Introduction
Generalist and omnivorous predators can rapidly establish in agro-ecosystems by using alternative prey (Murdoch et al., Reference Murdoch, Chesson and Chesson1985; Wiedenmann & Smith, Reference Wiedenmann and Smith1997; Symondson et al., Reference Symondson, Sunderland and Greenstone2002) and plants (Coll, Reference Coll1996; Coll & Guershon, Reference Coll and Guershon2002; Eubanks et al., Reference Eubanks, Styrsky and Denno2003) as food resources. When predators forage and develop in habitats where several prey species co-exist, direct and indirect interactions between prey species can occur. One mechanism of indirect interaction is competition mediated by a shared natural enemy, termed apparent competition (Holt, Reference Holt1977). The presence of one prey species can have a negative effect on the population of another prey species by allowing the population of a shared predator to increase, leading to a higher level of predation on both prey (Wootton, Reference Wootton1994; van Veen et al., Reference van Veen, Morris and Godfray2006; Blaustein & Chase, Reference Blaustein and Chase2007).
For biological control, apparent competition can induce an increase in predator densities prior to the arrival of pests in crops and allow predators limiting pest population growth following colonization of the crop by pests (Settle et al., Reference Settle, Ariawan, Astuti, Cahyana, Hakim, Hindayana, Lestari, Pajarningsih and Sartanto1996). The availability of alternative prey can also influence predator behaviour, leading to short-term indirect interactions between prey species (Holt & Lawton, Reference Holt and Lawton1994). The presence of alternative prey may lower predation on a focal prey species because of predator preference toward alternative prey (Eubanks & Denno, Reference Eubanks and Denno2000; Prasad & Snyder, Reference Prasad and Snyder2006) and potentially lead to positive effect on focal prey via a short-term apparent commensalism (Wootton, Reference Wootton1994; Abrams & Matsuda, Reference Abrams and Matsuda1996). How alternative prey affect the impact of generalist predators may depend in part on the occurrence of these short-term interactions. Identification of such multi-trophic interactions is of primary importance to the success of pest management programs that utilize biological control (Cook et al., Reference Cook, Khan and Pickett2007; Zehnder et al., Reference Zehnder, Gurr, Kuhne, Wade, Wratten and Wyss2007) in lieu of pesticides (to prevent side effects on natural enemies, Desneux et al., Reference Desneux, Decourtye and Delpuech2007) to regulate pests.
In soybeans in North America, generalist predators dominate the natural enemy community, attacking the recently introduced pest, the soybean aphid Aphis glycines Matsumura (Hemiptera: Aphididae) (Ragsdale et al., Reference Ragsdale, Voegtlin and O'Neil2004; Desneux et al., Reference Desneux, O'Neil and Yoo2006; Costamagna et al., Reference Costamagna, Landis and Brewer2008). In Midwest US soybean fields, one of the most common generalist predators is the anthocorid, Orius insidiosus Say (Hemiptera: Anthocoridae) (Rutledge et al., Reference Rutledge, O'Neil, Fox and Landis2004; Desneux et al., Reference Desneux, O'Neil and Yoo2006). Previous studies have reported that O. insidiosus limited soybean aphid population growth in microcosms (individual soybean plants encaged) (Rutledge & O'Neil, Reference Rutledge and O'Neil2005) and in the field (Desneux et al., Reference Desneux, O'Neil and Yoo2006). Orius insidiosus is typically present in soybean fields several weeks before the arrival of the soybean aphid (Rutledge et al., Reference Rutledge, O'Neil, Fox and Landis2004) feeding on plants (Lundgren et al., in press) and prey such as thrips (Isenhour & Yeargan, Reference Isenhour and Yeargan1981), spider mites, leafhoppers and other small arthropod prey (Rondon et al., Reference Rondon, Cantliffe and Price2004; Xu et al., Reference Xu, Borgemeister and Poehling2006). Indirect interactions are, therefore, likely to occur between the different prey species and may affect the dynamics of soybean aphid after its arrival into the field. Study of the life history characteristics of O. insidiosus-fed soybean aphid and the alternative prey, soybean thrips (Neohydatothrips variabilis [Beach] [Thysanoptera: Thripidae]), found that soybean thrips is higher quality food for that generalist predator (Butler & O'Neil, Reference Butler and O'Neil2007a). However, how alternative prey influence predation behaviour of O. insidiosus on soybean aphid is not known. In addition, O. insidiosus impact on A. glycines population growth depends of plant architecture complexity (Rutledge & O'Neil, Reference Rutledge and O'Neil2005), and it may also affect indirect interactions between A. glycines and alternative prey.
In a first experiment, we measured how different levels of the alternative prey, the thrips N. variabilis, could affect the efficiency of O. insidiosus to limit soybean aphid population growth in microcosm conditions. We also tested how plant complexity could affect O. insidiosus impact on aphids and indirect interactions between aphids and thrips by working with two different plant vegetative stages. In a second experiment, we characterized the foraging behaviour of O. insidiosus to (i) assess how the presence of thrips affects O. insidiosus predation behaviour on aphids, (ii) evaluate O. insidiosus prey preference in the two-prey system and (iii) measure how preference relates to the success of O. insidiosus in attacking the focal prey (soybean aphid) and alternative prey (thrips). Our aim was to document the short-term indirect effects of thrips on predation by O. insidiosus on soybean aphid and to help in understanding how generalist predators can be used as biocontrol agents in the presence of alternative prey.
