Introduction
Agriculture, in general, and the stability of management of insect pests, in particular, are subject to the vagaries of natural selection and evolution (Hendry et al., Reference Hendry, Kinnison, Heino, Day, Smith, Fitt, Bergstrom, Oakeshott, Jørgensen, Zalucki, Gilchrist, Southerton, Sih, Strauss, Denison and Carroll2011; Thrall et al., Reference Thrall, Oakshott, Fitt, Southerton, Burdon, Sheppard, Russell, Zalucki, Heino and Denison2011). The evolution of pesticide resistance has been a problem for management of insect populations ever since these were first used. It was expected that similar problems would bedevil plants that were engineered to express novel insecticidal toxins. Although recent studies suggest that some populations of at least five crop pests have evolved a degree of resistance to insecticidal toxins engineered into plants (Tabashnik et al., Reference Tabashnik, Dennehy and Carriere2005, Reference Tabashnik, Van Rensburg and Carriere2009; Bagla, Reference Bagla2010; Carrière et al., Reference Carriere, Crowder and Tabashnik2010; Tabashnik & Carriere, Reference Tabashnik and Carrière2010; Zhang et al., Reference Zhang, Yin, Zhao, Jin, Yang, Wu, Tabashnik and Wu2011), only three show field failures due to resistance: Busseola fusca to Bt corn producing Cry1Ab in South Africa, (Van Rensburg, Reference Van Rensburg2007), Spodoptera frugiperda to Bt corn producing Cry1F in Puerto Rico (Matten et al., Reference Matten, Head, Quemada, Romeis, Shelton and Kennedy2008) and Pectinophora gossypiella to Bt cotton producing Cry1Ac in India (Tabashnik & Carriere, Reference Tabashnik and Carrière2010). Thus, despite their deployment throughout the world, in some parts since the mid-1990s, resistance to transgenic insecticidal crops has not developed within the 5–7 years predicted by skeptics.
Transgenic cottons, expressing the Bacillus thuringiensis (Bt) toxin genes, target the pest noctuid Helicoverpa armigera (Hübner) (Noctuidae). Bt cotton has been widely adopted in Australia, where it currently comprises nearly 90% of all cotton crops (Zalucki et al., Reference Zalucki, Adamson and Furlong2009), as it does in other parts of the world (Wu et al., Reference Wu, Lu, Feng, Jiang and Zhao2008). This landscape level change began in the late 1990s with the introduction of one-gene cotton transformations (INGARD), followed by two-gene Bollgard II in 2004 (see discussion). At a landscape level, cotton has effectively become a sink crop, as virtually no offspring survive from eggs laid in the crop (Rochester et al., Reference Rochester, Zalucki, Ward, Miles and Murray2002), except in those cases where refuges of non-Bt cultivars or other crop hosts are deliberately planted across the landscape with cotton. From time to time, in some fields, H. armigera develop to adults on Bt cotton crops, although this survival does not appear to be due to physiological resistance to Bt per se, and most likely reflects poor expression (Lu, Reference Lu2011). The most commonly used deliberately planted non-Bt refuge in Australia is pigeon pea.
