Introduction
Death of a terrestrial vertebrate animal triggers intense competition among arthropods for use of the ‘fresh’ resource (Denno & Cothran, Reference Denno and Cothran1976; Kneidel, Reference Kneidel1984, Reference Kneidel1985). Insects are attracted to the body within minutes of death, and flies in the family Calliphoridae (blow flies, bottle flies) are among the first colonizers (Smith, Reference Smith1986). Adult females are initially drawn to the corpse as a result of odors emanating from decomposing tissues, while additional oviposition by conspecifics may be stimulated by pheromone or possibly kairomone signaling (Barton Browne et al., Reference Barton Browne, Bartell and Shorey1969; Eisemann & Rice, Reference Eisemann and Rice1987; Hammack et al., Reference Hammack, Bromel, Duh and Gassner1987; Hammack, Reference Hammack1990). Upon arrival, the females generally will oviposit in natural body openings (e.g. mouth, nose, ears, anus) or, when present, in wounds and lesions in the skin. Eggs are deposited in locations that favour neonate larval feeding on liquids rich in proteins. As the larvae mature into second and third stage larvae, feeding aggregations form in specific regions of the carrion (fig. 1). These aggregations are more commonly referred to as maggot masses and represent a dynamic ecological condition within an already unique ephemeral resource: the mass can vary in size, location (on body) and species composition dependent on geographic location, season, amount of sunlight, and stage of corpse decomposition. Within the mass, intense intra- and inter-species competition occurs, and some species of blow flies (e.g. Chrysomya rufifacies) and allospecifics (larviparous sarcophagids) are believed to possess adaptive life history characteristics that permit competitive advantages during larval development (Dennon & Cothran, Reference Denno and Cothran1976; Levot et al., Reference Levot, Brown and Shipp1979; Hanski, Reference Hanski1987; So & Dudgeon, Reference So and Dudgeon1990).
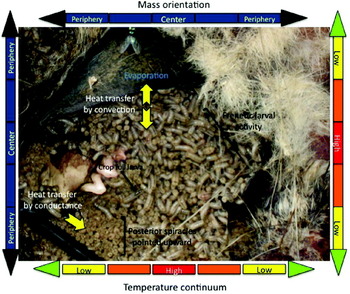
Fig. 1. Anatomy of a maggot mass. The mass shown is a multi-species larval aggregation formed from calliphorid eggs deposited in the mouth opening of an adult possum placed in a shady, urban location in Baltimore, Maryland, USA, during summer conditions. Peak internal mass temperatures are found in the centre of the aggregations and become cooler toward the periphery. A constant ‘stirring’ of the mass appears to occur at all times by the continuous locomotion or changing of position by larvae, commonly referred to as frenetic activity.
Perhaps the most distinctive feature of the maggot masses is heat generation. Internal temperatures of larval aggregations can vary considerably from ambient and ground conditions and, in some cases, have been reported to exceed the surrounding environment by as much as 10–30°C (Turner & Howard, Reference Turner and Howard1992; Anderson & VanLaerhoven, Reference Anderson and VanLaerhoven1996; Slone & Gruner, Reference Slone and Gruner2007). Most of the information concerning elevated maggot mass temperatures is derived from faunal succession studies with forensic entomology applications, so details on fly heat production and other physiological aspects of larval aggregations are lacking or not fully understood (Rivers et al., Reference Rivers, Ciarlo, Spelman and Brogan2010). Similarly, though several investigators have speculated on the costs and benefits of developing in maggot masses with elevated temperatures, or within large feeding aggregations in general, the predictions are mostly based on anecdotal observations rather than experimental evidence. Details about such important facets as whether cooperative feeding actually occurs among homogeneous (in terms of species composition) or heterogeneous feeding aggregations, what the source of heat production is in the maggot masses, how the flies avert heat stress, if at all, and if protection from predation or parasitism is afforded to larvae developing in maggot masses are not known. Our understanding of maggot masses, particularly the physiological ecology of the aggregation as a whole, is superficial at best. With the growing importance of necrophagous flies as evidence in criminal investigations, there is a need to explore this dynamic microcosm in depth to better understand abiotic and biotic influences on the flies present in the feeding aggregations.
In this review, an examination of what is known about the formation of maggot masses among flies in the families Calliphoridae and Sarcophagidae is presented based on research from field and laboratory experiments, and also from case studies. We also present arguments for the physiological benefits and limitations of developing in feeding aggregations that, at times, can represent regions of intense competition among and between species, overcrowded conditions, and which frequently experience sustained elevated temperatures that easily constitute a proteotoxic stress for most species of flies. Our goal is to also stimulate new investigations into areas that are poorly understood.
Reproductive strategies of Calliphoridae and Sarcophagidae
Competition among carrion breeding flies is intense, particularly among the feeding stages of larvae. The larval stages of calliphorids and sarcophagids are adapted for competitive intra- and inter-specific feeding battles: larvae have short feeding periods with rapid and efficient assimilation of food, immature development occurs quickly with few moults, digestive enzymes are modified for feeding on bacteria-infested foods, and individual larvae and feeding aggregations have high metabolic rates that generate internal heat (Roback, Reference Roback1951; Zdárek & Sláma, Reference Zdárek and Sláma1972; Hanski, Reference Hanski1977; Williams & Richardson, Reference Williams and Richardson1984; Terra & Ferreira, Reference Terra and Ferreira1994), further facilitating rapid growth rates (Campobasso et al., Reference Campobasso, Di Vella and Introna2001). Aspects of the reproductive strategies also are adaptive for maximizing carrion utilization and promoting some resource partitioning (Levot et al., Reference Levot, Brown and Shipp1979; Kneidel, Reference Kneidel1984; Ives, Reference Ives1991). For example, most necrophagous blow fly species oviposit on a corpse, and egg hatch occurs sometime later dependent on ambient conditions (Greenberg & Kunich, Reference Greenberg and Kunich2002). By contrast, sarcophagid females are viviparous, depositing young 1st stage maggots onto carrion. The different strategies result in higher fecundity yet increased risks (e.g. predation, drowning, temperature stress) for the calliphorids vs. lower fecundity with immediate resource acquisition (e.g. tissue penetration and food assimilation) for sarcophagids (Denno & Cothran, Reference Denno and Cothran1976). These differences would also be expected to influence the structure, composition, and possibly location (based on which species initially establish the aggregations) of maggot masses on carrion.