Materials and methods
Plants
Soybean (var. Beck 366NRR, Beck Hybrids, Atlanta, IN) was grown under greenhouse conditions (L:D 16:8, 23±1°C) in individual plastic pots (diameter 26 cm) using potting soil mix (Bennett's Greenhouse, Lafayette, IN). Plants were watered daily and fertilized with a slow release fertilizer once they reached cotyledon stage (N:P:K 14:14:14, Osmocote, The Scotts, Marysville, OH). Depending on the experiment, plants were either at the V2 (two unifoliate leaves and two trifoliate leaves) or the V4 vegetative stage (two unifoliate leaves and four trifoliate leaves), (Herman, Reference Herman1988). These two stages are within the range of early soybean vegetative development during which interactions between O. insidiosus and prey (soybean aphids and thrips) are likely to have the greatest impact on soybean aphid establishment in the field (Yoo & O'Neil, unpublished data).
Insects
We established colonies of soybean aphid, A. glycines, and soybean thrips, N. variabilis, from individuals that were collected in 2004 from a soybean field at Purdue University Agronomy Center for Research and Education (ACRE) in Tippecanoe County, IN, USA. Aphids were reared on potted soybean plants in a greenhouse (L:D 16:8, 22±1°C), and new individuals were added twice a year to the laboratory colony. The soybean thrips were reared on soybean leaves in environmental chambers (L:D 16:8, 22±1°C, 65±5% RH) using a method modified from Callan (Reference Callan1947). Orius insidiosus was reared in environmental chambers (L:D 16:8, 22±1°C, 65±5% RH) following the method described by Rutledge & O'Neil (Reference Rutledge and O'Neil2005). They were fed Ephesitia spp. eggs (Pyralidae: Lepidoptera), (Beneficial Insectaries, CA), and provided soybean stems with the cut end wrapped in cotton to provide moisture and oviposition sites. Colonies were initiated using adults collected from the field, and new O. insidiosus were added twice a year to the laboratory colony. The O. insidiosus colony was initiated two years prior to the start of the experiment. All tests were conducted with adult female O. insidiosus that were 2–3 days old. They were isolated individually in glass vials with a piece of soybean stem 24 hours before the beginning of the experiments.
Aphid growth in microcosm conditions
Following the design of Rutledge & O'Neil (Reference Rutledge and O'Neil2005), microcosms were created by placing a clear acetate cylinder over a potted soybean plant. Cylinders had a mesh top and windows for ventilation, and measured 50 cm high×15 cm in diameter. Sand was placed on the soil surface to provide a substrate into which the cylinder could be easily pushed to ensure a complete seal. The experiments were carried out at a temperature of 23±1°C and a L:D 16:8 photoperiod.
We measured aphid population growth on plants with and without O. insidiosus and in the presence of different levels of thrips. To investigate the impact of plant size, we ran two experiments using V2- and V4-stage plants. V2 plants had an average leaf area of 263.5 cm2 (SE±6.1 cm2), whereas V4 plants had an average leaf area of 507.5 cm2 (SE±13.0 cm2). For each experiment, groups were established according to a 2×3 factorial design. The two-level treatment consisted of the presence or absence of O. insidiosus in the microcosm. The three-level treatment varied the number of thrips available on the plants. To establish a consistent density of prey across the V2 and V4 experiments, we increased the number of aphids and thrips used. For V2 plants, we used 16 aphids with 0, 5 or 15 thrips per plant. For V4 plants, we used 32 aphids with 0, 10 or 30 thrips. The resultant prey densities for both V2 and V4 plants were 0.06 soybean aphids per cm2 leaf area, and 0, 0.02 and 0.06 thrips per cm2 leaf area.
To start an experiment, adult apterous aphids and thrips nymphs were distributed equally among the leaflets of the plant. We used thrips nymphs to avoid complications due to reproduction and because of the predominance of this stage in field populations (Desneux, unpublished data). Prey were allowed to settle for two hours on the plant's surface before one predator was introduced per plant. Experiments were run for four days, which previous experiments had shown was sufficient time for aphid populations to grow significantly in the absence of predation (Rutledge & O'Neil, Reference Rutledge and O'Neil2005). After four days, all aphids per node of plant were counted (a node consisted of the two unifoliates or single trifoliate leaf). Aphids were counted per node to also assess how the distribution of the aphids on the plants could have had an effect on predation pressure by O. insidiosus. The number of replicates undertaken with V2- and V4-stage plants was 117 and 124, respectively (19–21 and 19–23 replicates per group, respectively).
Each V2 and V4 experiment was analysed separately. We tested the effect of O. insidiosus, thrips and node on the number of aphids after four days of growth. For this, we used a generalized linear model for repeated measure design based on a Poisson distribution, a log-link function and an exchangeable correlation matrix. Such generalized linear models allow for deficiencies in statistical independence among counts made on the same plant but at different nodes (Proc Genmod with the GEE option to analyse repeated measures: SAS Institute, 1999).
Behavioural study
We characterized the foraging behaviour of O. insidiosus on single leaflet patches on uncaged V4 soybean plants at 23±1°C using a 2×2 factorial design. The first two-level treatment consisted of the presence or absence of aphids (0 or 20 aphids). The second two-level treatment consisted of the presence or absence of thrips (0 or 10 thrips). This resulted in an aphid-biased patch when both prey were present at the same time as has been observed in the field (Harwood et al., Reference Harwood, Desneux, Yoo, Rowley, Greenstone, Obrycki and O'Neil2007). To create a patch with aphid colonies, four apterous adults were enclosed on the adaxial surface of the last central trifoliate leaf using a clip cage (d: 4.5 cm, h: 1.1 cm) for four days. The number of aphids was adjusted to 20 individuals (two adults and 18 nymphs) before the behavioural observations. Thrips were added to the adaxial surface of the patch (i.e. the last central trifoliate leaf) 5 min before the experiments. Individual O. insidiosus were then placed on each patch. Orius insidiosus were observed for 20 min, or until they left the patch for more than two minutes or flew away. On each day of observation, 3–4 O. insidiosus per treatment were observed in a randomised order (n=32–36). Each O. insidiosus was tested on a new plant to avoid possible effects of previous foraging activity.
