Introduction
Rice planthopper (RPH) populations of Nilaparvata lugens (Stål) and Sogatella furcifera (Horváth) have periodically erupted across Asia. In outbreak years, this can result in heavy rice loss and almost total crop failure in many paddies (Zhai, Reference Zhai2011; Bottrell & Schoenly, Reference Bottrell and Schoenly2012; Otuka, Reference Otuka2013; Ma et al., Reference Ma, Wu and Peng2015). In China, RPH (refers to both studied species hereafter) are currently the most important agricultural insect pest and are responsible for damaging an area of about 20,000,000 ha rice each year (Hu et al., Reference Hu, Cheng, Qi, Wang, Lu, Zhang and Zhai2011, Reference Hu, Lu, Zhai, Lu, Liu, Zhu, Wu, Chen and Zhang2014; Cheng, Reference Cheng, Heong, Cheng and Escalada2015). Predicting RPH population dynamics and identifying their source areas are crucial for the management of this migratory pest in China. The origins of migratory RPH populations, both in temperate and subtropical regions, remain unclear (Zhai, Reference Zhai2011; Zhai et al., Reference Zhai, Zhou, Tao, Chen and Shen2011; Otuka, Reference Otuka2013; Otuka et al., Reference Otuka, Sakamoto, Chien, Matsumura and Sanada-Morimura2014). This is also a common problem for other migratory insects, due to very low population densities in one or more generations in the seasonal cycle (e.g. after overwintering) (Flockhart et al., Reference Flockhart, Wassenaar, Martin, Hobson, Wunder and Norris2013; Stefanescu et al., Reference Stefanescu, Páramo, Åkesson, Alarcón, Ávila, Brereton, Carnicer, Cassar, Fox, Heliölä, Hill, Hirneisen, Kjellén, Kühn, Kuussaari, Leskinen, Liechti, Musche, Regan, Reynolds, Roy, Ryrholm, Schmaljohann, Settele, Thomas, Swaay and Chapman2013; Chapman et al., Reference Chapman, Reynolds and Wilson2015).
Nilaparvata lugens and S. furcifera (Hemiptera, Delphacidae) are insect pest species that cannot overwinter in temperate climates. In China, only a few individuals can survive during winter in areas of Southern Yunnan, Southern Guangxi and Hainan (Cheng et al., Reference Cheng, Chen, Xi, Yang, Zhu, Wu, Qian and Yang1979; Hu & Xie, Reference Hu and Xie1988; Otuka et al., Reference Otuka, Matsumura, Watanabe and Dinh2008, Otuka, Reference Otuka2013; Hu et al. (Reference Hu, Fu, Han and Ye2015a , Reference Hu, Liu, Fu, Huang, Wang, Liu, Lü and Ye b ); ). Instead, infestations in temperate zones are initiated by windborne spring/summer migrants from the southern areas (Cheng et al., Reference Cheng, Chen, Xi, Yang, Zhu, Wu, Qian and Yang1979; Otuka et al., Reference Otuka, Matsumura, Watanabe and Dinh2008, Otuka, Reference Otuka2013). A northward migration of N. lugens and S. furcifera begins in March every year, and migrant progeny further expand population distributions to cover the rice-growing regions in China, Japan and the Korean Peninsula (Cheng et al., Reference Cheng, Chen, Xi, Yang, Zhu, Wu, Qian and Yang1979; National Coordinated Research Group for White Backed Planthopper, 1981; Hu & Xie, Reference Hu and Xie1988; Kisimoto & Sogawa, Reference Kisimoto, Sogawa, Drake and Gatehouse1995; Hu et al., Reference Hu, Cheng, Qi, Wang, Lu, Zhang and Zhai2011). DNA sequence analysis of mitochondrial genes (cox1, cox2) of both RPH species did not show much genetic differentiation among Asia populations, and this indicated a wide extent of genetic mixing between RPH populations in East and Southeast Asia (Matsumoto et al., Reference Matsumoto, Matsumura, Sanada-Morimura, Hirai, Sato and Noda2013).
Based on the rice-planting schedule, seasonal wind direction and topographic features, the Mekong River Delta was hypothesized to be the first source of N. lugens populations, whose flight to the Central Indochina Peninsula arrives in the Red River Delta in February and March (Wu et al., Reference Wu, Yu and Tao1997). However, evidence from RPH biotype data and trajectory analysis showed that RPHs in the Mekong River Delta do not undertake northward migration into Central or Northern Indochina Peninsula (Li et al., Reference Li, Luo, Wei and Huang1999; Zhai et al., Reference Zhai, Zhou, Tao, Chen and Shen2011; Otuka et al., Reference Otuka, Sakamoto, Chien, Matsumura and Sanada-Morimura2014). An alternative, and long-standing hypothesis, was that Northern Vietnam is a source region for RPH population outbreaks in China and other temperate regions. This hypothesis was supported by field investigations carried out in the Red River Delta in May (Otuka et al., Reference Otuka, Matsumura, Watanabe and Dinh2008; Zhai, Reference Zhai2011; Zhai et al., Reference Zhai, Zhou, Tao, Chen and Shen2011). However, light-trap catches recorded RPH in South China as early as March (Hu et al., Reference Hu, Xie, Lin, Xin, Huang, Chen, Zhang and Zhai2010). The immigration of RPH in South China before May has been termed as ‘early migration’, but the source of these immigrants has not yet been identified. Several field investigations have been carried out during March and April by Cheng in 1988 and Zhai in 2008–2013 (Zhai, Reference Zhai2011; Zhai et al., Reference Zhai, Zhou, Tao, Chen and Shen2011). These studies concluded that Northern Vietnam could not be the initial source area of early immigrants of RPH to China because during these two months the growth stage of rice plants in paddies in Northern Vietnam and South China were similar. Recently, backward trajectory analyses indicated that possible sources of the early migrations of N. lugens and S. furcifera in March and April were from Hainan Province, Northern and Central Vietnam and Southern Laos (Qi et al., Reference Qi, Lu, Hu, Wang, Cheng, Shen, Huang, Zhang and Zhai2010, Reference Qi, Zhang, Jiang, Sun, Yang and Cheng2011; Shen et al., Reference Shen, Chen, Hu, Cheng, Zhang and Zhai2011; Wang et al., Reference Wang, Qi, Lv, Hu and Yuan2011; Jiang et al., Reference Jiang, Wu, Qi, Zhang and Cheng2012). Hitherto, there was a lack of research data available from Central Vietnam and Laos to explore these questions. Furthermore, there is little knowledge about RPH population dynamics and migrations in these overwintering areas. Thus, the first source area of the migrants to subtropical regions, such as China and Japan, remains unclear.
