Introduction
A forest ecosystem is a complex unit of biodiversity, and its components include plants, animals, insects, microorganisms and their interactive relationships (Hunter, Reference Hunter1999). Due to their toxic effects on many beneficial organisms, chemical pesticides should not be used for insect pest control in sustainable forest ecosystems. Furthermore, the overuse of pesticides for pest control may result in the development of potential resistance to insecticides by the pest insects being targeted (Sánchez-Bayo et al., Reference Sánchez-Bayo, van den Brink and Mann2011). Thus, green pest-control methods such as microbial control and traps and beneficial insects should be used to replace pesticides.
Lymantria dispar (L.) (Lepidoptera: Lymantriidae), the gypsy moth, is a defoliator of mainly forest trees (Gould et al., Reference Gould, Elkinton and Wallner1990). It is of Eurasian origin and has a range that covers Europe, Africa, and North America (Keena et al., Reference Keena, Coté, Grinberg and Wallner2008). Gypsy moth larvae are known to feed on over 500 plant species within 73 families (Lance, Reference Lance and Ahmad1983; Liebhold et al., Reference Liebhold, Gottschalk, Muzika, Montgomery, Young, O'day and Kelly1995; Mrdaković et al., Reference Mrdaković, Mataruga, Ilijin, Vlahović, Tomanić, Mirčić and Lazarević2013). The larvae can cause economic damage and reduce forage production. The greatest impact of gypsy moths is the physiological stress in trees caused by defoliation (Humble & Stewart, Reference Humble and Stewart1994; Papadopoulou et al., Reference Papadopoulou, Chryssochoides and Katanos2009).
Biological control is an alternative approach to reducing populations of L. dispar, using natural enemies. Bacillus thuringiensis-based insecticides are in widespread use because of their specific toxicity against certain pests in the larval stage (Höfte & Whiteley, Reference Höfte and Whiteley1989). The microbial insecticide Bacillus thuringiensis var. kurstaki is often used to manage L. dispar (McCullough et al., Reference McCullough, Raffa and Williamson1999; Fabel, Reference Fabel2000). However, parasitoids play an important role in the biological control of L. dispar, and several European hymenopteran parasitoids of the gypsy moth have been established (e.g., Ooencyrtus kuvanae [Howard] [Encyrtidae] Anastatus japonicus Ashmead [=disparis Rushka] [Eupelmidae], Cotesia melanoscelus Ratzeburg [Braconidae], Phobocampe disparis [Viereck] [Ichneumonidae], Monodontomerus aereus Walker [Torymidae], Brachymeria intermedia [Nees]).
O. kuvanae (Howard) is a small encyrtid egg parasitoid that serves as a potential biological control agent of L. dispar. O. kuvanae was originally known to exist only in Japan, but now it is found to have nearly a Holarctic distribution. This parasitoid is an arrhenotokous and multivoltine species (Tadic & Bincev, Reference Tadic and Bincev1959; Brown, Reference Brown1984). The egg stage of L. dispar has a very long period, which can be used by O. kuvanae to go through several generations, each contributing to augmented parasitism rates in the field (Hofstetter & Raffa, Reference Hofstetter and Raffa1998; Wang et al., Reference Wang, Liu, Zhang, Wen and Wei2013). In addition, O. kuvanae can adapt to several different environmental conditions and is an abundant species (Brown, Reference Brown1984). However, it is not possible to rear this parasitoid on its natural host under laboratory conditions, because its natural host, L. dispar, is an univoltine species; additionally, the egg masses and urticacious hairs on the larvae of this host may cause allergic reactions in humans (Fabel, Reference Fabel2000; McCullough & Bauer Reference McCullough and Bauer2000; Tong et al., Reference Tong, Chun-xiang and Guo-cai2000). There are three main methods of rearing parasitoids, namely, on a natural host, on a substitute host and on an artificial diet (Consoli et al., Reference Consoli, Parra and Zucchi2000). Natural enemy rearing on substitute hosts is a determining factor for the success of many biocontrol programs, because this rearing option reduces production costs and increases the viability of large-scale use of the beneficial insect (Parra, Reference Parra, Parra and Zucchi1997).