For transgenic crops expressing Bt toxins at high levels for target pests, simple population genetic models (Kennedy et al., Reference Kennedy, Gould, Deponti and Stinner1987; Tabashnik, Reference Tabashnik1994; Gould, Reference Gould1998) and more complex models (Peck et al., Reference Peck, Gould and Ellner1999; Storer et al., Reference Storer, Peck, Gould, Van Duyn and Kennedy2003; Sisterson et al., Reference Sisterson, Carriere, Dennehy and Tabashnik2005; Shelton et al., Reference Shelton, Hatch, Zhao, Chen, Earle and Cao2008; Jongsma et al., Reference Jongsma, Gould, Legros, Yang, van Loon and Dicke2010) predict that refuges would substantially decrease the rate at which populations evolve physiological resistance to crops. The prediction is based on relative differences in population densities from different sources, a sink crop expressing toxin at a high enough level to kill any individuals that are heterozygous for resistance genes, extensive movement of moths in the landscape with random mating resulting in very few crosses between rare homozygous resistant individuals, should these arise from Bt crops. These models, by and large, do not directly incorporate information on oviposition behavior and host preferences of the target insects. Rather, they assume that adults do not shift their oviposition behaviour in response to the introduction of Bt crops into the landscape, and instead oviposit randomly with respect to the target crop, or maintain their previous preferences. When shifting host preferences are incorporated into a model (see Jongsma et al., Reference Jongsma, Gould, Legros, Yang, van Loon and Dicke2010) the resulting prediction is a preference shift in favour of alternative hosts. If moths were to avoid laying on sink crops, then physiological resistance should be slower to evolve, as a smaller fraction of the population will be subject to selection (Jongsma et al., Reference Jongsma, Gould, Legros, Yang, van Loon and Dicke2010). Concurrently, although growers of Bt cotton would enjoy a lower pest pressure, there should be a reduced sink effect of Bt cotton and, hence, less impact on population densities experienced throughout the landscape.
Host plant selection in Lepidoptera is generally undertaken by the ovipositing adults (Singer, Reference Singer, Vane-Wright and Ackery1984). Females may display a preference hierarchy among potential plant host species (e.g. Nylin & Janz, Reference Nylin and Janz1993; Midega et al., Reference Midega, Khan, Pickett and Nylin2011), which is thought to arise from the balance between attractants and deterrents (e.g. Renwick & Chew, Reference Renwick and Chew1994) and a number of proximate environmental effects on behaviour (e.g. learning: Cunningham et al., Reference Cunningham, West and Zalucki2001). Offspring can display the same oviposition preferences as their mothers, but this is not always the case (Thompson, Reference Thompson1988; Nylin & Janz, Reference Nylin and Janz1996; Mayhew, Reference Mayhew1997; Khan et al., Reference Khan, Midega, Hutter, Wilkins and Wadhams2006). Host preferences may be influenced by a number of evolutionary factors (West & Cunningham, Reference West and Cunningham2002), and it is generally argued that the evolution of more specialist oviposition behaviour would be more rapid in species that are host generalists, and potentially flip-flop between the two extremes (Janz & Nylin, Reference Janz, Nylin and Tilmon2008), if host selection is labile and populations become fragmented. Helicoverpa armigera (Hübner) (Noctuidae) is an extreme host generalist (Zalucki et al., Reference Zalucki, Daglish, Firempong and Twine1986, Reference Zalucki, Murray, Gregg, Fitt, Twine and Jones1994). There is experimental evidence for genetic variation in host preference among major lepidopteran cotton pests, including H. armigera (Schneider & Roush, Reference Schneider, Roush and Huettel1986; Jallow & Zalucki, Reference Jallow and Zalucki1996; Jallow et al., Reference Jallow, Cunningham and Zalucki2004; Wang et al., Reference Wang, Dong, Tang, Zhang, Li and Qin2004), providing the opportunity or raw material for evolution. Given this variation in oviposition behaviour and the assumption of a strong selection against laying on cotton, might we not expect a change in oviposition behaviour as a result of Bt cotton dominating the landscape?
One of the biggest problems with host-selection theory, explanations of why insects prefer to lay their eggs on particular plant species, is that empirical studies frequently fail to match with up with predictions (Mayhew, Reference Mayhew2001); in other words, insects appear to make bad choices. This lack of correlation, between insect choice and host suitability, may arise through constraints to the evolution of host-selection behaviour. Ecological (e.g. Walter & Benfield, Reference Walter and Benfield1994) or physiological (see Cunningham, Reference Cunningham2012) factors may prevent species, particularly generalists, from adapting to changes in host abundance or suitability within their environment. Given this theory of constraints, and the extensive host range of H. armigera, might we expect no change in oviposition behaviour as a result of Bt cotton dominating the landscape?