Formation of maggot masses
Maggot masses form when young fly larvae display what appears to be thigmotaxis to cluster in aggregations (Gennard, Reference Gennard2007). The aggregations are typically composed of calliphorid, sarcophagid, muscid and microdipteran larvae (Campobasso et al., Reference Campobasso, Di Vella and Introna2001). However, under most conditions examined in the field and in case studies, various species of blow flies dominate the composition of maggot masses (Goodbrod & Goff, Reference Goodbrod and Goff1990; Joy et al., Reference Joy, Liette and Harrah2006; Slone & Gruner, Reference Slone and Gruner2007). Exceptions do occur, as in cases in which several species (including some from different families) are represented in the feeding aggregations (Marchenko, Reference Marchenko2001), or in assemblages dominated by sarcophagids that can occur on human cadavers discovered indoors during summer months in parts of North America (Byrd & Castner, Reference Byrd, Castner, Byrd and Castner2010).
The exact stimulus for the formation of feeding aggregations is not clear. Oviposition is primarily stimulated in gravid calliphorids by ammonia-rich compounds emitted from the cadaver and, secondarily, by moisture associated with tissues, tactile stimuli, and possibly pheromones and kairomones (Eismann & Rice, Reference Eisemann and Rice1987; Hammack et al., Reference Hammack, Bromel, Duh and Gassner1987; Hammack, Reference Hammack1990; Ashworth & Wall, Reference Ashworth and Wall1994; Anderson, Reference Anderson, Byrd and Castner2010). Clustering eggs during oviposition suggests that one explanation for the formation of larval feeding assemblages is simply due to chance; the larvae feed in close proximity to the site of egg eclosion. Following egg hatch or larviposition, the subsequent larvae feed exclusively on soft tissue during all stages of immature development. This results in large numbers of flies feeding and developing in close proximity to one another since (i) oviposition behaviour of the adults tend to cluster eggs and newly deposited larvae together from the onset (Norris, Reference Norris1965; Barton Browne et al., Reference Barton Browne, Bartell and Shorey1969; Anderson, Reference Anderson, Byrd and Castner2010) and (ii) larvae are competing for the same food resources and the mere presence of hundreds to thousands of individuals on a finite ‘nutrient island’ will ‘force’ interactions. This explanation, however, is not entirely satisfactory. Though oviposition by multiple females frequently occurs in the same location on the carcass, following egg hatch, larvae of many species rapidly disperse from the site of oviposition seeking avenues to penetrate the interior of the carcass (Greenberg & Kunich, Reference Greenberg and Kunich2002). If the corpse is a large mammal, larvae may be separated by several centimeters to meters during all of the 1st and portions of the 2nd stage larvae or, in some cases, for the entire larval period. For most species of blow flies and flesh flies, maggot masses do not form until during the 2nd or 3rd larval stages; and, when they do, the larvae form rather tight masses at specific locations on the body. Overcrowding or food limitations do not account for the interactions among the first wave of colonizers on large carcasses, and the occurrence of hundreds to thousands of individuals in these aggregations would argue against random formation. It is possible that chemical cues, possibly serving as signals akin to pheromone trails used by social Hymenoptera, lead to the assemblages, although no direct evidence is available to support this speculation. Larvae from at least one species of sarcophagid, Sarcophaga (Neobelleria) bullata Parker, do demonstrate chemoattraction to carrion, as well as isolated bovine tissues (Christopherson & Gibo, Reference Christopherson and Gibo1996; Rivers & Warth, unpublished data), and the attraction appears to be stronger to food that has been previously fed upon, albeit time dependent by conspecifics (Rivers & Warth, unpublished data). Christopherson & Gibo (Reference Christopherson and Gibo1996) also demonstrated that the foraging behaviour of S. bullata was evident in late 2nd and early 3rd larval stages, consistent with the age of larval development in which maggot masses are most likely to form for this species (Rivers et al., Reference Rivers, Ciarlo, Spelman and Brogan2010). Similar behaviours have been anecdotally observed for the blow fly Protophormia terraenovae (Robineau-Desvoidy) (Rivers & Warth, unpublished data), suggesting that larval foraging using chemical cues may be common among calliphorids and sarcophagids.
Benefits of mass feeding
Cooperative digestion
An animal carcass represents a patchy, ephemeral resource that is nutrient rich (Hanski, Reference Hanski1987; Martínez-Sánchez et al., Reference Martinez-Sánchez, Rojo and Marcos-Garcia2000), but the window to use this ‘nutrient island’ by blow flies and flesh flies is very short. As a consequence, flies may deposit far more eggs or larvae than can be supported by the resource (Kneidel, Reference Kneidel1984; Al-Misned, Reference Al-Misned2002); and, in most cases, the tissues available to fly immatures are completely depleted in one generation. It is essential, then, that larvae of these flies be adapted for maximum utilization of the resource immediately upon egg hatch or as neonate larvae deposited on the food substrate. This is precisely what happens as newly hatched maggots penetrate the corpse with their mouth hooks and begin to feed voraciously during the larval stages, displaying rapid food conduction that is temperature dependent (Greenberg & Kunich, Reference Greenberg and Kunich2002) and which contributes to efficient food assimilation and short developmental duration (Roback, Reference Roback1951; Hanski, Reference Hanski1977).