In this study, Phylosamia ricini Donovan (Lepidoptera: Saturniidae) was selected as a new substitute host. P. ricini eggs have been used for the laboratory rearing of a number of parasitoids such as Trichogramma chilonis Ishii, Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae) and Anastatus japonicus (Hymenoptera: Eupelmidae) (Pu et al., Reference Pu, Liu and Zhang1988; Liu et al., Reference Liu, Zhang and Zhang1998). The host plants of P. ricini, Ligustrum vulgare (Lamiales: Oleaceae) and Ailanthus spp., facilitate the rearing of this lepidopterous species in the laboratory (Saito, Reference Saito1998; Osanai et al., Reference Osanai, Okudaira, Naito, Demura and Asakura2000; Tiradon et al., Reference Tiradon, Bonnet, Do Thi, Colombel, Buradino and Tabone2013). Females lay many eggs during their short lifespan (approximately 250 eggs per female); they are not subject to diapause and their eggs are large (1656.5 × 1143 µm2) (Tunca et al., Reference Tunca, Colombel, Sousan, Buradino, Galio and Tabone2015).
Host quality is a significant cause for the success of parasitism by parasitoid biocontrol agents. Host size (particularly for solitary parasitoids), host plant, host species and host age can affect host quality (Vinson & Iwantsch, Reference Vinson and Iwantsch1980; King, Reference King1987; Godfray, Reference Godfray1994; Campan & Benrey, Reference Campan and Benrey2004; Shuker & West, Reference Shuker and West2004; Ueno, Reference Ueno2005). Host age is an important factor influencing host acceptance and host suitability for the parasitoid egg (Vinson & Iwantsch, Reference Vinson and Iwantsch1980; Zhou et al., Reference Zhou, Abram, Boivin and Brodeur2014). Furthermore, the age of the female parasitoid is a determinant of reproductive rate and can affect parasitism (Amalin et al., Reference Amalin, Peña and Duncan2005; Aung et al., Reference Aung, Takagi and Ueno2010; Pizzol et al., Reference Pizzol, Desneux, Wajnberg and Thiéry2012). In this study, the age of the host and the age of the female parasitoid were investigated, with the aim of evaluating the new substitute host, P. ricini. We investigated the biological characteristics (offspring production ratio, development time, longevity, and fecundity) of O. kuvanae reared on eggs of this host. The establishment of new laboratory rearing methods of O. kuvanae on P. ricini will contribute to the laboratory rearing of this parasitoid.
Materials and methods
This study was performed at the INRA-PACA Mediterranean Forest and Entomology Unit, Laboratory of Biological Control, Antibes, France.
Rearing the host P. ricini
P. ricini eggs were collected daily in a Petri dish (5 cm) and kept inside an incubator at 25 ± 1 °C, 65 ± 5% relative humidity (RH), and a photoperiod of 16 : 8 h (light : dark [L : D]). Newly hatched larvae were transferred to plastic boxes (26 × 12 × 7 cm3) and were fed every day with fresh privet leaves, L. vulgare (Lamiales: Oleaceae). Different larval stages were reared in separate boxes and observed until pupation. Just after the pupal stage, individual pupae were shifted to adult rearing cages (30 × 39 × 30 cm3). This process was repeated on a daily basis.