From preliminary observations, O. insidiosus behaviours were characterized as: ‘walking’; ‘probing’, mouthparts extended and making contact with prey; ‘feeding’, mouthparts inserted into prey or the plant for at least ten seconds; ‘grooming’, using legs to clean antennae or other body parts; and ‘resting’, remaining motionless for at least two seconds. We recorded the time when the O. insidiosus walked on and off the patch. Attack events were defined as probing or feeding behaviours. A failed attack corresponded to feeding behaviour shorter than ten seconds or probing that was not directly followed by feeding behaviour. Observations were aided by the use of a ×10 lens magnifier (model 17110LG, Luxo ASA, Oslo, Sweden). Depending on the behaviour studied, the frequency (attack events) and/or duration (all other behavioural items) was recorded using the software, Etholog 2.25 (Ottoni, Reference Ottoni2000).
Effects of treatments (i.e. prey presence) on total patch residence time and the behavioural items recorded as durations were analysed using an ANOVA on log-transformed data (followed by Tukey's post-hoc test). To compare the time spent feeding on a given prey type in the presence or absence of a second prey (single-prey vs. two-prey patches), we used an ANOVA on log-transformed data. Finally, to assess impact of the presence of a second prey type in the patch on the predation of soybean aphid and thrips by O. insidiosus, ANOVA was used to compare the attack rate (attack per minute) between single-prey and two-prey patches.
We used Manly's formula (Manly et al., Reference Manly, Miller and Cook1972; Manly, Reference Manly1974; Weisser, Reference Weisser1994) to assess the preference of O. insidiosus for soybean aphids and soybean thrips when both prey were on a patch. In the Manly formula, preference is scored as a deviation in the number of individuals of a given prey type selected for a particular behaviour from the number of this prey type eligible for the action. For our study, we used ‘probing’ and ‘feeding’ as the selected behaviours and the number of prey per prey type on the patch as the number of available prey. As prey size can impact prey preference in generalist predators (Dixon, Reference Dixon1959; Evans, Reference Evans1976), we distinguished attacks occurring on aphid adults from those on aphid nymphs, as well as thrips. Manly's βj of the jth prey type for a particular action (with three prey types being considered) is estimated by
where Ai is the number of individuals of a given prey type i eligible for a particular action by the predator (![]() =N=total number eligible for this action) and xi is the number of a prey type i that have been selected for this particular action (with ri the number of a prey type i not selected and xi+ri=Ai). We considered the case in which a prey already selected for an interaction was still eligible for this action (Weisser, Reference Weisser1994). To account for the relative number of prey on the patch, expected values were 1/15 for aphid adults (two aphid adults on 30 total prey), 3/5 for aphid nymphs (18 on 30) and 1/3 on thrips (10 on 30). To test whether the estimated βj values were significantly different from expected values, we used a Student's t-test. We also compared Manly's Beta values for the aphid adults, aphid nymphs and thrips, using an ANOVA with Tukey's post-hoc-analysis.
=N=total number eligible for this action) and xi is the number of a prey type i that have been selected for this particular action (with ri the number of a prey type i not selected and xi+ri=Ai). We considered the case in which a prey already selected for an interaction was still eligible for this action (Weisser, Reference Weisser1994). To account for the relative number of prey on the patch, expected values were 1/15 for aphid adults (two aphid adults on 30 total prey), 3/5 for aphid nymphs (18 on 30) and 1/3 on thrips (10 on 30). To test whether the estimated βj values were significantly different from expected values, we used a Student's t-test. We also compared Manly's Beta values for the aphid adults, aphid nymphs and thrips, using an ANOVA with Tukey's post-hoc-analysis.
Finally, to assess potential differences in attack success on soybean aphid versus thrips, a Chi-square test was used to compare the proportion of failed attacks by O. insidiosus between aphids and thrips.
Results
Aphid growth in microcosm conditions
Overall, O. insidiosus significantly reduced aphid numbers in both V2- and V4-plant experiments (table 1, fig. 1a, b). In the absence of thrips, the mean number of aphids after four days' growth was significantly reduced by O. insidiosus on both V2 and V4 plants (fig. 1a, b). On V2 plants, the presence of the thrips significantly impacted aphid population growth (table 1; Thrips factor) and there was a significant interaction between O. insidiosus and thrips effect (Orius×thrips), showing that O. insidiosus's effect on aphid population was impacted by thrips presence. Specifically, O. insidiosus decreased aphid population growth only when five thrips per plant were present in the microcosm (fig. 1a), but not when 15 thrips were present (fig. 1a). On V4 plants, O. insidiosus had a significant effect on the number of aphids per plant, but both ‘thrips’ and interaction between ‘Orius’ and ‘thrips’ were not significant. In the presence of thrips (10 or 30 per plant), O. insidiosus was no longer able to significantly limit the number of aphids (fig. 1b).