Mouth hooks of a neonate fly larva are considered relatively delicate structures for puncturing holes through the integument of a corpse (Greenberg & Kunich, Reference Greenberg and Kunich2002), particularly if the skin is covered by hair or layers of fur. Consequently, blow flies tend to consume softer tissues first (e.g. lung and brain), presumably because these tissues are easier to penetrate with mouth hooks (Kaneshrajah & Turner, Reference Kaneshrajah and Turner2004) and not due to a higher nutrient content than other tissues (Kaneshrajah & Turner, Reference Kaneshrajah and Turner2004; Clark et al., Reference Clark, Evans and Wall2006). In the absence of a direct path (e.g. natural body opening or lesion) to the internal environment of the cadaver, a small individual larva is not likely to be capable of penetrating the tissues quickly enough to meet nutritional needs or prevent desiccation and starvation ensues. This would help explain why maggot masses of S. bullata and P. terraenovae need a minimum number of individuals to form a larval aggregation for normal growth and development to occur (Rivers et al., Reference Rivers, Ciarlo, Spelman and Brogan2010) and also would account for the tendency of necrophagous flies to oviposit in clusters, possibly using pheromones (Barton Browne et al., Reference Barton Browne, Bartell and Shorey1969), to ensure feeding success of their progeny. The advantage of cooperative feeding, however, appears to be age dependent, as female calliphorids do not oviposit in response to chemical cues emanating from existing larval masses (Erzinçlioglu, Reference Erzinclioglu1996).
Though larvae seemingly benefit from multiple individuals piercing cadaver tissues with mouth hooks, the more significant aspect of group feeding through larval aggregations is presumed to be liberation of nutrients from the corpse through mass release of digestive enzymes (Greenberg & Kunich, Reference Greenberg and Kunich2002; Anderson, Reference Anderson, Byrd and Castner2010). Day & Wallman (Reference Day and Wallman2006) compared larval development rates of Calliphora auger when reared on fresh vs. frozen (then thawed) sheep liver, the latter being tissue with weakened skin (Micozzi, Reference Micozzi1986), and thus should have been more easily accessible to larvae if mouth hooks were the key to feeding success. However, they found no differences in duration of development between the food types (Day & Wallman, Reference Day and Wallman2006). Similarly, Clark et al. (Reference Clark, Evans and Wall2006) found that food structure (‘liquidised’ (which is presumed to be homogenized in a food processor) vs. meat chunks) had no significant influence on larval development of Lucilia sericata. These observations suggest that features other than mass mouth hook use were more important to cooperative feeding among the flies. There is no doubt that large maggot masses breakdown and consume carrion tissues faster than smaller assemblages (Anderson, Reference Anderson, Byrd and Castner2010). However, group digestion, to our knowledge, has not been experimentally tested for any species of calliphorid or sarcophagid. Thus, the ideas of mass release of enzymes and cooperative mouth hook penetration of a corpse are intuitive speculation rather than from direct evidence. What has been shown is that larvae of necrophagous calliphorids do secrete salivary and gut fluids onto their food substrate (Price, Reference Price1974; Anderson, Reference Anderson1982; Young et al., Reference Young, Meeusen and Bowles1996; Muharsini et al., Reference Muharsini, Dalrymple, Vucolo, Hamilton, Willadsen and Wijffels2001), and these secretions contain an array of digestive enzymes including trypsin-like and chymotrypsin-like proteases, carbohydrases (i.e. amylase), and a pepsin-like enzyme presumed to be cathepsin-D like proteinase (Pendola & Greenberg, Reference Pendola and Greenberg1975; Bowles et al., Reference Bowles, Carnegie and Sandeman1988; Sandeman et al., Reference Sandeman, Feehan, Chandler and Bowles1990; Padilha et al., Reference Padilha, Pimentel, Ribeiro and Terra2009). Terra & Ferreira (Reference Terra and Ferreira1994) have speculated that the latter enzyme is present in all cyclorrhaphous Diptera that feed on food infested with bacteria. As Greenberg & Kunich (Reference Greenberg and Kunich2002) point out, a combination of heavy enzyme output with the ‘churning’ created by constant larval movement should quickly convert corpse tissues into a nutrient soup that bathes the larvae and promotes rapid consumption. More individuals in a feeding aggregation obviously should constitute greater enzyme output and also higher internal temperatures, promoting increased enzymatic activity up to a maximum threshold and, hence, more rapid breakdown and digestion of tissues. The circumstantial evidence is in place to support this view of cooperative pre-oral digestion of a corpse, but direct experimental evidence is lacking to substantiate an otherwise very plausible benefit of maggot masses.
Temperature regulation
During natural faunal succession of human and other animal carcasses, larvae from several species of calliphorids and sarcophagids form dense feeding aggregations that can generate internal heat (Deonier, Reference Deonier1940; Goodbred & Goff, Reference Goodbrod and Goff1990; Anderson & VanLaerhoven, Reference Anderson and VanLaerhoven1996). The heat production can exceed ambient air temperatures by several degrees, and in some instances, elevate to >30°C above environmental conditions. Despite temperature conditions that approach heat shock or even lethal levels (Deonier, Reference Deonier1940; Wigglesworth, Reference Wigglesworth1967), larvae of most calliphorids and sarcophagids appear to thrive in the hot, mass microclimate. The heat production may be viewed, then, as not just a byproduct of larval metabolism that must be dealt with; rather, it is an adaptive feature of the life history strategies of carrion breeding flies (Hanski, Reference Hanski1977; Cianci & Sheldon, Reference Cianci and Sheldon1990).