To investigate linkages between RPH population dynamics in China and Vietnam, a joint monitoring project was established in 2010 between the two countries (China–Vietnam Joint Project of Monitoring and Controlling Rice Migratory Pest). As part of this research framework, the present study aims to investigate early migration patterns of these pests from their purported overwintering ground. Early migration of RPH and population dynamics were investigated and analyzed in Vietnam and South China in 2012 and 2013 (March–April). Historical climate data recorded between 1977 and 2013 from Hepu (Guangxi Province, China) were analyzed to try to identify important factors potentially associated with RPH outbreaks.
Materials and methods
Population dynamics of RPH in Vietnam and South China
Systematic field investigations and light-trap monitoring were carried out between 2011 and 2013 at three sites in Vietnam (Thang Binh, Nam Dan and Hai Hau) and three in China (Hepu, Longzhou and Leizhou; see fig. 1). Field investigations were performed by the Department of Plant Protection, Vietnam and the China National Agro-Tec Extension and Service Center, China. At each experimental site, one or two paddies were selected that were moderately fertile and at least 600 m2 in area. Routine agricultural practices were carried out at the chosen sites, and ideally, no pesticides were applied during the rice-growing season. Field investigations of RPH populations were performed once every 5 days using the plant-shaking method. This entailed inserting a plate (39 × 29.5 × 2 cm) at the base of the rice plants, shaking the plant and collecting any RPH samples that fell from the plant (Hu et al., Reference Hu, Cheng, Qi, Wang, Lu, Zhang and Zhai2011). Individuals were counted and the number of N. lugens and S. furcifera per square meter was estimated.

Fig. 1. Location of experimental sites and list of regions of Vietnam (a). Starting points (+) of forward trajectories from Central Vietnam are presented in the inset (b).
At each experimental site, a black light-trap with 20 W fluorescent lamps (Jiaduo Science, Industry and Trade Co. Ltd., China) was used to monitor the population dynamics of RPH from 1 January to 15 November (2011–2013). In China, the black light traps were switched on at 19:00 h and off at 07:00 h the next morning (Bejing Time). In Vietnam, black light traps were switched on at 18:00 h and off at 06:00 h the next morning (Ho Chi Minh Time). Light-trap catches were collected every morning at 09:00 h and insects were identified using a stereomicroscope. To identify migration patterns, an abrupt (more than tenfold) increase in the daily light-trap catches was defined as the beginning of a peak period. Similarly, we determined that the peak period had ended when there was an abrupt decrease in catch (<1/10). All the days during a peak period were regarded as peak days.
In this study, early spring migration was the focus of our investigation, therefore only catch data from March and April were analyzed. The population dynamics of RPH could not be examined in 2011, because so few individuals were observed that year; thus, only data from 2012 and 2013 were considered. Population dynamics of N. lugens in Thang Binh and Dan Dam were similar with that of S. furcifera, but numbers of N. lugens in Hai Hau and Hepu were much smaller than that of S. furcifera. Trajectory analysis and regression models are based on the sum of both species.
Historical light-trap data from Hepu
Daily light-trap data were collected at Hepu between 1979 and 2013 by the China National Agro-Tec Extension and Service Center. Prior to 2005, traditional black light traps (20 W black light lamp) were used to catch RPH. From 2005 onwards, a new Jiaduo black light-trap with a fluorescent lamp was used instead. To our knowledge, no comparative studies for the efficacy of the two types of light traps for catching planthoppers have been carried out. Thus, the type of light trap was also included as a variable in the regression model to predict migration volume at Hepu with all data (1979–2013). Only data between 2005 and 2009 were selected to calculate migration trajectories.
Meteorological data and modeling
We implemented the Weather Research and Forecasting (WRF) Model in this study (Skamarock et al., Reference Skamarock, Klemp, Dudhia, Gill, Barker, Wang and Powers2005). The WRF is a mesoscale numerical weather prediction system designed to serve both atmospheric research and operational forecasting needs. We elected not to use the PSU/NCAR mesoscale model (MM5), because it was not supported since 2006 and the WRF now has all the capabilities that the MM5 system had. The WRF model provided hourly meteorological background data necessary for the trajectory analysis. The dimensions of the model domain were 99 × 84 grid points at a horizontal resolution of 30 km. Thirty-three vertical layers were available and the model ceiling was 100 hPa. The scheme selection and parameters used for the WRF are listed in table S1. National Centers for Environmental Prediction (NCEP) Final Analysis (FNL) Data were used as the meteorological data for the model input. FNL is a 6-hourly, global, 1-° grid meteorological dataset. The model forecast time is 72 h with data outputs at 1 h intervals, for horizontal and vertical wind speeds, temperature and precipitation.