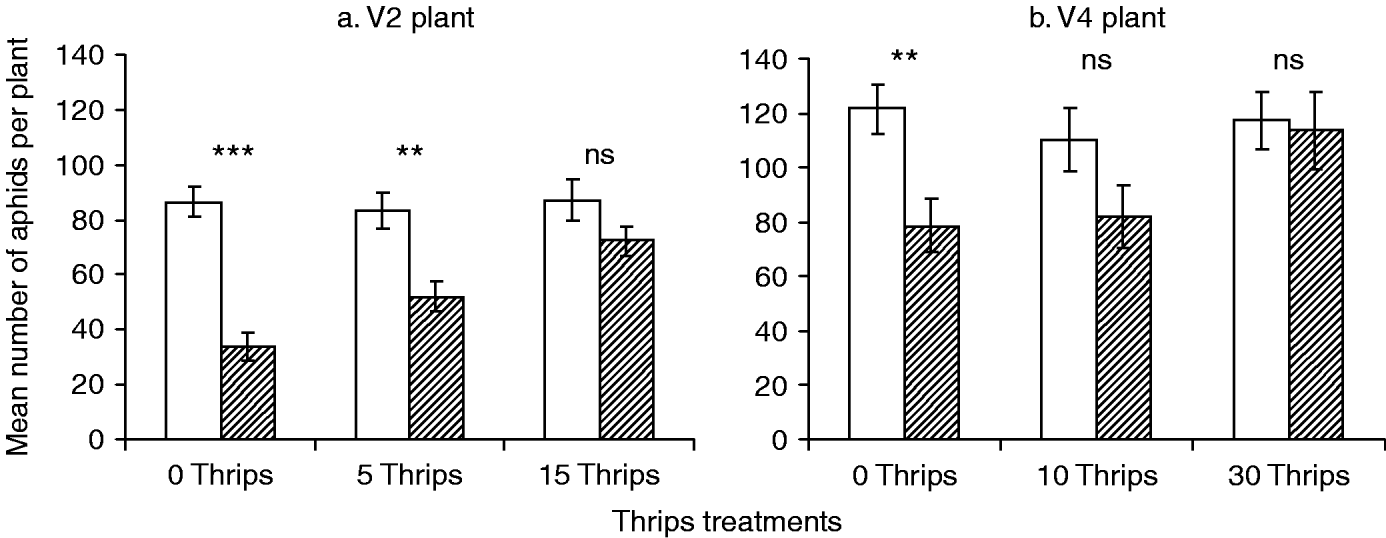
Fig. 1. Mean number (±SE) of aphids per plant after four days of growth in microcosm as function of thrips and O. insidiosus treatments. (a) Results for experiment with V2-stage plants (two unifoliate leaves and two trifoliate leaves) on which 0, 5 or 15 thrips and 16 aphids were placed. (b) Results for experiment with V4-stage plants (two unifoliate leaves and four trifoliate leaves) on which 0, 10 or 30 thrips and 32 aphids were placed (Student's t-test compared ‘with O. insidiosus’ to respective ‘without O. insidiosus’ groups. **, P<0.01; ***, P<0.001) (□, without Orius insidiosus; ![]() , with Orius insidiosus).
, with Orius insidiosus).
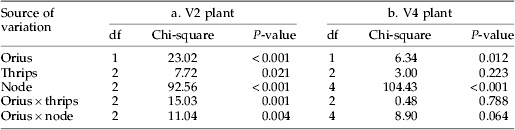
Table 1. Statistics from the generalized linear model used to analyse the number of aphids after four days of growth in microcosms as a function of plant nodes (Node), predator (Orius) and alternative prey (Thrips) presence. (a) Results for experiment with a V2 plant on which 0, 5 or 15 thrips and 16 aphids were used. (b) Results for experiment with a V4 plant on which 0, 10 or 30 thrips and 32 aphids were used.
Aphid numbers per node tended to increase from the bottom to the top of the plants (fig. 2, table 1; significant Node factor). The ‘node’ and ‘Orius’ factors interacted significantly for V2 plants (table 1). Orius insidiosus had less impact on aphids on the lower nodes of the plants probably because the predator foraged mostly on top parts of the plants as previously reported for O. insidiosus on various plant species (Atakan et al., Reference Atakan, Coll and Rosen1996). Interaction of ‘node’ and ‘Orius’ factors was not significant in the case of taller plants (V4).
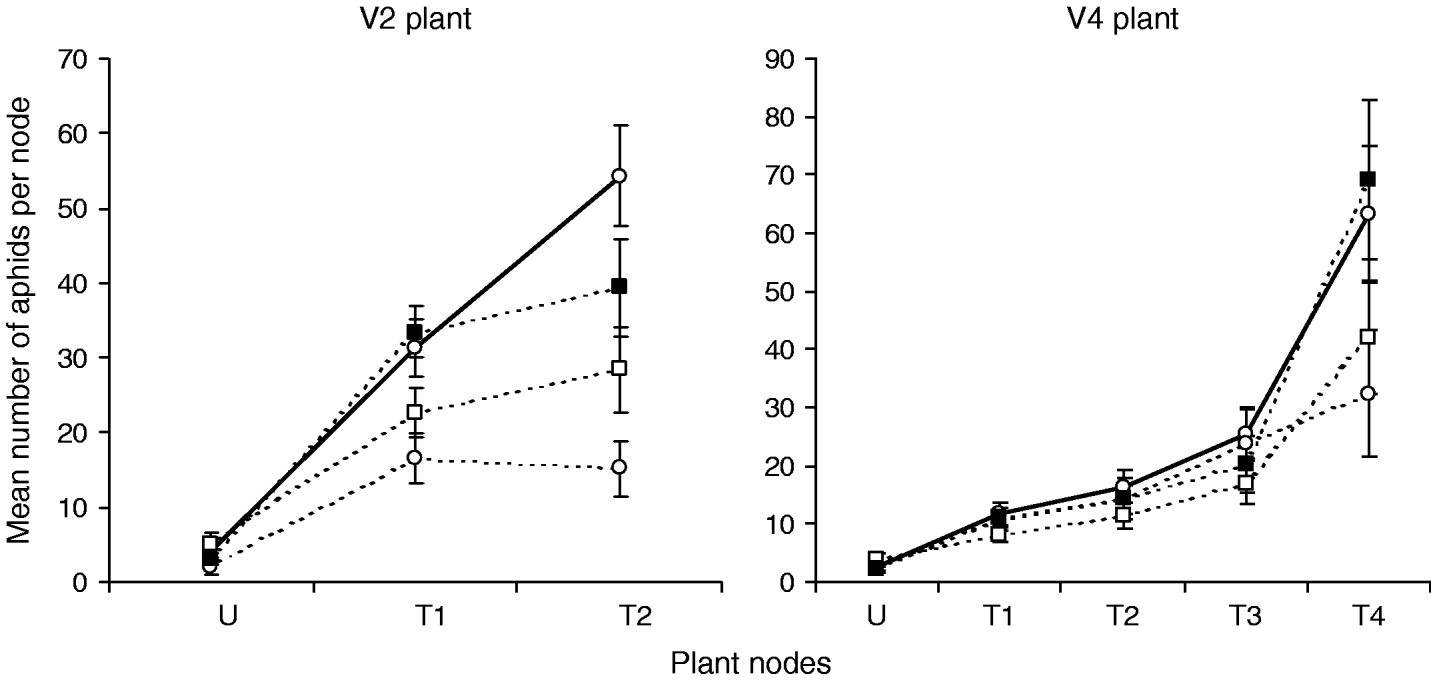
Fig. 2. Mean number of aphids (±SE) after four days of growth in microcosm as function of plant nodes (U, unifoliates=bottom of the plant; Tn, trifoliates n with the highest Tn corresponding to the top of the plant), thrips and O. insidiosus treatments on V2- and V4-stage plants. Control groups (without O. insidiosus) were pooled to simplify the figure because GLM analysis on control groups showed significant node effect but no significant effect of the thrips on aphid number (V2 plant: node: χ2=92.86, df=2, P<0.001; thrips: χ2=5.77, df=2, P=0.056 (—○—, control; - - -○- - -, Orius insidiosus – 0 thrips; - - -□- - -, Orius insidiosus – 5 thrips; - - -■- - -, Orius insidiosus – 15 thrips); V4 plant: node: χ2=103.94, df=4, P<0.001; thrips: χ2=2.72, df=2, P=0.257) (—○—, control; - - -○- - -, Orius insidiosus – 0 thrips; - - -□- - -, Orius insidiosus – 10 thrips; - - -■- - -, Orius insidiosus – 30 thrips).
Behavioural study
Residence time of the predator in patches with prey was more than double that when prey were absent (control group) (table 2). However, no significant difference in residence times was found between the various patches when prey were present. Compared to the control, time spent ‘walking on the patch’ and ‘grooming’ was significantly increased in treatments where soybean aphids were present but not when only thrips were present (table 2). In the control, the predator significantly ‘walked outside of the patch’ for a longer time than for other treatments. Finally, the time spent ‘feeding on plant’ and ‘resting’ was not influenced by prey presence.
Table 2. Mean time (±SE) spent for behaviours per O. insidiosus females on various prey patches (no prey, aphids alone, thrips alone or aphids and thrips together).
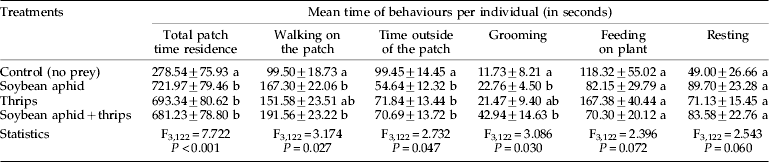
Values in same columns followed by the same letter are not significantly different (P>0.05, ANOVA on log-transformed data with Tukey's post-hoc analysis). Observations occurred over a 20 min time period.
There was a significant two-fold reduction in attack rate on aphids when thrips were present compared to when aphids were alone on the patch (fig. 3a; F1,69=6.114, P=0.016). In contrast, the presence of aphids did not reduce the attack rate on thrips (fig. 3b; F1,68=0.036, P=0.849). A similar significant reduction was observed in time spent ‘feeding’ on aphid when comparing aphid alone (360.06±77.73 s) and aphid in presence of thrips (114.86±44.65 s), (F1,69=5.418, P=0.023). In contrast, no effect on time spent ‘feeding’ on thrips by O. insidiosus was found when thrips were alone versus when they were in the two-prey patch (single-prey patch: 281.94±61.17 s; two-prey patch: 177.97±43.06 s; F1,68=0.963, P=0.330). The proportion of failed attacks to total number of attacks was significantly higher on aphid alone (45.27%) than on thrips alone (16.23%), (χ2=38.402, df=1, P<0.001).