Heterothermic heat production
The source of heat production is not known. Heat generation has been attributed to microbial activity (Rodriguez & Bass, Reference Rodriguez and Bass1985; Turner & Howard, Reference Turner and Howard1992) and/or metabolic heat production from the flies, a byproduct of larval activity including frenetic movement (apparent constant locomotion of larvae in the mass) and high metabolism linked to digestive processes (Campobasso et al., Reference Campobasso, Di Vella and Introna2001; Slone & Gruner, Reference Slone and Gruner2007), the latter being essentially associated with rapid rates of food consumption and muscle movement along the fore- and midgut regions (Williams & Richardson, Reference Williams and Richardson1984; Greenberg & Kunich, Reference Greenberg and Kunich2002). Slone & Gruner (Reference Slone and Gruner2007) found that the volume of a maggot mass developing on pig carcasses and how ‘tight’ the mass was packed together, but not species composition or age of larvae, profoundly influenced internal mass temperatures. By contrast, Rivers et al. (Reference Rivers, Ciarlo, Spelman and Brogan2010) demonstrated that age of the larvae and species did result in differences in mass temperature when flies were reared on bovine liver under laboratory conditions. However, the temperatures recorded in laboratory-generated maggot masses do not achieve the maximum temperature elevations observed from larval aggregations formed during natural faunal succession (Goodbrod & Goff, Reference Goodbrod and Goff1990; Turner & Howard, Reference Turner and Howard1992; Marchenko, Reference Marchenko2001). It is apparent that multiple factors, including several that are abiotic, contribute to heat generation in the larval aggregations (Turner & Howard, Reference Turner and Howard1992; Marchenko, Reference Marchenko2001; Joy et al., Reference Joy, Liette and Harrah2006; Slone & Gruner, Reference Slone and Gruner2007; Rivers et al., Reference Rivers, Ciarlo, Spelman and Brogan2010). However, no study has yet to decipher the exact contribution of these potential heat sources to the internal mass temperatures.
Contributing to the intrigue of heat production in larval aggregations are the unique conditions associated with thermoregulation within the maggot mass. The flies must cope initially with a terrestrial environment that gradually becomes liquefied as tissue decomposition progresses. This also means that the potential for thermoregulation within the mass changes over time. For example, oviposition and larviposition occur essentially in terrestrial conditions on the corpse, so that any initial heterothermic heat production by the maggots would result as a byproduct of aerobic metabolism and thus be limited mostly by the availability of oxygen (Schmidt-Neilson, Reference Schmidt-Nielsen1997). Heat loss from the young larvae would be expected to occur rapidly due to their large surface-area to volume ratio, short diffusional distance across the width of the body and high integumental conductance due to a lack of insulating barriers (Willmer et al., Reference Willmer, Stone and Johnston2000). These larval features likely yield nearly 1:1 direct heat transference to the mass and air surrounding the larvae, which has a high water vapor content (Withers, Reference Withers1992; Willmer et al., Reference Willmer, Stone and Johnston2000). However, as the tissues decompose, creating a semi-aquatic to aquatic larval environment, heat release is by convection and the heat capacity of water greatly limits the potential for temperature increases in the maggot mass. Heat is also expected to be lost from the system due to evaporation of liquid from the integument of the maggots exposed to air, and heat convection is further facilitated by the stirring effect created by constant larval movement from the center of the assemblages toward the periphery (Anderson & VanLaerhoven, Reference Anderson and VanLaerhoven1996). The direct effect is that the amount of heat collectively produced by all larvae in a given maggot mass does not yield maximum temperature gain or elevation in the aggregations (fig. 2). By contrast, if the larvae simply fed in stationary, tightly packed positions within the mass, temperature loss from the system would be expected to decrease as no or minimal heat conduction between individuals would occur (provided body temperatures are isothermal in relation to each other) and the rate of evaporative cooling should decline (Willmer et al., Reference Willmer, Stone and Johnston2000). The net impact would be even higher internal mass temperatures than are typically recorded in succession studies, minimally evoking heat shock conditions for most species and likely approaching or exceeding the upper end of the zone of temperature tolerance (Withers, Reference Withers1992; Higley & Haskell, Reference Higley, Haskell, Byrd and Castner2010; Rivers et al., Reference Rivers, Ciarlo, Spelman and Brogan2010).
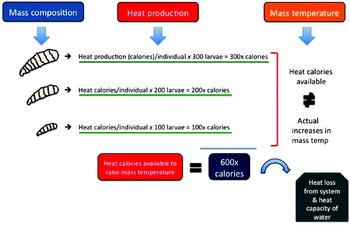
Fig. 2. Heat production in a hypothetical maggot mass. Metabolic heat generation is expected to be based on larval body size; thus, since fly larvae increase in size with age, older maggots (e.g. 3rd stage larvae) produce more heat than younger (1st stage larvae). All of the heat generated by larvae in a maggot mass is not available for elevation of aggregation temperatures, as some is lost to the environment by evaporation and radiation, and additional heat is used to overcome the heat capacity of fluids bathing the flies.