Trajectory analysis
Likely population sources and landing areas of migrating RPH were estimated by constructing both backward and forward flight trajectories. The trajectory analysis had the following assumptions: (i) RPH are displaced downwind (Deng, Reference Deng1981; Riley et al., Reference Riley, Cheng, Zhang, Reynolds, Xu, Smith, Cheng, Bao and Zhai1991, Reference Riley, Reynolds, Smith, Rosenberg, Cheng, Zhang, Xu, Cheng, Bao, Zhai and Wang1994; Furuno et al., Reference Furuno, Chino, Otuka, Watanabe, Matsumura and Suzuki2005; Hu et al., Reference Hu, Lu, Lu, Liu, Xu, Jiang and Zhai2013); (ii) take-off occurs mostly at dusk and partly at dawn (Chen & Cheng, Reference Chen and Cheng1980; Riley et al., Reference Riley, Cheng, Zhang, Reynolds, Xu, Smith, Cheng, Bao and Zhai1991, Reference Riley, Reynolds, Smith, Rosenberg, Cheng, Zhang, Xu, Cheng, Bao, Zhai and Wang1994; Furuno et al., Reference Furuno, Chino, Otuka, Watanabe, Matsumura and Suzuki2005; Hu et al., Reference Hu, Lu, Lu, Liu, Xu, Jiang and Zhai2013); (iii) migrants are likely to land at any time; and (iv) RPH cannot fly in an atmospheric temperature below 16.5°C (Riley et al., Reference Riley, Reynolds, Smith, Rosenberg, Cheng, Zhang, Xu, Cheng, Bao, Zhai and Wang1994; Furuno et al., Reference Furuno, Chino, Otuka, Watanabe, Matsumura and Suzuki2005; Otuka et al., Reference Otuka, Watanabe, Suzuki, Matsumura, Furuno and Chino2005; Hu et al., Reference Hu, Lu, Lu, Liu, Xu, Jiang and Zhai2013). During the peak days of light-trap catches, backward trajectories from light-trap locations were calculated hourly from 07:00 to 06:00 h the previous day, using seven different initial heights above mean sea level: 500, 750, 1000, 1250, 1500, 1750 and 2000 m. A total of 168 trajectories (24 h × 7 heights) were calculated for each location in 1 day. Backward trajectories were terminated at the take-off time of RPH (about 18:00 h) or when the air temperature fell below 16.5°C. Dawn take-off was not considered because the number of RPH flying at this time is much lower compared with at dusk (Chen & Cheng, Reference Chen and Cheng1980; Riley et al., Reference Riley, Cheng, Zhang, Reynolds, Xu, Smith, Cheng, Bao and Zhai1991). Trajectories were calculated for a flight duration of up to 36 h, but the total time was condition dependent (Cheng et al., Reference Cheng, Chen, Xi, Yang, Zhu, Wu, Qian and Yang1979; Hu et al., Reference Hu, Lu, Lu, Liu, Xu, Jiang and Zhai2013). Flight duration was considered to be: (i) below 13 h if migrants took off at dusk of the same night; (ii) 13–24 h if migrants took off at the previous dusk and landed during daytime; and (iii) above 24 h if migrants took off at the previous dusk and landed at night. A trajectory endpoint was considered a likely source if it was located in a place that satisfied the following two conditions. First, it had fine weather at dusk with an air temperature at 2 m above ground >17°C, an hourly precipitation <0.1 mm and a wind speed at 10 m above ground <4 m s−1, based on the WRF data (Chen & Cheng, Reference Chen and Cheng1980; Riley et al., Reference Riley, Cheng, Zhang, Reynolds, Xu, Smith, Cheng, Bao and Zhai1991). Second, if it was located in a rice planting area where the crops were at a late growth stage (Cheng et al., Reference Cheng, Chen, Xi, Yang, Zhu, Wu, Qian and Yang1979).
The calculation setup for the forward trajectory was the same as that for the backward trajectory except that the initial time for the forward trajectory was at dusk (about 19:00 h). The starting points of forward trajectories from Central Vietnam formed a grid at equal distance of 0.5° (see fig. 1b). During emigration periods, forward trajectories were calculated on each day except some rainy and/or windy days. Based on rice growth stage and RPH population dynamics at Thang Binh and Nan Dam, the emigration periods were from March to April in Southern Central Vietnam and from late March to late April in Northern Central Vietnam (see the Results section, fig. 5). Endpoints with a flight duration <4 h were regarded as short-distance dispersal and were ignored. The number of forward trajectories that arrived in Nam Dan, Hai Hau and Hepu were estimated by counting trajectories that passed through a circle with its center at these sites and within a radius of 100 km.
Backward and forward trajectories were calculated based on the method introduced by Zhu & Liao (Reference Zhu and Liao1992) with wind field and temperature data from the WRF output (for more details, see Hu et al., Reference Hu, Lu, Lu, Liu, Xu, Jiang and Zhai2013; Lu et al., Reference Lu, Zhai and Hu2013). The program for calculating trajectories was written in FORTRAN and run using Fedora 13, the Linux-based operating system (Fedora Project, http://fedoraproject.org/).