Our aim here is to utilize a ‘man-made’ change in host suitability for a highly polyphagous insect that occurs on a landscape level, and has existed over many generations, to investigate the extent to which change in host suitability can drive evolutionary change. Specifically, we are asking the question: Has there been a change in H. armigera egg distribution across different host plants, in populations from environments with widespread Bt cotton? To address this query, we used standard laboratory bioassays to test the behavior of first generation females (G1) from two major cotton-growing locations in Australia and, for a subset of G1 females, two subsequent further generations (using a slightly different but standard cage test methodology) to ascertain if females were consistent in their oviposition choices.
In order to evaluate whether there has been a change in H. armigera egg distribution, current evidence must be compared with data taken prior to the introduction of Bt cotton. Firempong & Zalucki (Reference Firempong and Zalucki1990b) tested a population from Narrabri for host oviposition preference among flowering plants of sunflower (Hysun 32), tobacco (Q46), maize (sweet corn), soybean (Davis), lucerne (Hunter River), cotton (Deltapine 61), linseed (unknown), pigweed and cabbage (Earliball). Insects were collected on two occasions as third–fifth instars from (non-Bt) cotton in February to March in 1985 and reared to adults. The results were consistent in both separate trials conducted with each cohort. Tests were conducted in a large hexagonal cage (1.6 m at the sides, 2.2 m in height and 4.3 m in diameter maintained within a glasshouse) with ca. 15 females in which glasshouse-grown potted plants were randomly repositioned throughout the period. Female moths showed a strong preference for ovipositing on tobacco, which received nearly 60% of eggs over five days consistently in two trials, followed by sunflower, lucerne, maize, cotton, soybean, linseed, pigweed and cabbage.
In an experiment designed to examine variation in host plant acceptance, Jallow & Zalucki (Reference Jallow and Zalucki1996) tested the post-alighting oviposition preference of individual moths from Narrabri collected from cotton, sorghum and sunflower crops. Plant material was presented to females as leaf discs cut from tobacco, lucerne, cowpea, maize, sorghum, and cotton (DP90 & HG660). Moths were tested using a tethered (stick) method (Jallow & Zalucki, Reference Jallow and Zalucki1995). Insects were collected as larvae and reared for 1–2 generations on a standard artificial diet in 1992 before being tested. Of the 20 tested moths from Narrabri, most females ranked maize, sorghum and tobacco as the most preferred. Some 45% of females ranked cotton (DP90 and HG660) as either first (n=4), second (n=3) or third (n=2) most preferred. Jallow & Zalucki (Reference Jallow and Zalucki1995) found that the stick test was a good predictor of oviposition choice by free-lying caged moths. This earlier work established a preference hierarchy at the population level (Firempong & Zalucki, Reference Firempong and Zalucki1990b) and demonstrated heritable differences among females in how hosts are ranked (Jallow & Zalucki, Reference Jallow and Zalucki1995, Reference Jallow and Zalucki1996). In our current experiments, we chose plants from the previously highly preferred group (tobacco), mid-ranked (obviously cotton) and low ranked hosts (cabbage). This in part gets around the problem of differences between cultivars across experiments. The changes are either going to be dramatic or not measurable.
Materials & methods
Collection and rearing of field insect material
Helicoverpa armigera eggs and larvae were collected from cotton fields and refuge crops (mainly pigeon pea) on three neighbouring properties (Genelg, Rosewood, Kilmarnock) in the Upper Namoi (Boggabri some 50 km from Narrabri, 30°19′S, 149°46′E) (mid-December 2009) and Bt cotton fields and refuge crops (pigeon pea and sorghum) on the Darling Downs (early February 2010). In each case, animals were sourced from multiple (6–18) widely separated fields, and immature stages were reared individually on a chickpea-based artificial diet (based on Teakle, Reference Teakle and Zalucki1991). Collection from widely separated fields means we are less likely to have collected closely related individuals. Male and female pupae were separated and each sex was housed in bulk in vermiculite. Temperature conditions were manipulated to ensure synchronous adult emergence.