Increased food assimilation
The elevated temperatures resulting from larval aggregations have been predicted to offer evolutionary advantages to at least some species comprising the maggot masses. Necrophagous flies are poikilotherms, and thus growth and development are dependent on ambient temperatures. Growth rates for calliphorid and sarcophagid immatures are linear within species-specific physiological limits (Byrd & Butler, Reference Byrd and Butler1998; Grassberger & Reiter, Reference Grassberger and Reiter2001, Reference Grassberger and Reiter2002; Greenberg & Kunich, Reference Greenberg and Kunich2002). Consequently, elevated internal temperatures of maggot masses confer accelerated rates of growth, presumably through increased metabolic rate (Wigglesworth, Reference Wigglesworth1967), which in turn, leads to increased efficiency of food assimilation (Hanski, Reference Hanski1976, Reference Hanski1977; Williams & Richardson, Reference Williams and Richardson1984). Ullyett (Reference Ullyett1950) speculated that the extent to which fly species effectively compete in a carrion community is dependent on growth rate; and, since this rate is temperature dependent, it is possible that some flies attempt to influence the composition of the mass with the ‘goal’ of achieving a desired internal aggregation temperature that maximizes conditions for larval development and potentially yields an environment that offers an advantage over competing species (Hanski, Reference Hanski1977; Cianci & Sheldon, Reference Cianci and Sheldon1990). This suggestion would be consistent with the differences in thermal tolerances among calliphorid species developing in the same maggot masses (Waterhouse, Reference Waterhouse1947; Williams & Richardson, Reference Williams and Richardson1984). Richards et al. (Reference Richards, Price and Villet2009) have speculated that for species with exceptionally high upper thermal limits (e.g. C. marginalis or C. ruffifacies), the larvae may facilitate temperature elevations to exceed temperature limits of competing species, essentially utilizing a mechanism akin to the defensive ‘heat-balling’ employed by the Asian honeybee Apis cerana to subdue the predatory wasp Vespa velutina (Matsuura & Sakagami, Reference Matsuura and Sakagami1973). The authors further contend that such thermal regulation of maggot masses could lead to spatial partitioning within the larval aggregations. Under this scenario, all species in the mass would seemingly need the ability to sense environmental temperatures approaching upper thermal limits and then have the capacity to engage appropriate cooling mechanisms (see discussion below) or face extinction in the maggot mass. According to Hanski (Reference Hanski1977), the latter condition does not appear to occur in natural fly populations on carrion.
Protection from low temperatures
As already discussed, the internal heat produced in larval aggregations yields a microclimate with temperatures well above ambient. This thermal microhabitat appears to confer some protection to the maggots from sudden, unexpected drops in temperature (Cragg, Reference Cragg1956; Campobasso et al., Reference Campobasso, Di Vella and Introna2001). The mechanism behind low-temperature protection has not been examined, but most likely fly larvae are buffered from sharp decreases in ambient temperatures by both high internal mass temperatures and the physical barrier of the carcass. However, heat production in larval aggregations would not be expected to protect larvae from chilling or cold shock injury if the masses were not large enough or of the appropriate species composition to generate sufficient heat to counter extreme temperature declines and/or long exposures to low temperature, nor would maggot heterothermy be a suitable method to cope with the harsh conditions of winter. The latter is an important consideration because, though larval masses have been found on carrion during winter in North America (Deonier, Reference Deonier1940; Cragg, Reference Cragg1956), most species of calliphorids and sarcophagids residing in temperate regions depend on highly evolved genetic programs that anticipate seasonal changes through adaptive preparatory physiological and morphological mechanisms that protect the flies from the extreme environment of winter (Denlinger, Reference Denlinger2002). Heat production from maggot masses, alone, is thus not a viable strategy for extending the reproductive cycle of necrophagous flies into seasons characterized by unfavourable temperatures for eggs and larvae, as has been speculated previously (Deonier, Reference Deonier1940).
Protection from predators and parasites
In some insect communities, group feeding by conspecifics is thought to promote spatial aggregation of competitors, thereby serving as a predator avoidance strategy (Krebs & Davies, Reference Krebs and Davies1996). One avenue by which aggregation may afford protection is at high population densities, i.e. crowded conditions of a maggot mass, in which the individuals have a reduced risk of attack by predators and parasites (Parrish & Edelstein-Keshet, Reference Parrish and Edelstein-Keshet1999; Hunter, Reference Hunter2000), particularly those feeding in the center of the group. Such adaptive strategies are employed by several species of Drosophila to avoid attack by hymenopteran parasitoids (Bernstein, Reference Bernstein, Hochberg and Ives2000; Rohlfs & Hoffmeister, Reference Rohlfs and Hoffmeister2004), but the idea of spatial aggregation in maggot masses as a predator avoidance strategy has not been examined for carrion inhabiting flies.
Cianci & Sheldon (Reference Cianci and Sheldon1990) have argued that the elevated maggot mass temperatures may also reduce predation on fly larvae by decreasing the period of time devoted to immature development (via increased growth rates) on a carcass, the logic being that vertebrate and arthropod predators would simply have less time available to locate and consume the fly prey if larval development time is reduced, since once feeding is complete, most species of blow flies and flesh flies wander from the food source to pupariate under the protection of soil (Putman, Reference Putman1983; Greenberg, Reference Greenberg1990; Gomes et al., Reference Gomes, Godoy and Von Zuben2006). While this idea is plausible, there is no empirical evidence to support or refute the contention. It is also possible that the incidence of parasitism by hymenopteran parasitoids is influenced by temperatures within the larval aggregations. For example, accelerated larval growth rates due to elevated mass temperatures leads to smaller puparia for some species (Ullyett, Reference Ullyett1950; Kamal, Reference Kamal1958; Rivers et al., Reference Rivers, Ciarlo, Spelman and Brogan2010). Several of these flies can withstand drastic reductions in pupal size, with only a modest reduction in eclosion or subsequent fecundity of adults (Kamal, Reference Kamal1958; Williams & Richardson, Reference Williams and Richardson1983), yet the nutritional value of small puparia is greatly diminished for some parasitoids, particularly those relying on a gregarious reproductive strategy (Rivers & Denlinger, Reference Rivers and Denlinger1995; Rivers, Reference Rivers, Rivers and Yoder2007; Voss et al., Reference Voss, Spafford and Dadour2009). An example is the gregarious ectoparasitic wasp Nasonia vitripennis (Walker) (Hymenoptera: Pteromalidae) that frequently rejects hosts deemed too small (Rivers & Denlinger, Reference Rivers and Denlinger1995; Rivers, Reference Rivers2004); or, if oviposition on such flies does occur, clutch sizes are reduced, sex ratios become more male biased, larval development is lengthened in duration, and adult body sizes are stunted, decreasing fecundity (Rivers & Denlinger, Reference Rivers and Denlinger1995; Rivers, Reference Rivers, Rivers and Yoder2007). These observations, alone, do not demonstrate that heat generation by maggot masses affords protection from parasitism because often the same conditions that lead to elevated internal temperatures create intensely competitive, overcrowded aggregations that can produce small puparia by non-proteotaxic stressors (Williams & Richardson, Reference Williams and Richardson1983; Rivers & Denlinger, Reference Rivers and Denlinger1995; Ireland & Turner, Reference Ireland and Turner2006).