Regression models with meteorological data
Based on the results of the trajectory analyses, Central Vietnam was identified to be a key source area for the RPH immigrating to South China in March and April (see the Results section). We explored the relationship between monthly cumulative light-trap catches at Hepu in April and weather factors in Central Vietnam, including Southern Central Vietnam (SCV) and North Central Vietnam (NCV). Our aim was to determine which factors had the most effect on immigration rates. Temperature is thought to be the most important factor for determining RPH population dynamics (Chen & Wang, Reference Chen and Wang1996; Hu et al., Reference Hu, Xie, Lin, Xin, Huang, Chen, Zhang and Zhai2010, Reference Hu, Cheng, Qi, Wang, Lu, Zhang and Zhai2011). A previous study recorded that the winter migration rates increased as temperature increased at Longzhou, Guangxi province in Central Indochina Peninsula (Hu et al., Reference Hu, Xie, Lin, Xin, Huang, Chen, Zhang and Zhai2010). Average monthly temperatures recorded from January to April in Central Vietnam were used in the analysis as potential variables. Previous studies also determined that the southerly wind was another very important factor for determining long distance windborne northward migration (Syobu et al., Reference Syobu, Otuka and Matsumura2012; Hu et al., Reference Hu, Lu, Lu, Liu, Xu, Jiang and Zhai2013). Thus, the number of days of southerly wind with speed of >4 m s−1 were summed for April and used as a variable in the analysis. The Indochina Peninsula is located in a tropical zone and the dry season extends roughly from November to April. In the dry season, precipitation is a major limiting factor on rice planting (Chang et al., Reference Chang, Wang, McBride and Liu2005; Phan et al., Reference Phan, Ngo-Duc and Ho2009; Zhai, Reference Zhai2011), and thus it might also have influence on the growth of RPH population. Wu et al. (Reference Wu, Yu and Tao1997) attempted to predict the occurrence of RPH in China based on the precipitation in the Mekong River Delta. Monthly precipitation recorded from January to April in Central Vietnam was also included as potential variables for the analysis. The type of light trap was also included as a variable in the regression model to rule out any difference between the two different light traps used in this study. This last variable was included because comparative studies for the efficacy of the two types of light traps for catching planthoppers were unavailable.
The monthly Climate Prediction Center (CPC) Merged Analysis of Precipitation (CMAP) data are available from 1979. The monthly temperature data and monthly days of southerly wind were derived from the NCEP/National Center for Atmospheric Research (NCAR) Reanalysis data from 1948 to 2011. The CMAP and NCEP/NCAR data, with a spatial resolution of 2.5° (i.e. 144 points in longitude and 72 points in latitude) are presented in the NETCDF format, which is self-explanatory and machine-independent.
Data exploration were carried out following the protocol described by Zuur et al. (Reference Zuur, Ieno and Elphick2010). The data were examined for the presence of outliers, auto-correlation in the response variable, collinearity and the type of any relationships identified. The variance inflation factor (VIF) was used to measure the multicollinearity, and a potential variable was excluded if its VIF value was >5 (Rogerson, Reference Rogerson2001; Zuur et al., Reference Zuur, Ieno and Elphick2010). As the initial analysis indicated that the residuals and the response variables were not auto-correlated, a Poisson General Linear Model (GLM) was therefore applied. Initial Poisson GLMs indicated overdispersion, thus we decided to apply a quasi-Poisson GLM. Due to the small sample size, a forward selection was applied using the quasi-AIC (QAIC) method (Burnham & Anderson, Reference Burnham and Anderson2002).
Results
Population dynamics of RPH in Vietnam in 2012 and 2013
Thang Binh
There were many peak days of light-trap catches of RPH at Thang Binh in March and April of 2012 and 2013. These peak days can be divided into five periods each year, namely in early March, middle March and early April, late March, middle April and late April. The interval between each periods was about 15 days (fig. 2). N. lugens and S. furcifera showed similar trends in their population dynamics. In paddies, rice was at booting stage in early March, and began to mature and harvested in late March (fig. 3). Late instar nymph (instar IV–V) and adults consistently accounted for a considerable proportion of the RPH population catches in the paddies during this time (fig. 3). Given the age structures of RPH and the maturity of the rice, this may indicate that many adults emerged from late instar nymphs and catches from light traps might be potential emigrants to other areas.

Fig. 2. Number of Nilaparvata lugens and Sogatella furcifera caught by light trap at experimental sites in 2012 and 2013. The Y-axes are on log10 scale. Peak days of catches are indicated by dot symbols (for details see the Methods section). Some data at Longzhou were not collected due to technical problems with the light trap (discontinuous line).

Fig. 3. Numbers of Nilaparvata lugens and Sogatella furcifera in paddies counted at experimental sites in Vietnam in 2012 and 2013. The letters on the top of each bar indicate the growth stage of rice: S-Seedling, T-Tillering, B-Booting, F-Flowering, R-Ripening and H-Harvest. In South China, only data from Hepu are shown. Population abundance of rice plant hoppers at Longzhou and Leizhou showed similar patterns to Hepu, but actual numbers of individuals were much fewer.
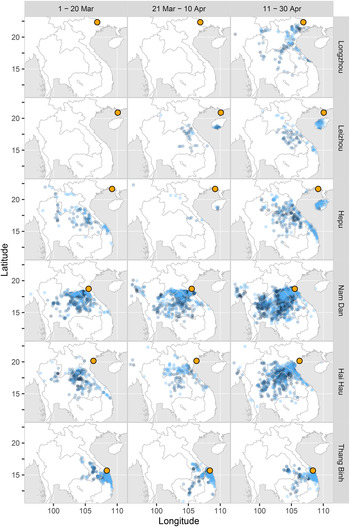
Results of the backward trajectories from Thang Binh also suggested that the macropterous catches from the light traps were from the surrounding area. A total of 56.04% (506/903) endpoints of the backward trajectories were located in the south part of Central Vietnam within a flight duration of 10.06 ± 0.59 h (95% confidence interval (CI)) (fig. 4). No backward trajectory could be traced back to Southern Vietnam, including the Mekong River Delta or Southeast Vietnam (fig. 4), but 59 trajectories (6.53%) on 13 peak days (32.5%, 13/40) had endpoints in Cambodia within 24.59 ± 2.44 h (95% CI). These results also suggest there was no direct exchange between RPH populations in Central and Southern Vietnam, but RPH in the Cambodian source area may have been the progeny of migrants from Southern Vietnam.

