Introduction
The larvae of click beetles within the genus Agriotes (Coleoptera: Elateridae), also known as wireworms, are abundant ground-dwelling insects which are predominantly herbivorous (Langenbuch, Reference Langenbuch1932; Furlan, Reference Furlan1998; Traugott et al., Reference Traugott, Schallhart, Kaufmann and Juen2008a). They feed on a wide range of plants in grasslands and arable fields, where they can cause considerable damage to crops such as potatoes and maize (Parker & Howard, Reference Parker and Howard2001). Throughout Central Europe, 18 Agriotes species have been recorded (Cate, Reference Cate, Löbl and Smetana2007); another two species can occur in adjacent regions in the south and east (L. Furlan, personal communication). Nine of these 20 species are widespread and of special agricultural importance: Agriotes brevis, Agriotes lineatus, Agriotes litigiosus, Agriotes obscurus, Agriotes proximus, Agriotes rufipalpis, Agriotes sordidus, Agriotes sputator and Agriotes ustulatus (Tóth et al., Reference Tóth, Furlan, Yatsynin, Ujváry, Szarukán, Imrei, Tolasch, Francke and Jossi2003; Furlan & Tóth, Reference Furlan and Tóth2007). The biology and ecology of some of these species have been studied in detail (e.g. Langenbuch, Reference Langenbuch1932; Kabanov, Reference Kabanov1975; Furlan, Reference Furlan1996, Reference Furlan1998, Reference Furlan2004; Traugott et al., Reference Traugott, Schallhart, Kaufmann and Juen2008a), revealing differences in species-specific traits, such as feeding behaviour and phenology. Therefore, an accurate identification to species level is a prerequisite for any study addressing Agriotes’ basic biology, pest status or control. Although genus-specific morphological characters (i.e. specific spiracles placed on the ninth abdominal segment) allow to clearly identify wireworms of the genus Agriotes, species-specific features are described for only eight of the 20 Central European species in a current larval identification key (Klausnitzer, Reference Klausnitzer and Klausnitzer1994). The intraspecific variability in these morphological identification characters is unknown. Additionally, some diagnostic features (e.g. certain structures of the mandible) cannot be used reliably in field-collected larvae as they are usually worn down. Hence, in field surveys the species-specific identification of Agriotes larvae implies several difficulties and is usually not considered. Adults, on the contrary, can be identified morphologically (Lohse, Reference Lohse, Freude, Harde and Lohse1979), and pheromone catches of males (Furlan & Tóth, Reference Furlan and Tóth2007; Vernon & Tóth, Reference Vernon and Tóth2007) can be used as a surrogate for the larval population present in the soil. Agriotes larvae, however, are long-lived, typically spending 2–5 years in the soil before pupating (Langenbuch, Reference Langenbuch1932; Kabanov, Reference Kabanov1975; Furlan, Reference Furlan1998, Reference Furlan2004), which means that the adult population does not necessarily reflect the species composition in the larval population. Moreover, as pheromones are available for only some Agriotes species, this approach does not allow the examination of the complete Agriotes species spectrum.
Molecular approaches have shown a great potential to taxonomically assign ‘difficult’ organisms, and they constitute a valuable tool in overcoming problems entailed with conventional morphological identification methods. Sequencing of taxon-specific DNA-fragments (DNA barcoding) provides one way for molecular taxonomic assignment (e.g. Greenstone et al., Reference Greenstone, Rowley, Heimbach, Lundgren, Pfannenstiel and Rehner2005; Antonini et al., Reference Antonini, Coletti, Serrani, Tronci, Cristofaro and Smith2009). This approach has resulted in a steadily increasing number of diagnostic sequences available in public databases such as GenBank or BOLD (Ratnasingham & Hebert, Reference Ratnasingham and Hebert2007). In DNA barcoding, one DNA sequence of particular interest is the mitochondrial cytochrome c oxidase subunit I (COI) gene, which has been proposed as a universal ‘barcode’ for animals (Hebert et al., Reference Hebert, Cywinska, Ball and deWaard2003). Aside from barcoding approaches, a number of polymerase chain reaction (PCR)-based techniques have been developed within the last decade, providing fast, yet reliable, species identification (Hinomoto et al., Reference Hinomoto, Muraji, Noda, Shimizu and Kawasaki2004; Traugott et al., Reference Traugott, Zangerl, Juen, Schallhart and Pfiffner2006; Hosseini, Reference Hosseini, Keller, Schmidt and Framenau2007; Rugman-Jones et al., Reference Rugman-Jones, Morse and Stouthamer2009a,Reference Rugman-Jones, Wharton, Van Noort and Stouthamerb), also within the genus Agriotes. Accordingly, Ellis et al. (Reference Ellis, Blackshaw, Parker, Hicks and Knight2009) recently utilized a terminal restriction fragment length polymorphism (T-RFLP) technique to identify the larval stages of three Agriotes species occurring in the UK.
