line with this, the review by Boisvert & Boisvert (Reference Boisvert and Boisvert2000) indicated that chironomids are 10 to 75 times less susceptible than culicids to Bti. Thus, the results of our study and of previous published studies show that Bti used for mosquito control induce no observable negative effects on the most sensitive of the non-target insect families, the Chironomidae, and no observable negative effects on the most sensitive of the non-target subfamilies, the Chironominae.
Further, we have identified all the chironomids from our study to species, making this the most detailed long-term study on these non-target insects ever performed. In the short-term view, we found no negative effects of Bti treatments at species level, but one species had significantly higher numbers in experimental wetlands than in reference wetlands. In the long-term view, four species showed significantly increased numbers, and one species had significantly decreased numbers, in experimental as compared to reference wetlands. The lack of negative effects by Bti-based mosquito control on chironomid species in the short-term view is consistent with results of previous studies (Boisvert & Boisvert, Reference Boisvert and Boisvert2000). The weakly significant decrease for one species (P=0.031) in the long-term view should be interpreted with caution because of the risk for false significances when performing multiple comparisons, and since the species belongs to subfamily Tanypodinae that is considered rather resistant to Bti (Boisvert & Boisvert, Reference Boisvert and Boisvert2000). Thus, our results from the long-term view analysis of chironomids at species level supports the general view of Bti being relatively benign to the environment when used in wetlands and at the dosage for mosquito control.
The chironomid production from these temporary flooded wet meadows and swamps of the River Dalälven flood plains was low compared with other wetlands, and particularly in comparison to permanent aquatic ecosystems. Annual chironomid biomass production (expressed as ash-free dry weight) in a Finnish wetland with similar climate, but with more aquatic conditions, was 5–30 times higher than in the River Dalälven wetlands (Paasivirta et al., Reference Paasivirta, Lahti and Perätie1988). Eutrophic lakes and reservoirs in England (Mundie, Reference Mundie1957), Wales (Potter & Learner, Reference Potter and Learner1974) and Scotland (Morgan & Waddell, Reference Morgan and Waddell1961) produced up to 50 times more biomass of chironomids annually. However, terrestrial or mainly terrestrial habitats, such as peatlands in Canada (Rosenberg et al., Reference Rosenberg, Wiens and Bilyj1988), agricultural soils in Germany (Weber, Reference Weber1992) and forest soils in France (Mollon, Reference Mollon1982), produce similar amounts of annual chironomid biomass as those measured in the River Dalälven wetlands. Since emergence traps affect the environment under the traps (Southwood & Henderson, Reference Southwood and Henderson2000), production estimates may deviate from real production. However, because this deviation is systematic, the comparisons between the wetlands under study are valid.
The chironomid production from the wet meadows and swamps of the River Dalälven flood plains was more similar to the reported chironomid production from mainly terrestrial conditions in peatlands, agricultural soils and forest soils, than to the reported chironomid production from eutrophic lakes and reservoirs. Aerial net catches in 2007 to estimate catch per unit effort (Southwood & Henderson, Reference Southwood and Henderson2000) revealed substantially higher catch of chironomids per unit time at the edge of the adjacent Lake Färnebofjärden (mean: 3.3 individuals min−1; range: 2.1–5.9) than in the wetlands (mean: 0.9 individuals min−1; range: 0.6–1.2). These additional data further emphasizes the major importance of stable aquatic environments for production of chironomids and the limited importance of the wetlands in this respect. Stable aquatic environments, such as lakes, produce higher abundance and biomass of chironomids than do either temporary wetlands or terrestrial environments (Mundie, Reference Mundie1957; Morgan & Waddell, Reference Morgan and Waddell1961; Potter & Learner, Reference Potter and Learner1974; Mollon, Reference Mollon1982; Rosenberg et al., Reference Rosenberg, Wiens and Bilyj1988; Weber, Reference Weber1992; Armitage et al., Reference Armitage, Cranston and Pinder1995). Therefore, the duration of the aquatic phase in temporary flooded wetlands may be important for the production of chironomids, and the chironomid production from such temporary wetlands might increase after floods of long duration. Our variance component analysis revealed that the number of weekly occasions with water under the traps explained 7.4% of the total variation in chironomid production in a short term view but only 0.71% of the variation in the long term view. The small effect of the aquatic phase might depend on a relatively short duration of the aquatic phase during floods in the River Dalälven flood plains. Alternatively, chironomid species that use the bottom sediments of lakes as their larval habitats may not find the temporary flooded environment sufficiently attractive as larval sites, leaving the temporary flooded environment to the chironomid species that could utilize terrestrial and semi-terrestrial environments as larval habitats. The variance component analysis revealed that the cause of the vast majority of the variation (90–96%) in production was a remaining residual variation. Thus, there is stochastic variation or other factors not recorded in this study that explains the variance in chironomid production. The combined effect of these unknown influences is much more important than the Bti treatment used in this study.
As we did not find any significant impact of Bti treatment on the overall chironomid production expressed as numbers or biomass, we expect impact neither on food availability for birds, bats nor on any other species which prey upon chironomids. This is an important issue as chironomids can be important food items for birds (Laursen, Reference Laursen1978; Cox et al., Reference Cox, Hanson, Roy, Euliss, Johnson and Butler1998; Buchanan et al., Reference Buchanan, Grant, Sanderson and Pearce-Higgins2006), bats (de Jong & Ahlén, Reference De Jong and Ahlén1991; Vaughan, Reference Vaughan1997; Encarnacao & Dietz, Reference Encarnacao and Dietz2006), frogs (Vignes, Reference Vignes1995) and other generalist predators. Our conclusion is that the chironomid production in the inundation wetlands of the River Dalälven offer a very limited food source to birds and bats, and that the mesotrophic Lake Färnebofjärden, adjacent to the studied wetlands, probably is a considerably better producer of chironomids as a food source.
Acknowledgements
We acknowledge grants from the Swedish Environmental Protection Agency to J.O.L., and the invaluable expertise and enthusiasm provided by Björn Forsberg (field sampling and identification to taxonomic order and sub-order), Axel Berglund (identification of Nematocera to family), Anna-Sara Liman (field sampling), Andreas Rudh (field-sampling and identification to taxonomic order and sub-order), Antti Vähäkari (field sampling), Anna Hagelin (field sampling and identification to taxonomic order and sub-order) and Fredrik Lundström (design and drawing of fig. 2).