Rearing the parasitoid O. kuvanae
The O. kuvanae colony was obtained from the parasitized eggs of L. dispar collected from the fields in Arbois-Avignon. Adult parasitoids were reared in glass tubes (1 × 7 cm2) and maintained in an incubator at 25 ± 1 °C, 65 ± 5% RH, and a photoperiod of 16 : 8 h (L : D). A drop of bio-honey was offered at 2-day intervals as a food source for adult parasitoids. The egg masses of P. ricini were collected and exposed daily to the mated female parasitoid. Then the offspring were allowed to emerge. Female and male parasitoids were collected at emergence. After mating, 90 females were kept to reach the different ages needed for the experiments. The remaining adult parasitoids were used to establish parasitoid cultures under laboratory conditions.
Experimental techniques
We tested the effects of age and number of P. ricini eggs and age of O. kuvanae females on offspring production ratio and development time. For this purpose, we used a 2 × 3 × 3 factorial design. There were two levels of treatment according to the age of P. ricini (1–2 days old and 3–4 days old), three levels of treatment for the number of P. ricini (40, 60, and 80 eggs), and three levels of treatment for the age of O. kuvanae (1, 3 and 5 days old). Furthermore, 40, 60 or 80 unparasitized eggs of P. ricini (either 1–2 or 3–4 days old) were exposed in a test tube (1 × 7 cm2) to single mated O. kuvanae females (1, 3 or 5 days old), with a drop of honey, for parasitism. The bottoms of each test tube were covered with cotton. A total of 180 host eggs per treatment and a total of 1080 host eggs per replication were used. The experiments were performed in five replicates. The emergence of adult parasitoids (at regular intervals of 24 h) and the dates of emergence of O. kuvanae adults were recorded to determine the offspring production ratio and development time. In addition, the longevity of the adult parasitoids was investigated under dietary (bio-honey) and non-dietary conditions. Longevity was measured from adult emergence until death. The experiment was performed in triplicate. A single mated O. kuvanae female (1-day old) reared from the eggs of P. ricini was introduced into a test tube (1 × 7 cm2) with 15 P. ricini eggs. Bio-honey was supplied as a nutrient for the female parasitoid. The P. ricini eggs were replaced daily with new batches of 15 eggs until the female died. The previous eggs were transferred to an incubator (25 ± 1 °C, 65 ± 5% RH and a photoperiod of 16 : 8 h [L : D]). Seventeen female parasitoids were tested, and we measured the realized fecundity and the means of the oviposition period and the post-oviposition period.
Data analysis
The offspring production ratio and development time were analyzed using a general linear model with the age of the hosts, number of hosts and the age of the female parasitoid as factors. Percentage data were normalized using an arcsine transformation (p′ = arcsine √p; Zar, Reference Zar1999). Longevity was analyzed using a two-sample t test (Minitab Release 14; SAS Institute, 2003; McKenzie & Goldman, Reference McKenzie and Goldman2005). The corresponding means (±standard error) for the oviposition and post-oviposition periods were calculated for the realized fecundity.
Results
There was a significant impact (P < 0.001) of both parasitoid age and host number on the offspring production ratio (table 1). The highest and the lowest offspring production percentages were obtained from a 5-day-old female with 40 host eggs (89.90%) and a 1-day-old female with 80 host eggs (27.60%) (table 2). Three factors (parasitoid age × host number × host age) showed a significant interaction (P < 0.001) on the development time of O. kuvanae (table 3). The minimum and maximum mean development times were 16.5 ± 0.08 days and 18.7 ± 0.08 days, respectively (table 4). Although these development times do not seem to differ much, a significantly shorter development time was observed in young (1–2-day-old) host eggs. The mean lifespan of adults was 51.10 ± 1.1 days with honey and 3.92 ± 0.14 days without honey (t60 = −42.05, P < 0.000). The mean number of offspring developed from the eggs laid by a single female reared on P. ricini (realized fecundity of female O. kuvanae) was 68.88 ± 3.22. Furthermore, the means of the oviposition and post-oviposition periods were 22.76 ± 1.37 days and 13.64 ± 1.40 days, respectively. Peak adult emergence occurred between 2 and 9 days. The emergence of the adults continued for about 3 weeks (fig. 1).