Host plants
We used tobacco (Nicotiana tabacum, SR1), cotton (Gossypium hirsutum, Sicot 71) and head cabbage (Brassica oleracea, var Warrior) to test oviposition preference. From previous studies, these plant species represent high, mid and low preference plants (Firempong & Zalucki, Reference Firempong and Zalucki1990b). Plants were grown in 15 cm diameter pots in standard potting mix, watered and fertilized as required.
Oviposition choice experiments
Upon emergence, adults were allowed to mate with conspecifics from the same location and host source and the females were subsequently used in oviposition choice trials. Two types of choice tests, hereafter referred to as small-cage tests and large-cage tests, were conducted. In all experiments, adults were given access to cotton wicks soaked in 10% sugar solution.
G1 tests
Mated H. armigera females, from field collected (G1) larvae were used in small-cage and, if they did not lay, in large-cage tests. In small-cage tests, females were released into 45 cm3 mesh cages, together with whole leaves of tobacco, cotton and cabbage, of approximately the same size, and with stems embedded in floral foam (Oasis). In large-cage tests, whole potted plants were used in choice tests for groups of moths tested in mesh cages that were 1 m3. For large-cage tests, cotton and tobacco of similar size (50–75 cm) were used. These plants had buds and flowers, and cabbage (non-flowering) was raised to the same height as the other two hosts to prevent height effects (Firempong & Zalucki, Reference Firempong and Zalucki1990a).
G1 moths from the Narrabri population were tested in small cages over two nights. The host plant source from which larvae were collected for this population (cotton or pigeon pea) was noted in individual choice trials, and moths were mated with individuals from the same source crop. If individual moths laid fewer than ten eggs, they were grouped by host source and used in a large-cage test, conducted over two nights.
For further experiments, we selected a subset of G1 females from Narrabri that laid more than ten eggs in small-cage trials that could be categorized as either ‘specialist’ females (n=2, one from each source), which placed a majority of eggs on a single host, or ‘generalist’ females (n=2, again one from each source), which distributed eggs more evenly amongst hosts (see results). The offspring of each of these females were reared on artificial diet and mated with sibs. These F1 females were then tested for oviposition preference using large-cage tests in sub-groups over eight nights. As no plants with open flowers were available for this test, large pre-flowering plants were used (50–60 cm). Both tobacco and cotton had either buds or squares, and such plants were used in this and the subsequent experiment (below). Cabbage plants were non-flowering but were raised to the same height as the other plants. The distribution of eggs among host plants was counted daily, and plants were randomised amongst cages daily, and we varied the locations within the cage each day. There were between 15 and 28 females in each cage for F1 and subsequent F2 tests.
On the final day of counting, a sample of eggs from each of the F1 groups displaying contrasting oviposition hierarchies was collected. The eggs were reared on artificial diet and subsequently re-tested as F2 adults, following the same (large-cage) test procedures outlined above for F1 adults.
As survival of larvae to the pupal stage was very low for the Darling Downs populations, we ran choice tests on this sample using small-cage tests only and grouped adults from all sources for the initial mating and subsequent testing of individual females.
Statistical analysis
Factors such as source of moths and host plants available that can affect egg-counts were tested for statistical significance using a regression approach that recognizes the possibility of correlations among egg counts (amongst plant parts or plants within cages, amongst days, amongst moths from the same source, etc.). Such correlations are usually ignored in these types of assays, but this effectively ignores the fact that if a moth lays on one plant first this may affect subsequent laying and so forth.
Estimation of regression coefficients
Predictors for the mean egg count were plant type (tobacco, cotton or cabbage) and the source of the female moth (cotton vs pigeon pea, or Narrabri vs Darling Downs).
A regression model for the i th egg-count, μi, is given by:
The use of an exponential link ensured that the expected egg counts were non-negative; namely, the exponentiation, on the right hand side of the formula above, ensured that the expected egg counts were always non-negative during the iterative process for estimating the regression coefficients. Each estimated regression coefficient (βi) was then tested for significance using a t-test, formulated by dividing each coefficient by its respective standard error.