Deleterious effects of larval aggregations
Chemoattraction to predators and parasitoids
Oviposition by calliphorid and sarcophagid females on a corpse is primarily stimulated by emission of ammonia-rich compounds from decaying tissues (Ashworth & Wall, Reference Ashworth and Wall1994) and is most likely influenced by microbial metabolites (DeVaney et al., Reference DeVaney, Eddy, Ellis and Harrington1973; Eisemann & Rice, Reference Eisemann and Rice1987; Hammack, Reference Hammack1990), although the exact chemical stimuli triggering oviposition have not been deciphered (LéBlanc & Logan, Reference LéBlanc, Logan, Amendt, Campobasso, Goff and Grassberger2010). The decomposing carcass appears to become even more attractive to flies once the initial colonizers have deposited eggs, presumably due to oviposition pheromones present on the newly laid eggs (Barton Browne et al., Reference Barton Browne, Bartell and Shorey1969; Anderson, Reference Anderson, Byrd and Castner2010). These same chemical cues draw the attention of a variety of predatory and parasitic insects that target the fly eggs and larvae feeding in aggregations (table 1). Chemoattraction is also probably mediated through chemicals associated with the fly larvae, thereby functioning as kairomones to predatory beetles and other sarcosaprophagous insects (Wertheim et al., Reference Wertheim, Vet and Dicke2003). The wide range of beetles, ants, wasps and even other fly species that locate the fly eggs and larvae can be voracious feeders, consuming several individuals each. Larvae of the hairy maggot blow fly, C. rufifacies, as well as some other species of calliphorids, rely not only on predation to subdue allospecifics feeding in shared maggot masses but also elevate internal mass temperatures to extremes not tolerable by most competing species (Waterhouse, Reference Waterhouse1947; Richards et al., Reference Richards, Price and Villet2009).
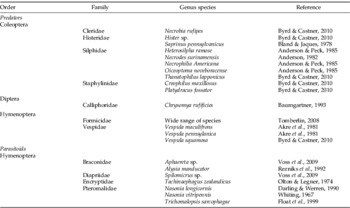
Table 1. Examples of predators and parasitoids of necrophagous fly larvae.

The list included in the table is not intended to be an exhaustive compilation of species functioning as predators and parasitoids, rather it serves to demonstrate that a wide range of insects are attracted to carrion to feed on necrophagous fly larvae and eggs.
How do the necrophagous flies cope with predation? The answer seems to be sheer numbers and rapid development rates; that is, high fecundity by individual females in clustered masses coupled with adaptations for rapid growth (see earlier discussion of cooperative feeding) offers the advantage of such bountiful food for predators that some fly larvae will survive. This reproductive strategy suggests that gravid females lay clutch sizes that maximize the mother's fitness, and that the conditions that favour the fitness of the adult female is in conflict with that of her progeny.
Parasitoids are also attracted to fly-infested carrion, although the cues that serve as attractants have not been determined. All carrion-specific parasitoids identified to date are parasitic Hymenoptera (Amendt et al., Reference Amendt, Krettek, Niess, Zehner and Bratzke2000; Disney & Munk, Reference Disney and Munk2004; Turchetto & Vanin, Reference Turchetto and Vanin2004; Voss et al., Reference Voss, Spafford and Dadour2009: table 1 provides some examples), and they parasitize either larvae or pupae/pharate adults. The most intensively studied of these wasps is the gregarious ectoparasitoid Nasonia vitripennis that attacks fly puparial stages but arrives at the corpse before wandering is initiated (Whiting, Reference Whiting1967). Wandering long distances from the corpse has been argued to be an adaptation of some necrophagous flies to avoid parasitism by N. vitripennis and other wasps (Legner, Reference Legner1977; Greenberg, Reference Greenberg1990). However, adult females have been observed ‘riding’ the maggots as they wander and then burrow into the soil, waiting for pupariation and pupation to be completed before ultimately attempting parasitism (Whiting, Reference Whiting1967). For some potential fly hosts, pupariation occurs on the corpse, leaving the fly exposed and seemingly unprotected from parasitoid attack. Despite not burrowing into the soil for pupariation, the incidence of parasitism for these fly puparia is low (Voss et al., Reference Voss, Spafford and Dadour2009), implying that, though mass feeding may attract parasitoids, the flies may utilize oviposition deterrents to avoid parasitism.
Thermal stress
Maggot masses generate heat, which can elevate internal temperatures to the point of evoking thermal stress responses in fly maggots (Rivers et al., Reference Rivers, Ciarlo, Spelman and Brogan2010). As the maggot mass grows in size, individual fly larvae may be exposed to rising temperatures that approach species-specific upper limits of the zone of tolerance (table 2: Withers, Reference Withers1992; Richards et al., Reference Richards and Villet2008). These upper environmental temperatures do not always reflect the actual internal temperatures of flies in the mass (Prange, Reference Prange1995; Rivers, unpublished data). This is an important distinction, as upper lethal temperatures for fly tissues represent conditions when proteins begin to denature and most features of aerobic metabolism are inhibited (Storey, Reference Storey and Storey2004). Within a short period, the damage is irreversible and cell death ensues (Prange, Reference Prange1995), most likely by oncosis (Manjo & Joris, Reference Manjo and Joris1995). In contrast, upper temperature limits within the mass environment are not absolute and can be influenced by humidity, length of exposure, level of hydration of the maggots and any means for heat dissipation used by the larvae (fig. 1: Prange, Reference Prange1995).