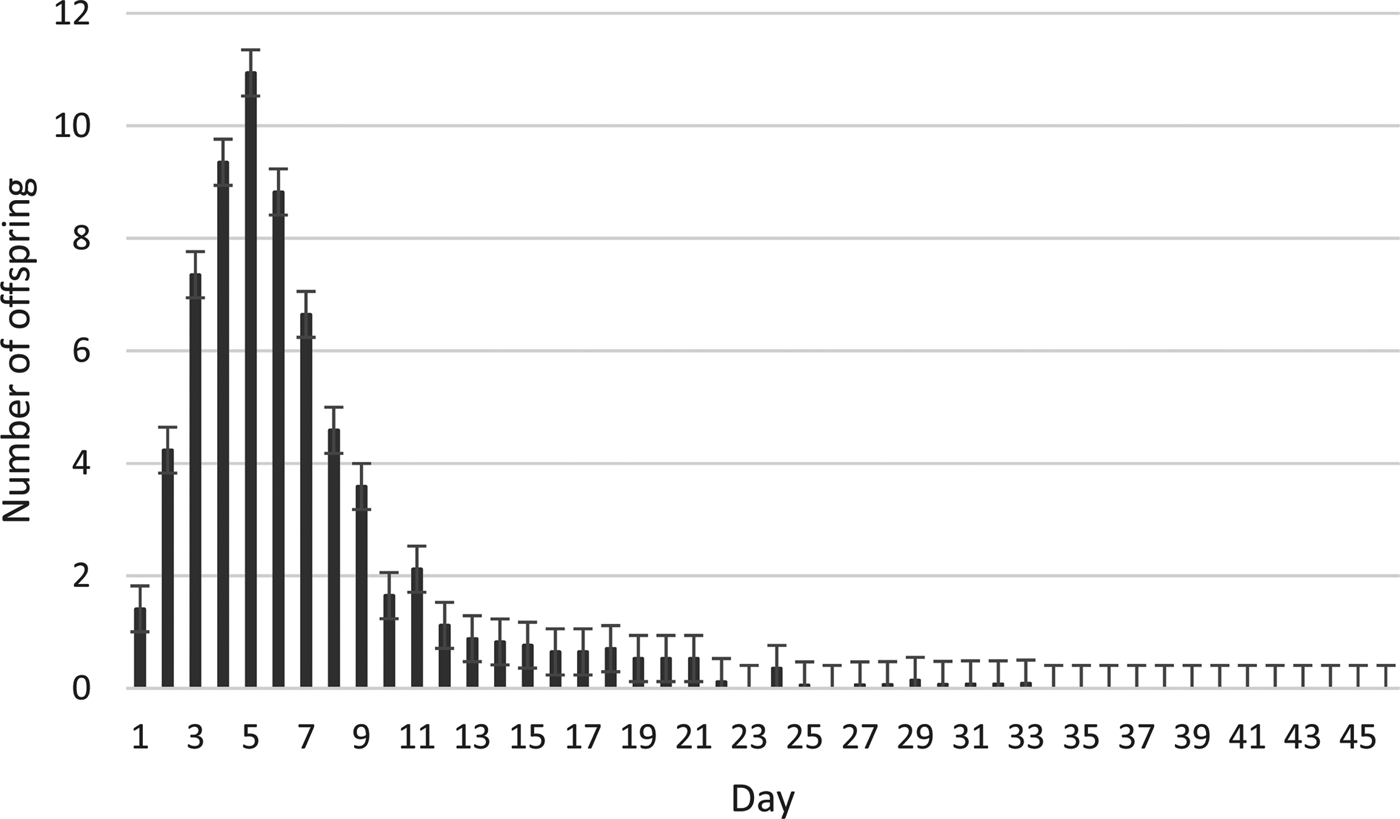
Fig. 1. Daily offspring number (mean ± SE) of Ooencyrthus kuvanae over the lifetime.