Allowance for correlations among egg-counts
Each set of three egg counts taken from the same female(s) within the same cage is considered a block of correlated egg counts. Egg counts are considered independent across blocks but not within blocks.
In order that the regression analysis be carried out in a way that allows for correlation within blocks, the ‘generalized estimating equations’ (GEE) method of data analysis was used (Liang & Zeger, Reference Liang and Zeger1986). This method allows simultaneous estimation of regression parameters for the mean and an estimation of regression parameters for the covariance. It also engenders no assumptions about the probability distribution of the data.
Denoting the covariance of egg counts i and j as Cij, the model for the variance/covariance matrix within a block is:
where α1 and α2 are parameters to be estimated from the data. Following Prentice (Reference Prentice1988) and Prentice & Zhao (Reference Prentice and Zhao1991), information about α1 and α2 is extracted from the cross-products of residuals: Sij=(yi−μi)(yj−μj).
Within each block of three egg counts, there are six such cross-products of residuals (including the diagonals: i=j). A variance/covariance for these six observations is estimated as the observed ‘sum-of-squares and products’ matrix among them, taken across all blocks. For further details, see Prentice (Reference Prentice1988) and Prentice & Zhao (Reference Prentice and Zhao1991).
We tested for difference in host plant preference between geographic source: Narrabri vs Darling Downs. Within Narrabri, we tested for differences between collection source: ex-refuge crop (pigeon pea) vs Bt cotton.
Evidence that preference is heritable
Evidence was sought for heritability of the distribution of egg counts across the three plant types across three generations. Four females that displayed apparent distinct oviposition patterns in G1 were selected and their F1 progeny tested and inbred to form an F2 generation. Three generations of egg counts across the three plants were thus recorded, producing four large blocks of (potentially) correlated egg counts, which will reflect heritability of preference due to genetic and maternal effects. Each block consisted of 39 sets of three egg counts.
One method to find evidence for heritability is to take moths in pairs and regress their phenotypic difference (the difference in their egg count distribution) against their kinship; a significantly negative relationship would indicate heritability. In order to get some handle on the concept of ‘preference’, each set of three egg counts was first divided by the total egg count for that set, converting it to three frequencies (fk, k=1, 3). This removed variation in fecundity (total egg count) from this particular analysis, placing the moths onto a common ‘preference’ scale. Information about the total egg count was not completely discarded, however, as the total egg count was used as a weight in the subsequent regression analysis (see below). Each resultant set of three frequencies was then contrasted to every other such set as the euclidean distance ‘d(ij)’ between the sets:
The degree of familial relationship was quantified using the ‘coefficient of kinshi’ (Lynch & Walsh, Reference Lynch and Walsh1998). Within blocks, kinships were calculated using the recursive formulas of Karigl (Reference Karigl1981) and are summarized as follows:

Kinship was assumed to be zero across blocks. The effects of kinship and day separation upon distance were assessed for statistical significance using the ‘glm’ module of the R statistical package. Each distance, being a sum-of-squares, was considered a gamma variate, i.e. a gamma error structure and an inverse link function were specified. Each distance was weighted in these analyses by the smallest of the two total egg counts relevant to that pair (ij). Given the multiple comparison being made, we use the 0.01 level of significance.
Results
Oviposition preference of G1 females
Of the moths sourced from the Narrabri, 11 females' ex-pigeon pea and 11 ex-cotton were set up in small cages to assess host preference. When females from both host sources from Narrabri are considered together, 77% of their eggs were laid on tobacco, 15% on cotton and 8% on cabbage (n=2068 eggs laid by all females across two nights). Five of seven (71%) ex-pigeon pea females that laid more than ten eggs showed a preference for tobacco, with one female preferring cotton (68% of 40 eggs) and one female preferring cabbage (54% of 13 eggs). Four of five (80%) ex-cotton females that laid more than ten eggs showed a preference for tobacco, and in one case cotton was clearly preferred (71% of 124 eggs laid). No ex-cotton females preferred to oviposit on cabbage.