Fig. 4. Distribution of endpoints of backward trajectories from experimental sites in 2012 and 2013 in Vietnam and South China (○).
Weather during peak days of light-trap catches in Thang Binh were sunny and calm in 2012 and 2013, providing suitable conditions for RPH to take-off. Between March and April (2005–2013), average wind speeds on the 850 hPa level (at altitude of about 1400 m) were <8 m s−1 during 526 days (95.81%) and <4 m/s during 372 days (67.76%), wind conditions that were not conducive for long-distance flight migrations. Nevertheless, nine years (2005–2013) of forward trajectory analyses suggested that RPH from Southern Central Vietnam can arrive in Northern Central Vietnam, the Red River Delta and South China. Many forward trajectories from South Central Vietnam arrived in China at Hepu, Hai Hau and Nam Dan in about 24 h (table S2).
Nam Dan
At Nam Dan, the curve of daily catches of RPH flattens out gradually and there were no distinctive peaks in March and April 2012, especially in S. furcifera populations. The number of S. furcifera changed gradually from several to 500 individuals (fig. 2). In paddies, rice was at tillering stage in early March and began booting in middle March and was matured by middle April (fig. 3). Brachypterous adults were predominant in the paddies population (fig. 3) thereby indicating that the rice habitat was suitable for the population growth of both planthoppers. However, as brachypterous adults cannot really fly they will settle locally. On the other hand, the light-trap catches also were increasing as the RPH population in paddies grew over time (figs 2 and 3). In 2012, the population density of both species of planthoppers remained low in March, but grew quickly in April (fig. 3). Correspondingly, light-trap catches increased with the course of time until they reached a maximum in the middle of April, 2012 (fig. 2). In 2013, the population density in paddies showed two growth phases during these two months and two peaks occurred in late March and late April (fig. 3). Results from the light-trap catches based on increasing catches, could be divided into two stages, before and after early April (fig. 2). Parallel trends between population dynamics inferred from light traps and those in paddies suggested that the light-trap catches might come from local populations. Nevertheless, it cannot be ruled out that these catches may have comprised of both local residents and immigrants from distant sources. However, based on the host plant status, most catches in early and middle March were probably immigrants, because as the rice was so young it would have been difficult for RPH to complete its nymph stage locally.
Backward trajectories suggested that the probable source of light-trap catches were located in Central Laos and Northeastern Thailand (fig. 4). Among the 58 peak catch days, 1038 trajectories (36.73%) on 41 days (70.69%) could be traced to Central Laos, and 1557 trajectories (55.10%) on 41 days (70.69%) to Northeastern Thailand, and the flight durations for each were 9.54 ± 0.40 and 16.51 ± 0.42 h (95% CI), respectively. Only 11 trajectories (0.39%) on 6 days (10.34%) had endpoints in the south part of Central Vietnam with flight durations of 24.09 ± 4.48 h. No trajectories had endpoints in the Mekong River Delta and Southeast Vietnam, and only 27 trajectories (0.96%) on 8 days (19.51%) had endpoints in Cambodia (fig. 4).
The weather during peak days of light-trap catches at Nam Dan were mostly sunny and calm in 2012 and 2013, suitable conditions for RPH to take-off. Forward trajectories from North and South Central Vietnam suggested that most migrants arrive in Central Laos, the Red River Delta and South China (fig. 5, table S2). A number of endpoints were located at Hepu, Hai Hau and Nam Dan with flight duration <18 h (Table S2).

Fig. 5. Distribution of endpoints of forward trajectories from Northern and Southern Central Vietnam in 2005–2013.
Hai Hau
At Hai Hau, rice was at the seedling stage in March and at the tillering stage in April. The population density of RPH was very low before late April of 2012 and 2013, and mainly comprised early instar nymphs (fig. 3). Thus, it can be assumed that the large number of adults caught by light traps, especially in mid to late April, were probably immigrants (fig. 2). Backward trajectories suggested that these adults might come from Central Laos, Northeastern Thailand and the north part of Central Vietnam (fig. 4). Analysis for previous subareas suggested that macropterous adults emigrated from Central Vietnam (see the Results sections – 3.1.1, 3.1.2). Among the 37 peak days of light-trap catches, 177 backward trajectories (17.05%) on 18 peak days (48.65%,) arrived in Northern Central Vietnam and the flight durations were 9.03 ± 0.88 h (95% CI; fig. 4). Only 15 trajectories (1.45%) on 6 peak days (16.22%) arrived in Southern Central Vietnam, and the flight durations were 28.00 ± 1.59 h (95% CI; fig. 4). Results seem to suggest that in these cases, RPH could have come from Northern Central Vietnam. The forward trajectories also confirmed that RPH arrived in Northern Central Vietnam in the Red River Delta (fig. 5, table S2). However, a greater number of backward trajectories arrived in Laos and Northeastern Thailand (fig. 4). In all, a total of 37.86% (393/1038) of trajectories on 24 days (64.86%) arrived in Laos within 13.00 ± 1.32 h (95% CI), while 40.17% (417/1038) of trajectories on 23 days (62.16%) arrived in Northeastern Thailand within 21.32 ± 0.71 h (95%) of flight duration (fig. 4).