Table 1. Results of a GLM analysis of the offspring production ratio of Ooencyrtus kuvanae.

Table 2. Effects of parasitoid age and host number on the offspring production ratio of Ooencyrtus kuvanae (mean ± standard error).
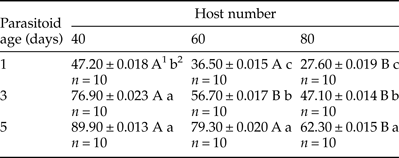
1 Means in each row followed by the same capital letter do not differ statistically.
2 Means in each column followed by the same lowercase letter do not differ statistically.
Table 3. Results of a GLM analysis of the development time of Ooencyrus kuvanae.

Table 4. Variation in development time (mean ± standard error) of Ooencyrtus kuvanae with parasitoid age, host number, and host age.
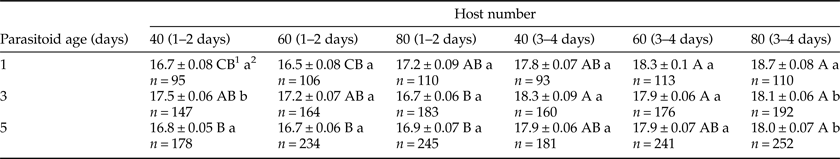
1 Means in each row followed by the same capital letter do not differ statistically.
2 Means in each column followed by the same lowercase letter do not differ statistically.
Discussion
We first determined some of the biological effects of the egg parasitoid O. kuvanae on a new substitute host, P. ricini. Substitute hosts are used to either decrease rearing costs or increase performance in rearing of parasitoids, and a careful selection of substitute hosts is important in biological control programs (Fedde et al., Reference Fedde, Fedde and Drooz1982); nevertheless, the suitability of these alternative hosts can vary greatly for a polyphagous parasitoid (Hoffmann et al., Reference Hoffmann, Ode, Walker, Gardner, van Nouhuys and Shelton2001). Initially, basic biological studies should be performed taking into account various factors (e.g., host age, host number and parasitoid age).
The percentage of offspring that emerged refers to the suitability of the host for insect parasitoid development (El Sharkawy, Reference El Sharkawy2011). In our study, the optimal offspring production rate was obtained from 40 host eggs and 5-day-old females. Our study demonstrates that the age of the host egg has no effect on the parasitoid's offspring production rate. O. kuvanae females were able to parasitize 1–2-day-old and 3–4-day-old eggs of P. ricini. The age of the host is one of the most important factors determining host acceptance by parasitoids (Vinson, Reference Vinson, Kerkut and Gilbert1985). According to Strand (Reference Strand, Waage and Greathead1986) and Vinson (Reference Vinson1998), hymenopter parasitoids can parasitize and develop on all host developmental stages, as they are able to adapt to a range of host conditions. However, the preference for young host eggs is important because the development of parasitoid offspring is influenced by the nutritional quality of the host eggs. Egg parasitoids often choose young or intermediately aged host eggs for parasitism (Reznik & Umarova, Reference Reznik and Umarova1990; Monje et al., Reference Monje, Zebitz and Ohnesorge1999).