For the Narrabri population, six females from cotton and four females from pigeon pea laid very few eggs (1–9) over two nights; but, when grouped in large cages with whole plants, these females ex-cotton and ex-pigeon pea laid 124 and 158 eggs, respectively. The distribution of eggs in these cages for ex-cotton females was 29% on tobacco and the remainder on cotton, while egg distribution by ex-pigeon pea females was 58% on tobacco, 35% on cotton and 7% on cabbage.
Similarly, the eight G1 females ex-Darling Downs tested in small cages laid 989 eggs over four nights with 71% on tobacco, 17% on cotton and 11% on cabbage. One female laid a higher proportion on cotton (51%), and two laid a substantial proportion on cabbage (36% and 37%), with tobacco in these cases having similar egg distributions (44–40%).
The variance of egg counts (as measured by α1) was significantly different from zero, as indicated by t-tests (t=2.911, <0.005 for Narrabri vs. Darling Downs populations, and t=4.553, P<0.001 for pigeon pea vs. cotton). Alpha two estimates, whether there is any correlation between egg counts amongst the three possible hosts within a cage, and these were not significant (i.e. there was no significant correlation; table 1).
Table 1. Regression parameters and associated t-statistics, for predictors of the mean egg count and for predictors of the covariance of egg counts. Results are presented separately according to whether the ‘source’ of the female moth refers to its host plant (pigeon pea vs. cotton) or to its geographical location (Darling Downs vs. Narrabri).

Although there was a significant difference in mean egg counts between the tested Narrabri and Darling Downs G1 females (table 1), this difference did not depend on host (i.e. the source by host interaction was not significant). Narrabri G1 females laid more eggs than Darling Downs G1 females, but moths from both sources ranked the hosts the same way: there were significantly more eggs on tobacco compared to cotton, which received significantly more eggs than cabbage (table 1). For Narrabri females, there was no significant difference between oviposition rankings of females ex-pigeon pea vs. ex-cotton (source and interaction terms were not significant; table 1). Moths from both sources preferred tobacco significantly to cotton, and the latter to cabbage.
Oviposition preference of F1 females: specialists vs. generalists
From G1 females ex-Narrabri, we selected a female from each collection host source that either showed a clear preference for tobacco or was more even in her distribution of eggs (table 2).
Table 2. Distribution of eggs (proportion) amongst three host plants over two nights by four H. armigera females collected as eggs from either cotton (COT) or pigeon pea (PP). These females were classified as either host specialist or generalists and their daughters re-tested as F1 and F2. Each female was given a designated number.
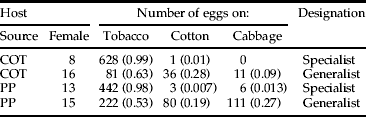
When F1 females from both host sources and host specialty types are considered together, there was a strong preference in the large-cage trials for oviposition on tobacco (65%), followed by cotton (27%) and then cabbage (8%); that is, the females behaved in much the same way as the G1 females (their mothers) from Narrabri.
The F1 offspring of the ex-cotton female that preferred tobacco (table 2) laid 5387 eggs, of which 75% were on tobacco, 22% were on cotton and 2% were on cabbage. The F1 offspring of the ex-pigeon pea host specialist behaved more like a cotton specialist/ generalist with 40% of eggs deposited on tobacco, 52% on cotton and 8% on cabbage (out of 18,545 eggs laid over eight days). The F1 offspring of the two females that were generalists laid a lower proportion on tobacco: 69% for the female ex-pigeon pea (out of 9630 eggs) and 63% for the ex-cotton female (6720 eggs). For these females, the remaining eggs were distributed as follows: 20% on cotton and 12% on cabbage for the ex-pigeon pea generalist and 35% on cotton and 2% cabbage for the ex-cotton generalist.