Population dynamics of RPH in South China
In South China at Longzhou, Hepu and Leizhou, rice was seeded in the middle of March and transplanted in early April. In 2012 and 2013, early instar nymphs of both RPH species were predominant in paddies in April and this suggested that the adults caught by light trap were immigrants to South China (fig. 3). Backward trajectories suggested that these adults might come from Central Vietnam, Central Laos, Hainan Island or Northeastern Thailand (fig. 4). Among the 30 peak days of light-trap catches at Hepu in March and April of 2012 and 2013, 13.51% (67/496) backward trajectories on 16 days (3.33%) arrived in Southern Central Vietnam within 25.22 ± 1.62 h (95% CI) flight duration (fig. 4). A further 10.89% (54/496) trajectories on 12 days (40%) arrived in Northern Central Vietnam. In early and middle March, 17.32% (24/127) backward trajectories on 6 days (60%, 6/10) arrived in Southern Central Vietnam, while 12.82% (45/351) trajectories on 11 days (73.33%, 11/15) arrived there in middle and late April. Only 1.71% (6/351) backward trajectories on 3 days (20.00%, 3/15) arrived in the Red River Delta in middle and late April. These results suggested that migrants from Central Vietnam could arrive in South China. Results of the forward trajectories further confirmed this finding (fig. 5). Interestingly, a greater proportion of backward trajectories could be traced to Laos and Northeastern Thailand. Among 30 peak catch days, 32.86% (163/496) trajectories on 22 days (73.33%) had endpoints in Laos within 22.82 ± 1.15 h (95% CI), while 20.36% (101/496) trajectories on 17 days (56.67%) arrived in Northeastern Thailand in 29.76 ± 1.12 h (95% CI) flight duration (fig. 4).
At both Leizhou and Longzhou, there were fewer peak catch days than at Hepu (fig. 2). The endpoints’ distributions of back trajectories from Hepu were similar to the results for Leizhou, but differed from Longzhou (fig. 4). At Longzhou, 27.40% (40/146) backward trajectories on 4 days (57.14%, 4/7) arrived in the Red River Delta (fig. 4). This suggests that the Red River Delta was potentially an important source area of migrants to Longzhou.
Results for the backward trajectory analysis of 9 years data (2005–2013) from Hepu were similar and also suggested that the macropterous adults migrated from Central Vietnam, central Laos, Hainan Island or Northeastern Thailand (fig. 6, table S3). In early and middle March, migrants most likely come from Southern Central Vietnam within 23.62 ± 2.51 h (fig. 6, table S3). In late March and early April, migrants most likely come from Northern Central Vietnam within 16.67 ± 1.07 h and the south part of Central Vietnam within 23.39 ± 1.21 h (fig. 6, table S3). Finally, in middle and late April migrants probably come from the north part of Central Vietnam within 17.22 ± 1.04 h (fig. 6, table S3). The forward trajectories also confirmed that RPH arrived from Central Vietnam to South China (fig. 5, table S2).

Fig. 6. Distribution of endpoints of backward trajectories from Hepu (○) in 2005–2013.
GLM regression
We explored the relationship between monthly cumulative light-trap catches at Hepu in April and weather factors in the Indochina Peninsula using regression models. Model covariates included monthly temperature and monthly precipitation in all potential source areas from January to April, and days of southerly wind with speed >4 m s−1 over the migration area in April. Results indicated that precipitation was the most important covariate at predicting the immigration volume of RPH at Hepu in April. Immigration volume in Hepu in April increased with increasing precipitation in Southern Central Indochina in January, and declined with increasing precipitation in Northern Central Indochina in January (table 1). The GLM model results suggested that wind did not have a significant influence on the RPH migration rates at Hepu in April.
Table 1. Results of the GLM model based on a quasi-Poisson distribution testing the effect of regional climatic parameters and light-trap type on cumulative light catches at Hepu in April.

Note: The type of light trap was a logical variable and it has two values, TRUE and FALSE. TRUE value means the black light trap with a fluorescent lamp and FALSE value means the traditional black light trap. Other variables included precipitations in Central Vietnam (NCV – Northern Central Vietnam, SCV – Southern Central Vietnam) in January.
Discussion
Our trajectory analyses results corroborate previous findings and support the hypothesis that RPH migrants in South China during March and April came from Central Vietnam (Shen et al., Reference Shen, Chen, Hu, Cheng, Zhang and Zhai2011; Wang et al., Reference Wang, Qi, Lv, Hu and Yuan2011). Many endpoints of backward trajectories from Nam Dan, Hai Hau and Hepu were located in Southern Laos, Central Laos and Northeastern Thailand. This suggested that the Central Indochina Peninsula may be the main source area of early spring migrants. RPH population dynamics and migration are poorly understood in Laos and Thailand. Northeastern Thailand is an essential production area for high-quality fragrant rice. The Chao Phraya River runs through this large rice-planting area and provides irrigation during the dry season (Zhai, Reference Zhai2011; Thanawong et al., Reference Thanawong, Perret and Basset-Mens2014). Lots of rice is also planted on the Vientiane Plain in Central Laos, and in the dry season this area is irrigated by reservoirs built near the Nan Ngum River (Zhai, Reference Zhai2011). In addition, our results indicated that RPH in the Mekong River Delta likely could not migrate to Central Vietnam directly, as the backward trajectory analysis from Central Vietnam showed no endpoints with an origin in the Mekong River Delta. These findings are in agreement with previous studies (Li et al., Reference Li, Luo, Wei and Huang1999; Zhai et al., Reference Zhai, Zhou, Tao, Chen and Shen2011; Otuka et al., Reference Otuka, Sakamoto, Chien, Matsumura and Sanada-Morimura2014).