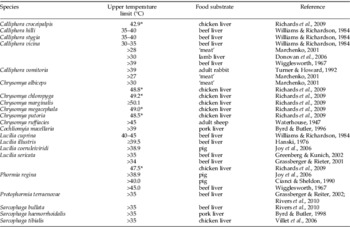
Table 2. Upper environmental temperature thresholds for calliphorid and sarcophagid larvae developing in maggot masses.

Upper temperature limits for feeding stages of larvae were either determined experimentally, in which temperatures are reported as a range or a LT50 (denoted by (*); LT50=upper lethal temperature at which 50% of individuals died under experimental conditions described by Richards et al. (Reference Richards, Price and Villet2009)) or extrapolated from maggot mass temperatures associated with faunal succession or laboratory rearing. In the case of faunal succession, internal temperatures in larval aggregations may have been from heterogonous masses.
Fly development at high temperatures can potentially be detrimental. The high internal temperatures of larval aggregations that have been reported under natural and laboratory situations constitute proteotoxic or thermal stress, capable of inducing the heat shock protein (hsp) response in several insect species, including the flies Drosophila melanogaster Meigen (Diptera: Drosophiladae), Sarcophaga crassipalpis Macquart (Diptera: Sarcophagidae) and S. bullata Parker (Chen et al., Reference Chen, Lee and Denlinger1990; Feder, Reference Feder, Johnston and Bennett1996; Korsloot et al., Reference Korsloot, van Gestel and van Straalen2004). Depending on the severity of high temperature elevation and length of exposure, hsp synthesis can occur at the expense of normal protein synthesis (Feder, Reference Feder, Johnston and Bennett1996), potentially compromising the development of the fly, which has indeed been reported for S. bullata and P. terraenovae when reared in large, laboratory generated maggot masses (Rivers et al., Reference Rivers, Ciarlo, Spelman and Brogan2010). Long before achieving conditions that evoke death, flies in larval aggregations may experience sufficient exposure to high temperatures that stimulate thermal stress and injury (Chen et al., Reference Chen, Lee and Denlinger1990; Paulie et al., Reference Paulie, Arrigo and Tissieres1992; Parsell & Lindquist, Reference Parsell, Lindquist, Morimoto, Tissieres and Georgopoulos1994; Feder et al., Reference Feder, Blair and Figueras1997). Non-lethal, high temperature stress can be manifested in necrophagous flies as an inhibition of feeding and/or growth, delay in the onset or completion of pupariation, suppressed puparial sizes, distortions in puparial shape, increased length in pupal and/or pharate adult development, and disruption of extrication behaviour, which can include shifting the peak day and time of day for adult eclosion (Chen et al., Reference Chen, Lee and Denlinger1990; Joplin et al., Reference Joplin, Yocum and Denlinger1990; Yocum et al., Reference Yocum, Zdarek, Joplin, Lee, Smith, Manter and Denlinger1994; Yoder et al., Reference Yoder, Benoit, Denlinger and Rivers2006).
Some speculation exists that the larvae may have the ability to self regulate body temperatures by moving in and out of the feeding aggregations (Deonier, Reference Deonier1940), conceivably allowing the maggots to avoid overheating during periods of elevated temperatures inside the masses by behaviourally driven spatial partitioning (Richards et al., Reference Richards, Price and Villet2009) and/or by releasing heat through evaporation (Villet et al., Reference Villet, Richards, Midgley, Amendt, Campobasso, Goff and Grassberger2010). Edney (Reference Edney1977) contends, largely based on the assumptions of Schmidt-Neilson (Reference Schmidt-Nielsen1964), that evaporative cooling is impossible for insects. However, Prange (Reference Prange1995) argues that evaporative cooling is used by insects that have access to essentially an unlimited water pool (either internally or in their environment) and that can generate heat to facilitate the water loss. Fly larvae developing in semi-liquid to liquid conditions of a maggot masses seem to fit those criteria. Such thermoregulatory abilities would also appear to depend on the fly larvae first experiencing high, potentially stressful, temperatures before cooling can occur (Prange & Modi, Reference Prange and Modi1990). Richards et al. (Reference Richards, Price and Villet2009) have observed behavioural patterns in larvae of Chrysomya albiceps (toward periphery) and Ch. marginalis (in core) leading to spatial aggregation in larval aggregations that appear to reflect larval thermal tolerance. Despite the merits of the argument, there is no experimental evidence to support the contention that maggots can regulate body temperature to avert heat stress.
Overcrowding
Overcrowding in a maggot mass is inevitable, at least with carcasses involving mammals (Kneidel, Reference Kneidel1984). As mentioned earlier, oviposition pheromones present on the egg chorion stimulates conspecific, and perhaps allospecific, oviposition/larviposition in clusters on the corpse. To compensate for a rapidly diminishing resource, the flies are predicted to deposit far more eggs or larvae than can be supported by the corpse (Kneidel, Reference Kneidel1984; Al-Misned, Reference Al-Misned2002), a situation that attempts to maximize maternal fitness at the expense of the progeny's. The net result is that maggot masses forming during natural faunal succession are typically composed of hundreds to thousands of individuals from a variety of calliphorid, sarcophagid, muscid and microdipteran species (Denno & Cothran, Reference Denno and Cothran1976; Hanski, Reference Hanski1977). These conditions do not favour offspring achieving maximum or even optimal larval weights (Kamal, Reference Kamal1958; Levot et al., Reference Levot, Brown and Shipp1979; Ireland & Turner, Reference Ireland and Turner2006). Obviously, overcrowding means decreased food availability per individual, which in turn, is expected to increase the length of larval development since it takes a longer period of time to acquire the critical weight/nutrients associated with the next moult (Ullyet, Reference Ullyett1950; Williams & Richardson, Reference Williams and Richardson1984). Overcrowding is likely countered by elevations in internal mass temperatures; up to a threshold number of individuals in the mass and a threshold upper temperature limit, larval growth rates are accelerated by increased temperatures (detailed explanations have already been discussed earlier). This helps to explain why some calliphorids appear to have faster rates of development under ‘crowded’ conditions (Saunders & Bee, Reference Saunders and Bee1995; Ireland & Turner, Reference Ireland and Turner2006).