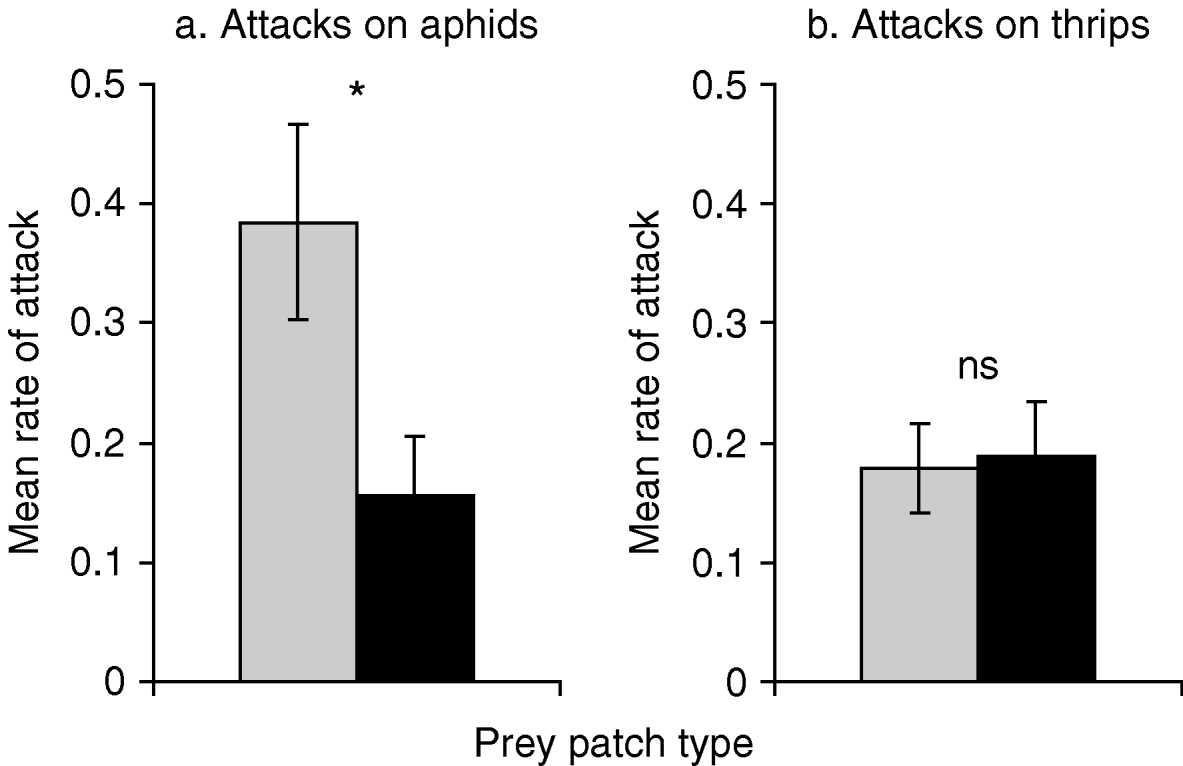
Fig. 3. Mean attack rate (±SE), (number of attack per minute) by Orius insidiosus on (a) aphids, and (b) thrips in single-prey and two-prey patches (n=32–36; ANOVA; *, P<0.05) (![]() , single-prey patch; ■, two-prey patch).
, single-prey patch; ■, two-prey patch).
Orius insidiosus ‘probed’ and ‘fed’ on thrips, aphid nymphs and adults within the patch to varying extent (fig. 4; probed as function of number of prey in the patch: F2,77=10.979, P<0.001; fed as function of number of prey in the patch: F2,65=6.533, P=0.003) and regardless of the proportion of the different prey present on patch in the case of aphid nymphs and thrips. Specifically, the thrips were preferentially attacked by the predator regardless of attack behaviour considered and also regardless of the density of the various prey available on the patch (fig 4; probed as function of number of thrips in patch: t=3.344, df=25, P=0.003; fed as function of number of thrips in patch: t=2.603, df=21, P=0.017). Attacks on aphid adults were not significantly different from expected βj values (probed as function of number of aphid adults in patch: t=1.127, df=25, P=0.270; fed as function of number of aphid adults in patch: t=1.278, df=21, P=0.215) but nymphs were significantly less attacked than expected (fig. 4; probed as function of number of aphid nymphs in patch: t=−4.828, df=25, P<0.001; fed as function of number of aphid nymphs in patch: t=−2.603, df=21, P=0.001).
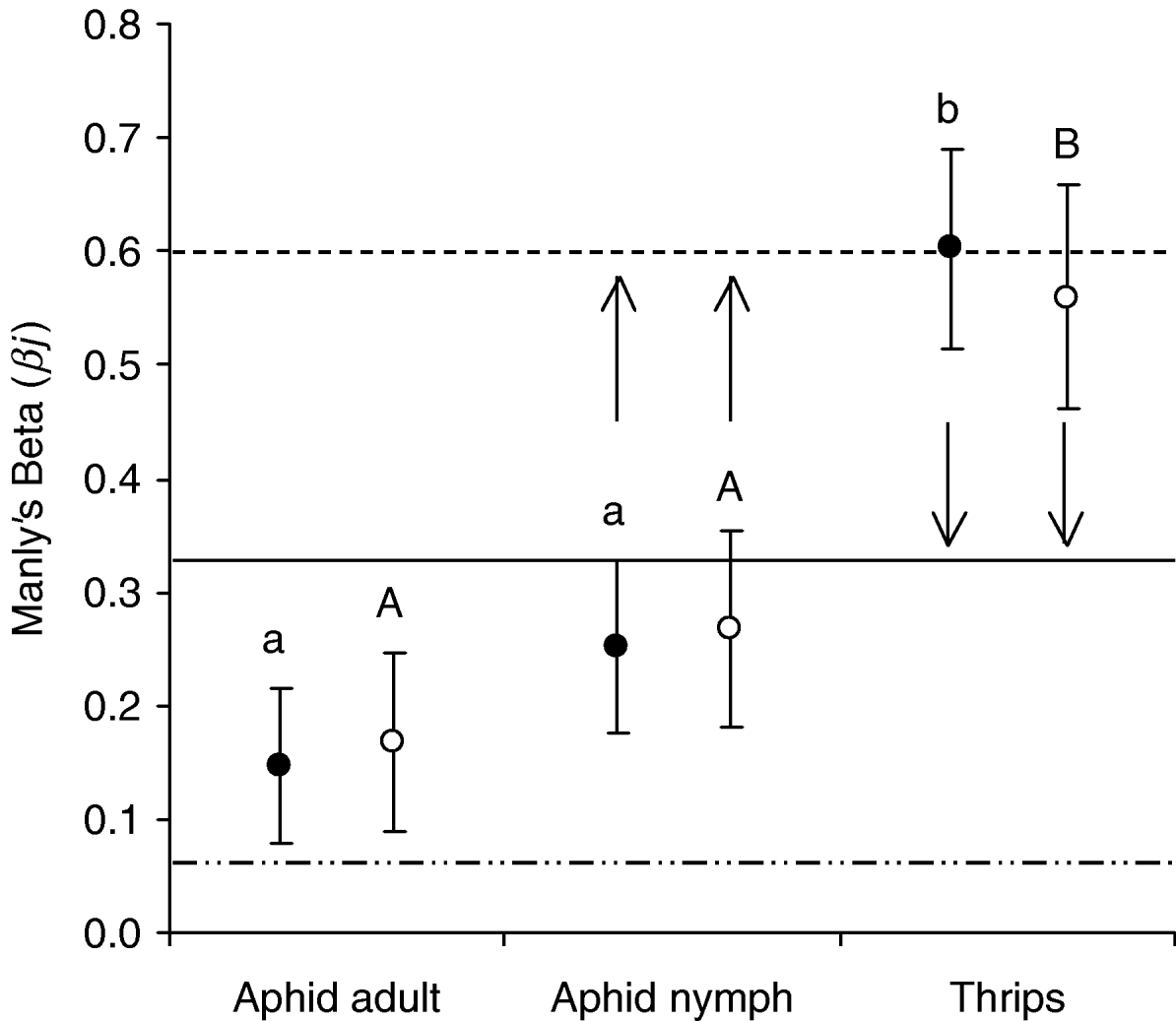
Fig. 4. Manly's Beta values (±SE) for O. insidiosus behaviour in two-prey patch (2 aphid adults+18 aphid nymphs+10 thrips). •, Probed as function of number of prey in the patch; ○, fed as function of number of prey in the patch. Lines represent the expected βj values against which calculated βj values for each prey are compared (Student's t-test, significance difference with expected βj values are indicated by arrows, at the 0.05 level). Different letters indicate significantly different βj values between the prey (lower case letters: probed as function of number of prey in the patch; upper case letters: fed as function of number of prey in the patch) (P>0.05, ANOVA with Tukey's post-hoc analysis) (- - - - -, expected βj for aphid nymph; – ·· – ·, expected βj for aphid adult; ——, expected βj for thrips).
Discussion
Our study confirmed the impact of O. insidiosus on the population growth of the soybean aphid (as reported by Desneux et al. (Reference Desneux, O'Neil and Yoo2006) and Rutledge & O'Neil (Reference Rutledge and O'Neil2005) ) and that its efficiency depends of plant size and initial number of aphids, with lower impact of O. insidiosus on taller plants (owing to higher complexity: Casas & Djemai, Reference Casas, Djemai, Tscharntke and Hawkins2002) and higher initial number of aphids (Rutledge & O'Neil, Reference Rutledge and O'Neil2005). In addition, we demonstrated that the presence of thrips could affect the efficiency of O. insidiosus to limit soybean aphid population