The population dynamics and migration of RPH in Southern Central Vietnam were investigated in detail for the first time in this study. Many macropterous adults emerged and emigrated in March as rice matured (see Thang Binh in fig. 3). Both forward and backward trajectories suggested that these migrants arrived in Northern Central Vietnam, Northern Vietnam and South China. Thus, Southern Central Vietnam was one of the first source areas of migratory RPH. In Southern Central Vietnam, RPH always had shorter flight distance simulations due to the weak winds during the sunny weather conditions, characteristic of this and other tropical areas like the Mekong River Delta and the Philippines (Riley et al., Reference Riley, Reynolds and Farrow1987; Otuka et al., Reference Otuka, Sakamoto, Chien, Matsumura and Sanada-Morimura2014). In tropical areas, RPH has a shorter pre-ovipositional period and seems to have less migratory tendencies (Wada et al., Reference Wada, Ito, Takahashi and Tang2007; Wada, Reference Wada, Heong, Cheng and Escalada2014). However, our results revealed that planthoppers located in tropical areas do undertake long-distance migration over sea areas. We recorded simulated migration from Southern Central Vietnam over the South China Sea to South China. Similarly, tropical populations of N. lugens in the Philippines are estimated to migrate across the sea to Taiwan (Otuka et al., Reference Otuka, Watanabe, Suzuki, Matsumura, Furuno and Chino2005, Reference Otuka, Huang, Sanada-Morimura and Matsumura2012). Radar observation also showed that a small number of RPH detected at night may have the potential to migrate longer distances (Riley et al., Reference Riley, Reynolds and Farrow1987). The ecological and evolutionary drivers for long-distance migration of RPH remain a key question to answer. Similar population dynamics of N. lugens and S. furcifera at Thang Binh suggested that interspecific competition was not a factor to force them to emigrate. Previous studies suggested that macropterous migratory adults emerge under crowding or deteriorating host conditions (Kisimoto, Reference Kisimoto1956; Chen & Cheng, Reference Chen and Cheng1980; Sogawa, Reference Sogawa1982; Xu et al., Reference Xu, Xue, Lu, Zhang, Zhou, He, Ma, Jiang, Fan, Xu, Ye, Pan, Li, Bao, Nijhout and Zhang2015). In tropical areas, the maximum density of the two RPH species was 810 per 10 m2 at Thang Binh and 5800 per 10 m2 at Nan Dam (fig. 3). These densities of RPH are relatively low compared with the subtropical Yangtze River Delta area, where numbers of N. lugens were frequently greater at 13,500–16,500 per 10 m2 (Hu et al., Reference Hu, Lu, Zhai, Lu, Liu, Zhu, Wu, Chen and Zhang2014). Our results concurred with the observation that RPH population density in tropical regions was always much lower than that in subtropical regions (Wada, Reference Wada, Heong, Cheng and Escalada2014). Hence, population densities and overcrowding during emergence did not appear to be a factor forcing emigration of RPH from tropical areas.
In Southern Central Vietnam, host rice plants are always available at different growth stages because rice is planted all year-round (Zhai et al., Reference Zhai, Zhou, Tao, Chen and Shen2011). Thus, in tropical areas RPH can easily find a suitable host plant within short dispersal distances and need not emigrate. Host deterioration should therefore not have been a factor to force RPH to commence long-distance migration. During the investigative periods at Thang Binh, it was noted that mirid bugs (Cyrtorhinus lividipennis Reuter), a natural enemy of RPH, were abundant in rice paddies. Previous studies have shown that unsprayed, irrigated rice fields can have relatively few insect pest problems (such as RPH), probably due to predation by their natural enemies such as spiders and C. lividipennis that occur in abundance under these conditions (Sigsgaard, Reference Sigsgaard, Toft and Scharff2002; Wada, Reference Wada, Heong, Cheng and Escalada2014). Migration may confer benefits through the successive colonization of new habitats that temporarily provide an ‘enemy-free space’, or at least a significant reduction in predation, parasitism and/or pathogen infection compared with remaining permanently at the same location (Altizer et al., Reference Altizer, Bartel and Han2011; Chapman et al., Reference Chapman, Reynolds and Wilson2015). RPH cannot completely escape their predators, however, because C. lividipennis migrate long distances to follow their food source (Riley et al., Reference Riley, Reynolds and Farrow1987; Zhai, Reference Zhai2001). Nevertheless, the population abundance of spiders and C. lividipennis was much less in China than at the RPH source areas in tropical regions (Hu et al., Reference Hu, Cheng, Qi, Wang, Lu, Zhang and Zhai2011; Wada, Reference Wada, Heong, Cheng and Escalada2014). Predator avoidance may therefore have played an important role in driving the evolution of RPH to migrate long distances.
Previous studies have shown that N. lugens establishes its population on rice plants at the late stage of development after S. furcifera emigrates (Cheng et al., Reference Cheng, Chen, Xi, Yang, Zhu, Wu, Qian and Yang1979; National Coordinated Research Group for White Backed Planthopper, 1981; Hu & Xie, Reference Hu and Xie1988; Yang et al., Reference Yang, Zheng, Zhao, Wang, Xu, Qi, Xu, Wu, Zhang, Cheng and Zhai2011). In this study, S. furcifera consistently arrived and settled 1 month earlier in China than N. lugens did. The abundance of N. lugens was much less than S. furcifera at Hai Hau, Hepu, Longzhou and Leizhou in 2012 and 2013. This provides more evidence that S. furcifera established its population earlier than N. lugens. Conversely, in Central Vietnam both data from light traps and paddy surveys in 2012 and 2013 showed that N. lugens and S. furcifera had similar population dynamics in March and April. One possible explanation may be that S. furcifera has stronger migratory tendencies and capabilities than N. lugens. S. furcifera can fly for longer periods compared with N. lugens as demonstrated in flight mill experiments (Feng et al., Reference Feng, Zhai and Zhang2001; Wang & Zhai, Reference Wang and Zhai2004). Whilst both RPH species commence their northward migration at the same time from Central Vietnam or others parts of the Central Indochina Peninsula, it appears that S. furcifera’s longer-distance flight advantage provides it with the opportunity to establish its population in new habitats before N. lugens arrives. Interestingly, when both species are established in the same habitat S. furcifera is at a competitive disadvantage (Wang et al., Reference Wang, Chang and Zou1997, Reference Wang, Chang and Zou1998; Cheng et al., Reference Cheng, Zhao, Lou and Zhu2001; Yang et al., Reference Yang, Zheng, Zhao, Wang, Xu, Qi, Xu, Wu, Zhang, Cheng and Zhai2011). It therefore seems likely that preemptive migration of S. furcifera before N. lugens to new habitats is to compensate for later interspecific competition.