Once the maggot mass reaches a critically high number of individuals, development is compromised by depleting nutrients and increased larval waste products; and, consequently, growth slows (Levot et al., Reference Levot, Brown and Shipp1979; Greenberg & Kunich, Reference Greenberg and Kunich2002; Rivers et al., Reference Rivers, Ciarlo, Spelman and Brogan2010). Slower growth rates have been argued to increase predation on fly larvae since a longer period of time is devoted to immature development on a carcass (Cianci & Sheldon, Reference Cianci and Sheldon1990). Optimal body weights are not achieved in extremely large maggot masses, resulting in reduced puparial sizes as well (Kamal, Reference Kamal1958; Levot et al., Reference Levot, Brown and Shipp1979; Saunders & Bee, Reference Saunders and Bee1995). For some species, the strong inter-specific competition for food between larvae does not result in high mortality (Erzinçlioglu, Reference Erzinclioglu1996), yet during intraspecies competition, particularly in overcrowded conditions, significant species-specific mortality occurs (Hanski, Reference Hanski1977; Hanski & Kuusela, Reference Hanski and Kuusela1977; do Reis et al., Reference dos Reis, von Zuben and Godoy1999; Rivers et al., Reference Rivers, Ciarlo, Spelman and Brogan2010). Kamal (Reference Kamal1958) has shown that, for several necrophagous fly species, extreme reductions in puparial size, and thus in subsequent adults, only modestly altered fecundity. However, Rivers et al. (Reference Rivers, Ciarlo, Spelman and Brogan2010) demonstrated that, for S. bullata and P. terraenovae, large maggot masses result in overcrowding and heat stress conditions, yielding high larval mortality, reduced puparial weights and a high incidence of pupal/pharate adult mortality. It should be noted that in the latter study, the authors were not able to distinguish between the effects of overcrowding and heat stress on the flies.
Overcrowding has been extensively studied in D. melanogaster, and the impact of larval crowding is very similar to that with necrophagous flies: larval development is extended; mortality from egg to adult increases; sizes of larvae, puparia and subsequent adults are smaller; and adult fecundity is reduced (Lints & Lints, Reference Lints and Lints1969; Scheiring et al., Reference Scheiring, Davis, Ranasinghe and Teare1984; Zwaan et al., Reference Zwaan, Bijlsma and Hoekstra1991). Depletion of nutrients, as well as build up of wastes (urea, uric acid), are thought to contribute to the overcrowding effects; but, perhaps more intriguing, larval crowding triggers the heat shock response (Buck et al., Reference Buck, Nicholson, Dudas, Wells, Force, Baker and Arking1993), which actually leads to prolonged adult longevity and increased thermal hardiness in the resulting adults (Sørensen & Loeschcke, Reference Sørensen and Loeschcke2001). This appears to be in sharp contrast to the deleterious effects reported for P. terraenovae and S. bullata reared in overcrowded maggot masses (Rivers et al., Reference Rivers, Ciarlo, Spelman and Brogan2010), although alterations in longevity and fecundity of adults were not tested.
Spatial aggregation among necrophagous flies may be a means for these flies to minimize interspecies overcrowding on carrion (Ives, Reference Ives1991; Kouki & Hanski, Reference Kouki and Hanski1995; dos Reis et al., Reference dos Reis, von Zuben and Godoy1999). Utilization of different reproductive strategies, inoculation of carrion with species-specific bacteria and regulation of the internal mass temperature (i.e. elevation to heat shock temperatures) all have been implicated as mechanisms for resource partitioning between species (Waterhouse, Reference Waterhouse1947; Denno & Cothran, Reference Denno and Cothran1976; Williams & Richardson, Reference Williams and Richardson1984; Hammack et al., Reference Hammack, Bromel, Duh and Gassner1987; Richards et al., Reference Richards, Price and Villet2009). However, it is still unclear whether these are indeed factors leading to spatial aggregations on carrion or to what degree inter- and intra-specific aggregations are associated with resource partitioning in carrion communities.
Conclusions
In this review, we have presented arguments for the physiological benefits and limitations of necrophagous fly larvae developing in large feeding aggregations on vertebrate carrion. As discussed, these maggot masses represent regions of intense competition among and between species, overcrowded conditions, and microhabitats that experience sustained elevated temperatures that easily constitute a proteotoxic stress for most species of flies. Despite the intriguing conditions of the maggot mass, surprisingly little is known about the physiological ecology of this feature of carrion communities. Much of the information that forms the basis for our understanding of larval aggregations is derived from faunal succession studies that obviously were not designed to experimentally test physiological parameters of fly biology or maggot mass adaptations. As a consequence, several features of necrophagous larval physiology are in need of new research, including detailed studies on cooperative feeding in maggot masses to understand the contributions of mass mouth penetration and group extra-oral digestion to facilitate assimilation of carrion tissues. The source of internal heat in larval aggregations has been attributed to heat production by fly larvae, yet the precise source of heat generation has not been determined nor the tissues in fly larvae that yield heat or the biotic factors (e.g. tissue and carcasses differences, competing species) that alter a maggot's heterothermic ability. These temperature elevations result in conditions that constitute thermal stress for most species of necrophagous flies examined, yet whether larvae in maggot masses actually experience heat stress is unknown. In fact, though anecdotal observations have lead to claims that larvae avert overheating by behavioural adaptations and possibly evaporating cooling, there is no experimental evidence to support such contentions.
From a physiological perspective, maggot masses represent an experimental system with an almost endless array of basic questions waiting to be addressed, and the findings of such studies have the potential to be applied to criminal investigations in which necrophagous flies are evidence.
Acknowledgements
This work was supported in part by a faculty development grant from Loyola University Maryland to D.B.R.