growth. On small plants (V2-stage plant), the ‘Orius’ and ‘thrips’ factors interacted in microcosm conditions. Soybean aphids establish in field with O. insidiosus when plants are small and thrips are already present (Yoo & O'Neil, unpublished data). The effect of O. insidiosus on soybean aphids depends on the associated thrips density, with increasing thrips numbers significantly reducing the predator impact on aphid population growth. When plant size and initial number of aphids increased (V4-stage plant, 32 aphids), both ‘Orius’ and ‘thrips’ factors do not interact. The negative effect of thrips on the capacity of O. insidiosus to limit aphid population growth may occur mostly when high numbers of both prey are present. Indeed, both prey types tend to aggregate on the top of the plant (significant ‘node’ factor for aphids counts, see fig. 2: Atakan et al., Reference Atakan, Coll and Rosen1996; Desneux, unpublished data) on which the impact of O. insidiosus on aphids appears to be the strongest, whereas predator's effect is lower on other lower parts of plants. Therefore, on taller plants (like V4-stage plant), the effect of thrips is localized only on the top of the plant, whereas it tends to also occur in the middle of small plants (V2-stage plant). Orius insidiosus is less efficient in limiting soybean aphid population growth on taller plants (Rutledge & O'Neil, Reference Rutledge and O'Neil2005; present results), which diminishes any disruptive effects of thrips prey. Further research is needed to identify the relative roles of aphid number, density, growth rates and plant size and complexity on O. insidiosus predation on soybean aphid.
As a generalist predator, O. insidiosus can feed on alternative prey that are available at the same time as the target species (Isenhour & Yeargan, Reference Isenhour and Yeargan1981; Kiman & Yeargan, Reference Kiman and Yeargan1985; Harwood et al., Reference Harwood, Desneux, Yoo, Rowley, Greenstone, Obrycki and O'Neil2007). As such, a potential decrease of predation pressure on the targeted pest may occur at least in the short term (Holt & Lawton, Reference Holt and Lawton1994; van Veen et al., Reference van Veen, Morris and Godfray2006). Predation of soybean aphid by O. insidiosus in microcosm conditions was adversely affected by the presence of alternative prey and the negative impact increased as the density of alternative prey increased. During the four-day experiments in microcosms, O. insidiosus did not have enough time to reproduce; so, we demonstrate here that at least short-term indirect interactions can occur between the two prey species (aphids and thrips). Other studies have shown hemipteran preference for non-target prey in other multi-prey systems (Rosenheim et al., Reference Rosenheim, Wilhoit and Armer1993; Eubanks & Denno, Reference Eubanks and Denno2000). Our results highlight the limitations of using single prey species experiments to estimate the impact of a generalist insect predator on a focal prey population.