Temperature is thought to be the most crucial factor for controlling brown planthopper population dynamics by its direct effects on survival, reproduction and foraging (Chen & Wang, Reference Chen and Wang1996; Cheng & Zhu, Reference Cheng and Zhu2006; Hu et al., Reference Hu, Cheng, Qi, Wang, Lu, Zhang and Zhai2011). Similarly, temperature may regulate and control the population dynamics of both RPH species and a previous study has shown that catch sizes of N. lugens at Longzhou were positively correlated with winter temperature in the Central Indochina Peninsula, especially in May (Hu et al., Reference Hu, Xie, Lin, Xin, Huang, Chen, Zhang and Zhai2010). From November to April, most tropical areas of the Indochina Peninsula experience a dry season (Chang et al., Reference Chang, Wang, McBride and Liu2005; Phan et al., Reference Phan, Ngo-Duc and Ho2009). Our weather data confirmed that during this period it was always warm and sunny, with only a few days of rain. Temperature does not appear to be a limiting factor for RPH in the Indochina Peninsula because during the study period it was always warmer than 10°C and hence above the survival threshold of both N. lugens and S. furcifera (Cheng et al., Reference Cheng, Chen, Xi, Yang, Zhu, Wu, Qian and Yang1979; Hu & Xie, Reference Hu and Xie1988). For example, the mean temperature in January in Central Vietnam was 21.37°C during 1979–2013.
Our study revealed that winter precipitation in the Central Indochina Peninsula has the most influential role on migration volumes of S. furcifera at Hepu in April, as indicated by the GLM. In general, RPH prefer humid environments (Cheng et al., Reference Cheng, Chen, Xi, Yang, Zhu, Wu, Qian and Yang1979) and presumably, precipitation or humidity is a crucial factor for the survival of RPH, including overwintering populations. Rice plants do not grow well during dry winters and under these conditions few planthoppers were observed on rice plants from regeneration or fallen seeds in paddies in Guangdong, Yunnan and Sichuan Provinces (Yang et al., Reference Yang, Liu, Kong and Lin1982; Zhu & Wu, Reference Zhu and Wu1982; Gao et al., Reference Gao, Zhu, Wang and Zhu1988). Winter precipitation would be advantageous to RPH overwintering populations by providing a habitat with suitable and abundant rice plants and high humidity conditions that they prefer. Wu et al. (Reference Wu, Yu and Tao1997) proposed that the Mekong River Delta was the original source area of RPH emigrating to China. They suggested that precipitation might be very important in regulating RPH populations in this area, and that this information might be used to forecast RPH population abundance in China. Studies have shown that the N. lugens biotype in Southern Vietnam is different to that in Northern and Central Vietnam and China (Zhai et al., Reference Zhai, Zhou, Tao, Chen and Shen2011; Li et al., Reference Li, Luo, Wei and Huang1999). It is therefore assumed that N. lugens populations in Southern Vietnam do not frequently link to populations in Northern and Central Vietnam and China. Correspondingly, our study also provides strong evidence from the results of the trajectory analyses that the Mekong River Delta is not a source population for China. Furthermore, our study is reliable as it was both large in scope and time. The similarities in RPH population dynamics detected between the Mekong River Delta and Central Indochina regions by Wu et al. (Reference Wu, Yu and Tao1997) were probably due to a correlation in precipitation levels between the adjacent regions at that time that influenced local RPH population abundances, rather than any actual biological linkages between populations in these areas. The findings were based on limited data from 6 years only (1991–1996), thus we reject the conclusions drawn by Wu et al. (Reference Wu, Yu and Tao1997).
In summary, we provide new insights into RPH population dynamics and migration routes from source populations in Vietnam to South China. Some evidence suggested that Laos and Thailand might also be important source populations. We shed new light on our understanding of the interspecific relationships between RPH species, their establishment in new areas and the primary drivers that could have caused them to evolve their long-range insect migration. Our results provide valuable information for forecasting pest outbreaks, information that is necessary for implementing control measures against these migratory pests. Only Central and Northern Vietnam were studied here, further work across the whole Indochina Peninsula is required to deliver a more detailed and thorough migration map of RPH movements across the region. Future international joint pest monitoring programs between Eastern Asia countries might achieve this, as well as create further opportunities for information sharing relating to these pests.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485316001024
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant no. 31471763), the cooperative project on the surveillance and management of rice migratory pests between China and Vietnam (grant no. 2030114), the Fundamental Research Funds for the Central Universities (grant no. KJQN201434) and The National Key Technology R&D Program (grant no. 2012BAC19B01). GH's visiting scholarship at Rothamsted was funded by Nanjing Agricultural University and the Priority Academic Program Development of Jiangsu Higher Education Institutions. Rothamsted Research is a national institute of bioscience strategically funded by the UK Biotechnology and Biological Sciences Research Council (BBSRC).