The oviposition behaviour of F2 offspring (grand-daughters of G1) from generalists vs. specialists was statistically similar to that of F1 offspring from generalists vs. specialists (their mothers; table 3). In the F2 offspring, ex-cotton tobacco specialist laid 64% of eggs on tobacco, whereas for the generalist this was 44%. In the F2 test, offspring from the ex-pigeon pea female originally classified as a tobacco specialist continued to lay more eggs on cotton (74%) as it had done in the F1 test. In the F2 test, offspring from the ex-pigeon pea generalist did not show a strong preference for a particular host (e.g. 51% on tobacco; table 3). There was a significant effect of kinship on egg-laying behaviour, but the day of egg lay did not affect this relationship (table 4 and fig. 1).

Fig. 1. Histogram of ‘distance’, separately for each level of kinship for G1, F1 and F2 females. When moths are unrelated, the histogram is flatter, shifting left (i.e. the oviposition preferences are more similar), as moths are more related (see table 4).
Table 3. Distribution of eggs for females selected in G1 and retested as F1 and F2 in larger cages over eight days. The females are numbered as in table 2.
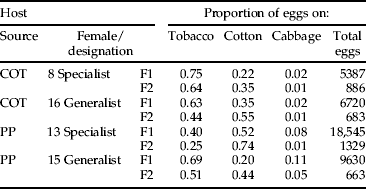
Table 4. Evidence that preference is heritable based on a kinship analysis. Parameters of the ‘distance’ between distributions of egg-counts across three generations (see text for details).

Discussion
Host preference trials performed in the 1980s and 1990s with H. armigera female moths from Narrabri and the Darling Downs demonstrated that tobacco was highly preferred next to cotton and that cabbage received very few eggs (Firempong & Zalucki, Reference Firempong and Zalucki1990b; Jallow & Zalucki, Reference Jallow and Zalucki1996).
Since this work was conducted, nearly the entire irrigated cotton crop in Australia has shifted from being conventional varieties to those with either one or, more recently, two Bt genes expressing Cry toxins in all green leaf tissue (see Zalucki et al., Reference Zalucki, Adamson and Furlong2009, fig. 2). Consequently, the cotton crop has effectively become a sink for the main targets of this technology, Helicoverpa spp. Yang et al. (Reference Yang, Johnson and Zalucki2008) found that 97.5% of first instars died within 72 h on two gene Bt cotton, compared to 60% on non-Bt cotton. Has this effective sink or trap crop changed the host plant selection behaviour of the H. armigera population in cotton growing areas?

Fig. 2. Area of cotton grown in Australia from 1996/97 to 2009/10 (histogram), as well as the proportion of one (INGARD, sold line) and two-Bt gene (BOLLGARD II, dashed line). Note the collapse of the area grown reflects drought conditions and the lack of water for irrigation. Records sourced from Cotton Yearbook series published by Australian Cotton Grower.
Based on the results of the experiments reported herein, the short answer to the question appears to be ‘no’. As in tests performed in the late 1980s and early 1990s on populations of H. armigera from both geographic locations tested herein, overall tobacco continues to be the preferred host for oviposition, despite the fact that it is not grown as a commercial crop in Australia. Female H. armigera moths continue to lay eggs on cotton, and some females even preferred it among the options that we offered. This was true for both geographic regions. Collection sources within a region also made no difference. This result is regardless of the fact that the current landscape is comprised of a variety of cotton on which virtually all of a moth's offspring will die. We have not tested oviposition by H. armigera on Bt cotton vs. non-Bt cotton; we intend to conduct such work shortly. However, work done on Heliothis virescens (Fabricius) (Noctuidae) and Helicoverpa zea (Boddie) (Noctuidae) in the USA demonstrates no oviposition preference of female moths for non-Bt cotton vs. Bt cotton after almost a decade of widespread planting of Bt cotton (Torres & Ruberson, Reference Torres and Ruberson2006). Moreover, in both pest species, there were no differences in the spatial distribution of eggs within plants between the two types of cotton, indicating that moths had not shifted their behaviour to deposit on structures of Bt cotton that may be more or less toxic to pests (Torres & Ruberson, Reference Torres and Ruberson2006). In India in a field study, Kumar & Stanley (Reference Kumar and Stanley2010) found H. armigera did not discriminate between Bt and non-Bt cottons in their tests. Anecdotally, in the Australian system, large numbers of Helicoverpa spp. eggs continue to be recorded on Bt cotton fields.