Results from the behavioural experiment support the hypothesis of a prey preference toward thrips. On the aphid-biased patch, the predator preferentially attacks the thrips; and switching behaviour did not occur toward the most available prey (aphids) (see Manly's Beta values; fig. 4). The O. insidiosus foraging pattern observed during the behavioural assay, likely, also occurred in the microcosms because both prey distributions overlapped within same microcosms (Desneux & O'Neil, unpublished data); and, therefore, predators foraged on patches containing both prey. For instance, the predator's preference for thrips may be due to a differential ability to detect thrips compared to aphids. During observations, we frequently saw predators walking close to aphids without attacking them. Predators always attacked the thrips when in the same proximity. Soybean thrips have been shown to exhibit escape behaviours in response to approaching O. insidiosus (Isenhour & Yeargan, Reference Isenhour and Yeargan1981), which appear to induce a tracking behaviour in the predator (Desneux, personal observation). In contrast, soybean aphids moved only after experiencing a predator's attack or in response to a nearby attack. Other studies have demonstrated that hemipteran predators preferentially attack more mobile prey when given a choice (Rosenheim et al., Reference Rosenheim, Wilhoit and Armer1993; Eubanks & Denno, Reference Eubanks and Denno2000; Venzon et al., Reference Venzon, Janssen and Sabelis2002). It is still unclear whether the hemipteran predators detect movement visually (Eubanks & Denno, Reference Eubanks and Denno2000) or through substrate-borne vibrations (Pfannenstiel et al., Reference Pfannenstiel, Hunt and Yeargan1995). However, O. insidiosus, like many hemipteran predators (Readio & Sweet, Reference Readio and Sweet1982), present acute vision capabilities (Henaut et al., Reference Henaut, Alauzet, Dargagnon and Lambin1999) and are primarily diurnally active in soybean fields (Pfannenstiel & Yeargan, Reference Pfannenstiel and Yeargan2002). It is likely, therefore, that O. insidiosus are visually-oriented predators that react to moving prey more rapidly than sessile or less mobile prey (like aphids) at short distances.
Alternatively, the predator's preference could be mediated through the relative ease in attacking thrips versus soybean aphids. In our observations, thrips did not exhibit obvious defensive behaviours except to escape attack by running away. In contrast, soybean aphid exhibits defensive mechanisms against O. insidiosus (Butler & O'Neil, Reference Butler and O'Neil2006) and parasitoids, including kicking behaviours and cornicle secretions (Wyckhuys et al., Reference Wyckhuys, Stone, Desneux, Hoelmer, Hopper and Heimpel2008). Impact of these defensive behaviours is reflected in the rate of failed attacks observed on aphids (45%) versus thrips (16%). A low rate of successful attacks on aphids by the hemipteran predator, Anthocoris nemorum (Linnaeus), a predator similar in size to O. insidiosus, has been reported by Evans (Reference Evans1976), who demonstrated that the success rate was positively correlated with the predator/prey size ratio. Moreover, in predator-prey systems, predator/prey size ratio has been shown to influence foraging success and subsequent prey preference (Lafferty & Kuris, Reference Lafferty and Kuris2002). Our results are in concordance with these findings, as soybean aphids are much larger than soybean thrips.
Although O. insidiosus failed in nearly one-half of its attacks, it has been shown that soybean aphids probed by this predator invariably die (Butler & O'Neil, Reference Butler and O'Neil2006), probably owing to a toxin known to be injected by hemipteran predators when attacking prey (Edwards, Reference Edwards1961; Cohen, Reference Cohen1990). Thus, although O. insidiosus may fail to consume attacked aphids, it readily kills encountered aphids, suggesting that preference mediated by the ease of capture may not have a significant impact on the population growth rate of soybean aphids per se. However, failed attacks may partially reduce the predator's impact on aphid populations at a trans-generational level because of the lack of a direct relationship between aphid death and food uptake by the O. insidiosus and consequent O. insidiosus population growth.
Our results are of interest in understanding how O. insidiosus establishes and sustains populations in soybean fields. The presence of thrips early in the soybean season may help O. insidiosus colonize fields and initiate reproduction by feeding on thrips as O. insidiosus population dynamics in soybean fields have been linked to thrips population levels (Isenhour & Marston, Reference Isenhour and Marston1981). Then, as soybean aphids colonize, they may in turn be attacked by O. insidious, the impact of which will be mediated by Orius numbers, the relative numbers of soybean aphid and alternative prey and the preference of O. insidiosus for prey in the system. Thus, a potential decrease in the predation rate on soybean aphid may be induced by short-term apparent commensalism (+, 0) between soybean aphid and thrips (Holt, Reference Holt1977), although apparent competition with indirect negative effect of thrips on soybean aphid may offset this at longer (trans-generational) time-scales. That last point may likely occur because, in laboratory conditions, a diet constituted of soybean thrips improves O. insidiosus fitness-related parameters compared to a diet constituted of aphids (Butler & O'Neil, Reference Butler and O'Neil2007b). Ultimately, whether attacks on soybean aphids is sufficient to prevent or suppress an aphid outbreak will depend on the relative growth rates of O. insidiosus and soybean aphid, which in turn depends upon how O. insidiosus uses alternative prey (mostly thrips) and soybean aphids to support its population growth (see Butler & O'Neil, Reference Butler and O'Neil2007a, Reference Butler and O'Neilb).
Acknowledgements
This paper is in remembrance of Bob O'Neil who passed away in February 2008. Regretfully, we lost a great specialist in biological control, and I lost a dear friend. We would like to thank George Heimpel, Ho Jung Yoo, Douglas Richmond, Marc Rhainds and Steve Yaninek for their comments on the manuscript, and Benjamin Isambert and Edwige Desneux for technical assistance. This work was supported by a grant from USDA/CSREES NRI (2003-03334), as well as through support of the Indiana Soybean Alliance and the North Central Soybean Research Program. This is Purdue's Agricultural Research Program manuscript number 2007-18164. All experiments described in this paper were done in the USA according to the rules of the ethical board for animal experiments complying with the current laws of this country.