Here, we combine DNA barcoding and multiplex PCR to molecularly identify Agriotes species. The objectives of our study were: (i) to generate species-specific sequence information for all Central European Agriotes species; (ii) to develop a multiplex PCR assay for rapid identification of the nine most widespread species in Central Europe (the ‘core’ Agriotes species); and (iii) to evaluate the molecular identification protocol by screening a large number of field-collected Agriotes larvae. We aimed to develop a simple protocol which is cheap, reliable and can be performed with standard molecular equipment to maximize its practical applicability.
Materials and methods
Origin of adult beetles
Adults of each Agriotes species occurring in Central Europe were used to establish the molecular identification system. The majority of these beetles was stored in 70–90% ethanol; rare species (A. acuminatus, A. infuscatus, A. medvedevi, A. modestus, A. pallidulus, A. paludum, A. pilosellus, A. turcicus, as well as A. aequalis and A. gurgistanus, occurring in regions bordering in the south and east, respectively) were dried and pinned and came from private beetle collections (P. Cate, L. Furlan, G. Platia), including some individuals dating back as far as 1963. Beetles were identified using a standard identification key based on morphological characters (Lohse, Reference Lohse, Freude, Harde and Lohse1979). To account for the genetic variation within and between species, beetles collected from all over Europe were examined (table 1). Special attention was paid to the nine core Agriotes species, including specimens from Canada, where some of these species have been introduced from Europe more than a century ago (Vernon et al., Reference Vernon, La Gasa and Philip2001).
Table 1. The 20 Agriotes species (except for two larvae, indicated by ‘*’, adults only) investigated in this study, including the nine agriculturally most important species (in boldface). For each species, the sampling localities, number of DNA extracts and COI (mtDNA) sequences obtained (not all specimens were sequenced) are provided. A ‘+’ indicates species whose larvae are covered by the morphological identification key of Klausnitzer (Reference Klausnitzer and Klausnitzer1994). Sequence superscript ‘ht’ refers to those haplotypes used for phylogenetic analyses (see fig. 1).

DNA extraction and PCR
Total DNA was extracted from adult beetles using tissue from legs or the abdomen. From each Agriotes species, several individuals were used, including, if possible, specimens collected at different localities. A CTAB-based protocol described in Juen & Traugott (Reference Juen and Traugott2005) was employed with the following modifications to obtain amplifiable DNA from badly preserved beetles (specimens stored in ethanol <70% or dried/pinned specimens): beetles’ tissue was fully immersed in 430 μl TES buffer (0.1 M TRIS, pH 8, 10 mM EDTA, 2% sodium dodecyl sulphate) and 10 μl Proteinase K (20 mg ml−1, AppliChem, Darmstadt, Germany) in 1.5 ml reaction tubes, homogenized with pestles followed by an overnight incubation at 58°C. To extract DNA from pinned beetles and voucher specimens, we used a non-destructive DNA extraction method, which avoids conferring external morphological damage to the beetle. In this case, the whole beetle was placed in a 1.5 ml reaction tube containing the TES buffer–Proteinase K mix described above, incubated overnight and removed afterwards, leaving a sufficient amount of DNA for further analysis. Specimens were flushed with ethanol (99.8%) several times before replacing them to the collection. Voucher specimens for each species were either deposited in our laboratory or returned to the owner in case of rare specimens from beetle collections. All extractions were done in a separate pre-PCR laboratory; one extraction negative control was included in each batch of beetles extracted and tested with universal primers (Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994: PCR conditions see below).