Researchers have also found that old host eggs (≥4 days) are less suitable for some Ooencyrtus species, although Ooencyrtus females are able to adapt to any host egg (Nechols et al., Reference Nechols, Tracy and Vogt1989; Takasu & Hirose Reference Takasu and Hirose1993; Hofstetter & Raffa Reference Hofstetter and Raffa1998). Our results are in agreement with those of Zhao et al. (Reference Zhao, Zeng, Xu, Lu and Liang2013), who found that all ages (2, 3, 4, 5, 6 and 7 days old) of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) pupae can be successfully parasitized by the parasitoid Pachycrepoideus vindemmiae (Rondani) (Hymenoptera: Pteromalidae). Zhou et al. (Reference Zhou, Abram, Boivin and Brodeur2014) reported that Telenomus podisi Ashmead (Hymenoptera: Scelionidae) can successfully develop in all ages of Podisus maculiventris (Say) (Hemiptera: Pentatomidae) eggs. Pak et al. (Reference Pak, Buis, Heck and Hermans1986) and Jacob et al. (Reference Jacob, Joder and Batchelor2006) showed that old host eggs do not have any negative effect on parasitoid preference or offspring fitness. On the other hand, King (Reference King1998) demonstrated that the number of offspring decreases with increasing host age (1-, 2-, 3-, 4- and 5-day-old housefly pupae) for the pupal parasitoid Spalangia cameroni (Wiki) (Hymenoptera: Pteromalidae). Da Rocha et al. (Reference Da Rocha, Kolberg, Mendonça and Redaelli2006) observed that offspring emergence of the egg parasitoid Gryon gallardoi (Hymenoptera: Scelionidae) decreases with increasing host age. Furthermore, different ages (1, 2 or 3 days old) of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) eggs affect the percentage emergence of Telenomus remus Nixon (Hymenoptera: Scelionidae) (Peñaflor et al., Reference Peñaflor, De Moraes Sarmento, Da Silva, Werneburg and Bento2012). We conclude that the effects of host age vary according to parasitoid species.
In our study, we observed minimal decreases in development time with increases in the age of P. ricini eggs. Several studies have found that development time varies with host age in many parasitoids, such as Nasonia vitripennis (Walker) (Hymenoptera: Chalcidoidea) (Wylie, Reference Wylie1964), Dinarmus basalis (Rond.) (Hymenoptera: Pteromalidae) (Islam, Reference Islam1994), Brachymeria lasus (Walker) (Hymenoptera: Chalcididae) (Husni et al., Reference Husni, Yooichi and Hiroshi2001) and Diadromus collaris (Hymenoptera: Ichneumonidae) (Wang & Liu, Reference Wang and Liu2002). Peverieri et al. (Reference Peverieri, Furlan, Benassai, Caradonna, Strong and Roversi2013) reported that the development time of the egg parasitoid Gryon pennsylvanicum (Hymenoptera: Platygastridae) is longer in older eggs of Leptoglossus occidentalis Heidemann (Heteroptera, Coreidae), and Da Rocha et al. (Reference Da Rocha, Kolberg, Mendonça and Redaelli2006) had the same result for Gryon gallardoi (Brethes) (Hymenoptera: Scelionidae). Crossman (Reference Crossman1925) and Kamay (Reference Kamay1976) reported that the development time of O. kuvanae was 21 days at 25 °C and 14 days at 30 °C.
In our study, an optimal offspring production rate was obtained using 5-day-old female O. kuvanae. The reproductive strategies of parasitoids range from synovigenic to proovigenic. Characteristics of the female such as age may also affect offspring production, particularly in synovigenic parasitoids. If a female parasitoid is synovigenic, such as O. kuvanae, she is born with immature eggs (this contrasts with the proovigenic strategy, where adult females have a fixed number of oocytes within their ovarioles) and needs to feed as an adult to sustain egg production. Therefore, increased egg production draws on the energy reserves that could be allocated to extending longevity (Francisco, Reference Francisco2001; Mondy et al., Reference Mondy, Corio-Costet, Bodin, Mandon, Vannier and Monge2006; Jervis et al., Reference Jervis, Ellers and Harvey2008). Heimpel et al. (Reference Heimpel, Rosenheim and Kattari1997) reported that synovigenic Aphytis females emerge with a fraction of the eggs that can potentially mature during a lifetime. Ueno & Ueno (Reference Ueno and Ueno2007) showed that the rate of oviposition strongly depends on female age; immature eggs are low in number in the earliest stage of the synovigenic female Itoplectis naranyae Ashmead (Hymenoptera: Ichneumonidae) and subsequently increase with increasing female age.