Our results demonstrate that, as found by Firempong & Zalucki (Reference Firempong and Zalucki1990b) and Jallow & Zalucki (Reference Jallow and Zalucki1996), genetic variation for oviposition still exists in H. armigera and that this variation is heritable. There are still moths that show more specialized patterns of oviposition and others that distribute their eggs more evenly. Yet, there appears to have been no change in oviposition behaviour in this generalist pest species. This finding supports the theory of constraints, either ecological or physiological, to the evolution of preference.
Recent molecular studies suggest that populations of H. armigera are probably not structured geographically, with high gene flow between populations (Weeks et al., Reference Weeks, Endersby, Lange, Lowe, Zalucki and Hoffmann2010; see also Endersby et al., Reference Endersby, Hoffmann, McKechnie and Weeks2007). Additionally, persistence of H. armigera populations throughout a number of cropping seasons might present a barrier to an evolutionary shift in host preference (Jongsma et al., Reference Jongsma, Gould, Legros, Yang, van Loon and Dicke2010), as subsequent generations of insects are presented with varying abundances of crop and wild host species (Wardhaugh et al., Reference Wardhaugh, Room and Greenup1980; Walter & Benfield, Reference Walter and Benfield1994). If movement is extensive, population mixing and random mating could ensure that no local directional change in behaviour to exploit better hosts or avoid bad ones can be achieved.
Constraints to evolution in host preference may be inherent in the behavioural mechanism of host selection. Recent advances in insect neurophysiology suggest that constraints in olfactory perception might prevent insects from adapting towards (or away from) particular hosts (Cunningham, in press). It is also possible that oviposition preference in generalists is not related to offspring performance, as host quality is not predictable, and variability in neonate performance even within host plants is large (Zalucki et al., Reference Zalucki, Clarke and Malcolm2002).
Quantitative theoretical models (Gould, Reference Gould1984; Kennedy et al., Reference Kennedy, Gould, Deponti and Stinner1987; Castillochavez et al., Reference Castillochavez, Levin and Gould1988) suggest that natural selection for behavioural avoidance as a mechanism of herbivore adaptation to classical host-plant resistance could render a high-dose plus refuge strategy ineffective. Our finding that Helicoverpa spp. moths do not avoid cotton as hosts for oviposition verifies the appropriateness of adopting a stringent plan in Australia, which includes mandatory planting of dedicated refuge crops, to retard the evolution of resistance to Bt by this pest. Physiological resistance to Bt cotton has not yet developed in Australia, and between 2002 and 2010 the proportion of the H. armigera population that carried genes conferring resistance to either of the two toxins in Bollgard II did not increase (Mahon et al., Reference Mahon, Olsen, Downes and Addison2007; S. Downes, unpublished data). Is the resistance management strategy working or has the relatively low area of cotton (fig. 2), and hence the relatively small proportion of the population subject to selection, resulted in relatively low selection for resistance? Since rainfall levels and the price of cotton have improved considerably, the area of cotton grown in Australia is expected to increase. With greater exposure of populations to Bt cotton, the vigilance of resistance monitoring needs to be maintained.
Acknowledgements
This work was supported in part by ARC grant DP0988150 to MPZ. T. Parker, K. Stanford, W. Finn, N. Winters, D. Jones, L. Howie, A. Hulthen and A. Marcora provided valuable technical assistance. Collection of insect material was supported by CSIRO, Cotton Communities Catchment Cooperative Research Centre through CCC0005 and the Cotton Research and Development Corporation (CRDC) through CSE0002. Dr B. Pyke, CRDC, generously provided many years of records on area of cotton grown.