A part of the 5′-end of the mitochondrial cytochrome c oxidase subunit I (COI) gene (approx. 660 bp) was amplified by PCR using the universal invertebrate primers LCO1490 and HCO2198 (Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994). To facilitate the amplification of degraded DNA from badly preserved beetles, the following intermediate primers were employed: C1-J-1859 (Simon et al., Reference Simon, Frati, Beckenbach, Crespi, Liu and Flook1994) and newly designed general Agriotes primers Agr-gen-A501 (5′-GATTYCTGTTGATCGYATATTAAT-3′), Agr-gen-A500 (5′-TGTTCCTGCDCCRTTTTC-3′) and Agr-gen-S500 (5′-GTTATYGTRACAGCACATGCWTTC-3′). The forward primer LCO1490 was used in combination with reverse primers A501 and A500, C1-J-1859 with HCO2198 and S500 with A501; amplifying approx. 350–500 bp fragments. Each 10 μl PCR contained 1.5 μl of DNA extract, 0.25 U HotStarTaq® DNA Polymerase (Qiagen, Hilden, Germany), 1 μl of 10× PCR Buffer (Qiagen), 5 mM MgCl2, 0.2 mM dNTPs (Genecraft, Lüdinghausen, Germany), 1 μM of each primer, 5 μg bovine serum albumin (BSA) and 2.55 μl of PCR-grade RNase-free water (Qiagen). The thermocycling program (executed on a Mastercycler Gradient, Eppendorf, Hamburg, Germany) consisted of an initial activation step of 15 min at 95°C, followed by 35 cycles of 1 min at 94°C, 1 min at 50°C, 1 min at 72°C and a 10 min final extension at 72°C. PCR products were electrophoresed on 1.5% agarose gels stained with GelRed™ (Biotium, Hayward, USA) and visualized under UV light.
Sequencing and phylogenetic analysis
PCR products of specimens from several localities were purified with ExoSAP®-IT (GE Healthcare, Little Chalfont, UK) following the manufacturer's recommendation and subjected to cycle sequencing PCR (BigDye® Terminator v3.1 Cycle Sequencing Kit, Applied Biosystems, Foster City, USA) using the general primers described above. Following precipitation and cleanup, the resuspended PCR products were sequenced on a 3130 Genetic Analyzer (Applied Biosystems) in both forward and reverse directions. Sequences were aligned and edited manually using BioEdit Sequence Alignment Editor v7.0.9.0 (Hall, Reference Hall1999) and representative sequences submitted to GenBank and BOLD (GenBank accession numbers listed in table 1). For the design of the species-specific primers a sequence alignment was generated comprising the Agriotes species (table 1) and the non-target elaterids (table 2).
Table 2. Non-target elaterid beetles and other soil invertebrates used to evaluate the specificity of the PCR assays.

* , outgroup for phylogenetic analysis (fig. 1).
The phylogenetic relationships among the nine core Agriotes species were examined using PAUP* v.4.0b10 (Swofford, Reference Swofford2002). As tree-building method, maximum parsimony was selected and Hemicrepidius niger served as an outgroup. The most parsimonious trees were inferred by a heuristic search with 100 random-addition-sequence replicates and the tree-bisection-reconnection (TBR) option. Node supports were evaluated by running 1000 replicates of bootstrap resampling (Felsenstein, Reference Felsenstein1985) and a 50%-majority-rule-consensus tree refined using MEGA v. 4.0.2 (Tamura et al., Reference Tamura, Dudley, Nei and Kumar2007). Sequence divergences within and between species (same sequence alignment as used for phylogenetic tree, outgroup excluded) were calculated using the uncorrected p-distance option (Nei & Kumar, Reference Nei and Kumar2000).
Primer design and multiplex PCR
Primer Premier 5 (Premier Biosoft International, Palo Alto, USA) was used to design several primers targeting the nine core Agriotes species. We aimed to generate primers that amplify DNA fragments between 100–600 bp, which allows separation by simple agarose gel electrophoresis. Primers targeting conserved sites within two or more species were preferred in order to reduce the total number of primers in the reaction.