Adult nutrition can have important effects on the lifetime reproductive success of female parasitoids (Hagen, Reference Hagen, Boethel and Eikenhary1986; Jervis et al., Reference Jervis, Kidd and Heimpel1996). Synovigenic parasitoid species can utilize both host hemolymph and non-host foods such as nectar, honeydew and pollen in natural conditions (Jervis et al., Reference Jervis, Kidd, Fitton, Huddleston and Dawah1993, Reference Jervis, Kidd and Heimpel1996; Heimpel & Collier, Reference Heimpel and Collier1996; Jervis & Kidd, Reference Jervis and Kidd1996, Reference Jervis, Kidd, Hawkins and Cornell1999; Gilbert & Jervis, Reference Gilbert and Jervis1998). Under laboratory conditions, different adult diets such as different sugars or honey increase longevity and egg maturation (Jervis & Kidd, Reference Jervis and Kidd1986; Heimpel & Collier, Reference Heimpel and Collier1996). Carbohydrate sources affect the longevity of adult parasitoids (Jacob & Evans, Reference Jacob and Evans1998). A significantly increased mean lifespan for O. kuvanae (51 days) may be achieved in the presence of honey, and in the absence of honey, parasitoids died within 3.91 days. Different sugars (solutions of glucose, fructose and sucrose), undiluted bee honey and distilled water have been assessed for adult Trichogrammatoidea bactrae Nagaraja (Hymenoptera: Trichogrammatidae) longevity. The longevity of females fed honey is significantly increased (Perera & Hemachandra, Reference Perera and Hemachandra2014). Tunca et al. (Reference Tunca, Gökçek and Özkan2002) evaluated glucose, fructose, sucrose and honey diets for Chelonus oculator Panzer (Hymenoptera: Braconidae) and determined that honey is a better diet for parasitoids than other sugars. Similar observations have been made for Catolaccus grandis (Burks) (Hymenoptera: Pteromalidae) (Ramos & Cate, Reference Ramos and Cate1992) and Venturia canescens (Hymenoptera: Ichneumonidae) (Eliopoulos et al., Reference Eliopoulos, Stathas and Bouras2005). Crossman (Reference Crossman1925) noted a 28–42-day longevity for O. kuvanae females, and the same researcher recorded a longevity of up to 130 days for females and 105 days for males under laboratory conditions. On the other hand, Hérard & Mercadier (Reference Hérard and Mercadier1980) reported the greatest longevity of female and male parasitoids as 57 and 37 days.
In the current study, a mean of 68.88 ± 3.22 adults developed from the eggs laid by a female reared from P. ricini. This value was 68.1 ± 5.0 on Antheraea pernyi Guerin-Meneville (Lepidoptera: Saturniidae) and 32.8 ± 2.8 on L. dispar in a previous study (Wang et al., Reference Wang, Liu, Zhang, Wen and Wei2013). The mean oviposition and mean post-oviposition periods of the female O. kuvanae reared on P. ricini were 22.76 ± 1.37 days and 13.64 ± 1.40 days, respectively. According to Wang et al. (Reference Wang, Liu, Zhang, Wen and Wei2013), the mean oviposition period is 25 ± 1.7 days and 21.9 ± 1.9 days for A. pernyi-reared O. kuvanae females and L. dispar-reared O. kuvanae females. Several factors may influence offspring production in O. kuvanae females. However, more offspring are produced using P. ricini, which is an important finding for L. dispar biological control programs. Usually, it is more advantageous to use substitute hosts for parasitoids; however, Yang et al. (Reference Yang, Achterberg, Choi and Marsh2005) argued that parasitoids are more effective when reared on their original host than on a substitute host. Our results indicate that P. ricini is ideal for rearing O. kuvanae.
Acknowledgements
Great appreciation is extended to Jean Claude Martin (INRA-Unité Expérimentale Forestière Méditerranéenne) for his critical review of the manuscript.