A diagnostic multiplex PCR assay was developed and optimized by gradient PCR, testing of different primer combinations and concentrations. Compared to standard singleplex PCR, multiplex PCR offers the advantage of being able to simultaneously screen for several species within one reaction, saving time and money. The optimized multiplex PCR was conducted in a total volume of 10 μl containing 1.5 μl of DNA extract, 5 μl of 2×Multiplex PCR Master Mix (Qiagen), 1 μl of 10×primer mix (primer concentrations provided in table 3) and 2.5 μl of PCR-grade RNase-free water (Qiagen). The thermocycling protocol included an initial activation step of 15 min at 95°C, followed by 35 cycles of 30 s at 94°C, 90 s at 61°C, 1 min at 72°C and a final step of 10 min at 72°C. Each PCR included both negative (PCR-grade RNase-free water instead of DNA) and positive controls (DNA of the Agriotes species under investigation). Multiplex PCR products were electrophoresed on 3% agarose gels stained with GelRed™ (Biotium) at 90 V for 35–40 min and band patterns visualized under UV light to identify the species. The peqGOLD Ultra LowRange DNA-Ladder II (25–700 bp; PeqLab, Erlangen, Germany) was used as a fragment size standard.
Table 3. Specific primer pairs designed from COI sequences (mtDNA) to detect Agriotes species. Columns show the primer targets, primer names (S and A denotes forward and reverse primers, respectively), primer sequences, expected product size and concentration (Con.) of the primers in the multiplex PCRs (I, II).

Specificity testing of the multiplex PCR
The specificity of the multiplex PCR was evaluated using the 20 Central European Agriotes species (table 1). A minimum of ten individuals of each of the nine core species was tested, except for A. rufipalpis and A. proximus (seven and eight individuals only).
Non-target elaterid beetles (e.g. Agrypnus murinus, H. niger) and other soil invertebrates (mainly insect larvae), which typically can be found in the wireworms’ environment, were collected in Agriotes-infested field sites (table 2), their DNA extracted as described above and tested.
Evaluation of the molecular identification protocol
The practical applicability of the protocol was evaluated by screening 905 Agriotes larvae, which were collected in several field sites in Austria. Larvae of unknown identity were stored in 96% ethanol prior to molecular analysis. A simple Chelex-based DNA extraction protocol (Traugott et al., Reference Traugott, Bell, Broad, Powell, Van Veen, Vollhardt and Symondson2008b) was used to extract the DNA from these wireworms: the larval abdomen was cut to take a small piece of tissue, which was homogenized in a 1.5 ml reaction tube containing 20 μl PBS (pH 7.2, Sigma-Aldrich, St. Louis, USA) and 5 μl Proteinase K (20 mg ml−1, AppliChem). Thereafter, 200 μl of 10% Chelex solution (Bio-Rad, Hercules, USA) was added, and the samples were incubated overnight at 58°C on a rocking platform followed by 15 min incubation at 94°C. Within each batch of 30 samples, two extraction negative controls were included to check for potential carry-over of DNA.
Results
Agriotes barcoding sequences and phylogenetic analysis
In total, 196 adult Agriotes, collected from 60 locations, were used to develop the molecular identification system (table 1). Fifty-three individual COI sequences were deposited in GenBank and BOLD (GenBank accession numbers see table 1), covering all Agriotes species investigated except A. medvedevi, A. modestus and A. turcicus, where sequence generation was not possible due to the low quality of the DNA. These reference sequences allow identifying DNA barcodes of 17 Agriotes species commonly found in Central Europe.
Basic phylogenetic analysis was conducted for the nine core Agriotes species, comprising 22 scorable haplotypes (table 1) with a mean interspecific sequence variability of 11.4%. Using 508 nucleotide-long-sequences, maximum parsimony tree reconstructions showed that conspecific sequences clustered together (fig. 1; 346 nucleotides were constant, 27 variable, but parsimony-uninformative, 135 parsimony-informative; tree length: 354 steps). Haplotypes of A. brevis and A. sputator, as well as A. lineatus and A. proximus, however, could not be separated clearly due to low interspecific variability between the species pairs (bootstrap-support values for nodes for A. brevis/A. sputator and A. lineatus/A. proximus were 100% and 99%, respectively). Interspecific sequences divergences between A. brevis and A. sputator ranged from 2.0% to 2.4% (intraspecific variability of A. brevis-haplotypes: 0.4–0.6%; A. sputator: one haplotype only). Likewise, interspecific sequences divergence between A. lineatus (intraspecific variability: 0.2–0.8%) and A. proximus (one individual only) ranged between 0.4–0.8% only, prohibiting the development of species-specific primers (see below).

Fig. 1. Maximum parsimony tree of COI (mtDNA) sequences of Agriotes species. Bootstrap-support values (1000 replicates) greater than 50% are indicated above branches. Numbers beside Agriotes species indicate haplotypes (ht); numbers in parentheses refer to the total individual sequences. ![]() , outgroup Hemicrepidius niger.
, outgroup Hemicrepidius niger.
The multiplex PCR-based identification protocol
For the nine core Agriotes species, primers were designed: species-specific primers for A. litigiosus, A. obscurus, A. rufipalpis, A. sordidus and A. ustulatus, as well as group-specific primers targeting A. brevis/sputator and A. lineatus/proximus. Eleven primers were put together in a first multiplex PCR (I), yielding DNA-fragments between 168–516 bp in size (table 3, figs 2a and A1 in Appendix) allowing to discriminate between these species/groups within a single-step reaction. Beside the desired PCR products amplified by the specific primers, additionally 530 bp and 455 bp fragments were amplified in A. sordidus and A. lineatus/proximus, respectively. These fragments resulted from the combination of the general Agriotes forward primer S212 with the reverse primers A216 and A213 (figs 2a and A1 in Appendix). Moreover, some variations in band patterns were found in several individuals: a ∼180 bp fragment appeared in four out of the 11 A. sordidus tested, while the additional 530 bp fragment mentioned above was missing in another four larvae. In A. ustulatus, an additional ∼500 bp fragment appeared in five out of ten individuals. These variations in band patterns were also observed when field-collected Agriotes larvae were assayed. Occasionally, additional non-specific faint bands could be observed (e.g. in A. rufipalpis; fig. 2a). The diagnostic bands, however, were always present.

Fig. 2. (a) Multiplex PCR (I) products of the nine taxa targeted using specific primers. Lane 1: fragment size standard (25–700 bp). Samples from left to right: Agriotes brevis/sputator, 168 bp (2), A. rufipalpis, 192 bp (3), A. sordidus, 225 and 530 bp (4), A. lineatus/proximus, 293 and 455 bp (5), A. ustulatus, 323 bp (6), A. obscurus, 464 bp (7), A. litigiosus, 516 bp (8). (b) Multiplex PCR (II) products to identify A. brevis and A. sputator. Lane 1: fragment size standard (25–700 bp). Lane 2 and 3, A. brevis (168 and 462 bp) and A. sputator (168 bp), respectively.
To assign specimens testing positive for the A. brevis/A. sputator-primer pair to their respective species, a second multiplex PCR (II) was employed. The reaction mix and thermocycling conditions were the same as for the PCR (I), except that only three primers (S212, A215 and A522, for primer concentrations see table 3) were used and that the annealing temperature was 55°C for 3 min. Within this assay, A. sputator gave a single 168 bp PCR product (serving as an internal PCR control), whereas an additional 462 bp fragment was amplified with the DNA of A. brevis (figs 2b and A1 in Appendix).
The specificity of both multiplex PCR assays was tested with DNA extracts from all 20 Agriotes species (table 1), as well as from the other elaterid species and non-target invertebrates (table 2). No cross-reactivity was found within the corresponding size range (100–600 bp) of the diagnostic PCR products. Occasionally, an extra non-specific band of ∼700 bp was observed for H. niger and two earthworm species (Lumbricus terrestris, Octolasion lacteum).
The application of the diagnostic PCR assays was evaluated using 905 field-collected Agriotes larvae. Eighty-three percent of the larvae showed specific band patterns, allowing assignment of these specimens to one of the nine core Agriotes species (A. lineatus/A. proximus as a group). DNA extracts which failed in the multiplex PCR were retested in singleplex PCR using universal COI primers (see above). The amplified COI fragments were sequenced to assign the specimens to their species-specific DNA barcode using the blast algorithm (Altschul et al., Reference Altschul, Gish, Miller, Myers and Lipman1990). This approach revealed that several ‘Agriotes’ larvae were Adrastus sp. (Coleoptera, Elateridae), which are morphological very similar to Agriotes in larval stage. Less than 2% of the field-collected larvae (17 out of 905) did not provide amplifiable DNA.
Discussion
The molecular identification system developed in the present study comprises the provision of diagnostic barcoding sequences for 17 Agriotes species which occur in Central Europe as well as a multiplex PCR-based identification protocol for the nine most widespread and hence agriculturally most important species. These assays allow, for the first time, to reliably identify the Central European Agriotes species in pre-adult stage.
The present Agriotes barcoding is based on the 5′-end of the COI gene, which has been proposed as a universal ‘barcode’ (Hebert et al., Reference Hebert, Cywinska, Ball and deWaard2003; Hajibabaei et al., Reference Hajibabaei, Singer, Hebert and Hickey2007; Mitchell, Reference Mitchell2008), resulting in a rapidly growing COI sequence database (e.g. Lindroth & Clark, Reference Lindroth and Clark2009 for Melanotus wireworms occurring in the midwestern United States). Sequencing PCR products amplified with the universal COI primers (Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994) offers reliable identification of Agriotes species by comparing the generated sequences with those we have deposited in GenBank. No barcodes could be generated for A. medvedevi, A. modestus and A. turcicus due to bad DNA quality. However, these three species have been taken into consideration for the establishment of the specificity of the multiplex PCR assays.
For the multiplex approach, we initially aimed at identifying all nine core Agriotes species within a single PCR. This was hampered by the limits to the number of primers which can be used within one PCR, especially with short fragments. In the present case, a subsequent, second multiplex PCR was required to separate between A. brevis and A. sputator. Likewise, Rugman-Jones et al. (Reference Rugman-Jones, Morse and Stouthamer2009a) identified six hemipteran species by multiplex PCR and were able to diagnose two additional species after digestion of PCR products with restriction enzymes. Similarly, PCR products obtained with general primers can be directly subjected to restriction enzyme digestion to provide diagnostic band patterns (e.g. Miller et al., Reference Miller, Allsopp, Graham and Yeates1999; Roehrdanz et al., Reference Roehrdanz, Olson, Fauske, Bourchier, Cortilet and Sears2009; Rugman-Jones et al., Reference Rugman-Jones, Wharton, Van Noort and Stouthamer2009b; Sumer et al., Reference Sumer, Tuncbilek, Oztemiz, Pintureau, Rugman-Jones and Stouthamer2009). Ellis et al. (Reference Ellis, Blackshaw, Parker, Hicks and Knight2009) were the first to molecularly discriminate between three Agriotes species found in the UK (A. obscurus, A. lineatus and A. sputator) by applying a T-RFLP technique. However, methods in addition to PCR require further investment of time and money. Moreover, Noel et al. (Reference Noel, Tessier, Angers, Wood and Lapointe2004) found that the identification success rates were significantly lower in PCR-RFLP compared to multiplex PCR, especially when degraded DNA was examined. Our protocol, on the contrary, should also be suitable to identify badly preserved specimens and even semi-digested DNA (Lindahl, Reference Lindahl1991; King et al., Reference King, Traugott, Read and Symondson2008), as we have chosen primer pairs amplifying rather short, yet easily distinguishable, fragments ranging from 168 to 530 bp. Another advantage inherent to PCR-based identification techniques is that only small amounts of tissue are necessary for successful analysis. The Agriotes larvae can still be used for other types of analysis (e.g. morphological examination) stored as voucher specimens or even re-analyzed after molecular analysis.
Multiple individuals from each Agriotes species collected from different regions in Europe and Canada were used to establish the molecular identification. This assures that both the barcoding and the multiplex PCR-based identification are not corrupted by intraspecific sequence variability. The multiplex PCR assay proved to be highly specific as the diagnostic band patterns were obtained only with the target species. Also, no cross-amplification was found with non-agrioted elaterid beetles, as well as with other soil-dwelling invertebrates. Other insect larvae, for instance those mistakenly identified as Agriotes, will not lead to false positive results in the case of being assayed.
The multiplex PCR-based identification protocol (see Appendix) allows rapid identification of large numbers of samples at low costs. Using this protocol, one person can process approximately 100 larvae within two days. Besides, it is simple enough to be implemented in most molecular labs, requiring only standard equipment, and is ideally suited for dealing with large numbers of samples, such as typically encountered in field surveys and/or routine diagnostic work. By replacing conventional agarose gel electrophoresis with automatic electrophoretic techniques such as QIAxcel (Qiagen) (Macfadyen et al., Reference Macfadyen, Gibson, Raso, Sint, Traugott and Memmott2009), the efficacy of our approach can be enhanced even further. Similarly, Saccaggi et al. (Reference Saccaggi, Krüger and Pietersen2008) developed a molecular identification protocol which allowed differentiating between three mealybug species within four hours, demonstrating the feasibility of multiplex PCR for species identification.
The phylogenetic analysis of the nine core Agriotes species conducted within this study showed that sequence data is generally consistent with the current morphological species assignments (Lohse, Reference Lohse, Freude, Harde and Lohse1979). The analysis showed a high similarity between A. lineatus and A. proximus. These species were almost identical when the complete COI gene and additional parts of the COII were examined (data not presented). Accordingly, there is also hardly any evidence for species separation when comparing morphological characters, as differences used in adult taxonomy are minor and restricted to pronotum hair coat and prothorax characteristics only (Lohse, Reference Lohse, Freude, Harde and Lohse1979). These two species were also found to be attracted by the same pheromone baits in Portugal and Bulgaria (Subchev et al., Reference Subchev, Toshova, Furlan and Tóth2005; Tóth & Furlan, Reference Tóth and Furlan2005; Tóth et al., Reference Tóth, Furlan, Xavier, Vuts, Toshova, Subchev, Szarukán and Yatsynin2008). Unfortunately, the larvae of A. proximus have not yet been described (Klausnitzer, Reference Klausnitzer and Klausnitzer1994), making a comparison of these two species in their larval stage impossible. Further research is required to provide deeper phylogenetic resolution, as these results raise the question of whether A. lineatus and A. proximus should still be seen as two separate species.
Our study has shown that both DNA barcoding and multiplex PCR are versatile tools for species-specific assignment of Agriotes larvae. This approach now facilitates studying wireworm ecology at a species-specific level, hence contributing to the development of novel control strategies against these soil pests.
Acknowledgements
This research was supported by a grant of the University of Innsbruck awarded to KS, the BBK project 1448 and the Austrian Science Fund project P20377. We would like to thank Renata Bazok, Rod Blackshaw, Nina Brunner, Markus Clodius, Albert Ester, Manuela Kaub, Bernhard Kromp, Marion Landl, Bill Parker, Giuseppe Platia, Enrico Ricchiardi, Harald Schneller, Klaus Schrameyer, Mitko Subchev, Robert Vernon and Jozsef Vuts for providing adult Agriotes specimens for analysis. Moreover, we are grateful to Jasmin Klarica for phylogenetic advice as well as Corinna Wallinger, Lorna Raso and two anonymous reviewers for commenting on the manuscript.
Appendix: The multiplex PCR-based identification protocol for Central European Agriotes
1 Storage of field-collected wireworms
• Store larvae individually in 70–90% ethanol or frozen.
2. Chelex-based DNA extraction
• Cut larval abdomen and take a small piece of tissue;
• Homogenize sample in a 1.5 ml reaction tube containing 20 μl PBS and 5 μl Proteinase K (20 mg ml−1) and cool at 4°C;
• Add 200 μl of 10% Chelex solution (slurry continuously mixed on magnetic stirrer) and incubate sample overnight at 58°C on a rocking platform;
• Incubate sample at 94°C for 15 min;
• Store DNA extract at –28°C.
3. Multiplex PCR (I) and (II)
• Spin samples at 13,000 rpm for 5 min before using them in PCR;
• PCR master mix:

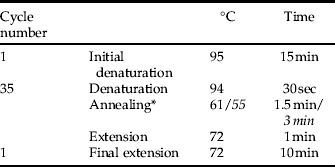
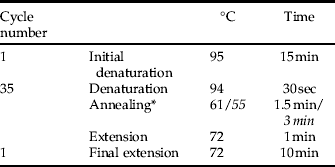
• Thermocycling conditions:

* , Second multiplex PCR (II) with differing annealing temperature and duration in italic face.
4. Agarose gel electrophoresis
• Use 3% agarose gel and LowRange DNA-Ladder to separate PCR products.

Fig. A1. Schematic overview of Agriotes-specific PCR products and corresponding primers (full primer names and sequences listed in table 3) targeting the COI gene (mtDNA). LCO1490 and HCO2198 are universal primers (Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994) comprising a 658 bp fragment. S and A denotes forward and reverse primers, respectively. A ‘*’ indicates the PCR product which appears in the second multiplex PCR (II) only.