Introduction
Insects inhabit almost every environment, so they interact with a wide range of pathogens such as viruses, bacteria, fungi, protozoans and nematodes (Mahy, Reference Mahy2004; Pennacchio & Strand, Reference Pennacchio and Strand2006; Vega et al., Reference Vega, Kaya and Tanada2012). Because of the high risk of infection, insects have evolved various mechanisms to keep pathogens from entering their bodies. The first group of mechanisms involves all of the mechanical barriers that prevent pathogens from entering the insect body (Siva-Jothy et al., Reference Siva-Jothy, Moret and Rolff2005; Vega et al., Reference Vega, Kaya and Tanada2012). The second group of mechanisms that protect insects against infections is the immune system, which consists of humoral and cellular immune responses. The humoral response operates through immune-related molecules that are released from immune cells to the hemolymph. Those molecules may be antimicrobial peptides (AMPs) or phenoloxidase (PO) (Lauwers et al., Reference Lauwers, Twyffels, Soin, Wauquier, Kruys and Gueydan2009). Pro-PO is activated by elicitors derived from microbial cell wall components such as peptidoglycan, beta-1,3-glucan and lipopolysaccharide (Marmaras et al., Reference Marmaras, Charalambidis and Zervas1996). The final products of PO activity are melanin and quinones, but during the process of melanization, many intermediate products, which are important during defense against bacterial (both Gram (+) and Gram (−)), fungal, and viral agents, are synthesized (Marmaras et al., Reference Marmaras, Charalambidis and Zervas1996; Tsakas & Marmaras, Reference Tsakas and Marmaras2010; Gonzalez-Santoyo & Cordoba-Aguilar, Reference Gonzalez-Santoyo and Cordoba-Aguilar2012; Muturi et al., Reference Muturi, Blackshear and Montgomery2012). In contrast, cellular immunity is based on hemocyte participation in processes such as phagocytosis, nodulation and the encapsulation of pathogens (Boman & Hultmark, Reference Boman and Hultmark1987; Rolff & Siva-Jothy, Reference Rolff and Siva-Jothy2004; Strand, Reference Strand2008; Charles & Killian, Reference Charles and Killian2015). The first step in the immunological response cascade is the recognition of the pathogens, which leads to the activation of signal transduction pathways such as Toll, Imd or Jak/Stat; this leads to the activation of appropriate processes such as phagocytosis, melanization, encapsulation, nodulation, lysis, virus destruction by RNAi mediation, autophagy or apoptosis (Charles & Killian, Reference Charles and Killian2015; Jupatanakul et al., Reference Jupatanakul, Sim, Angleró-Rodríguez, Souza-Neto, Dasm, Potim, Rossi, Bergren, Vasilakis and Dimopoulos2017). Additionally, changes in hemocytes’ activity can also be observed when environmental conditions, including temperature, food availability or population density are changed (Urbański et al., Reference Urbański, Czarniewska, Baraniak and Rosinski2014).
Yamamarin, also known as the Any-GS or the growth-suppressing pentapeptide, is a peptide (DILRG-NH2) isolated from the silk moth Antheraea yamamai larvae. The yamamarin is known as a diapause regulatory factor in silk moths (Yang et al., Reference Yang, Abe, Zhao, An and Suzuki2004). On semi-isolated hearts of the beetle Tenebrio molitor, yamamarin shows a strong cardioinhibitory effect (Yang et al., Reference Yang, Abe, Zhao, An and Suzuki2004; Kuczer et al., Reference Kuczer, Dziubasik, Midak-Siewirska, Zahorska, Luczak and Konopinska2010). Although its physiological function in insects remains unclear, yamamarin shows some interesting additional properties, such as the suppression of rat hepatoma cell growth (Yang et al., Reference Yang, Abe, Sato, Yamashita, Matsuda, Hamayasu, Imai and Suzuki2007; Kamiya et al., Reference Kamiya, Sato, Yokoyama, Wang, Aizawa, Kumaki, Mizuguchi, Imai, Demura, Suzuki and Kawano2010). Moreover, Sato et al. (Reference Sato, Yang, An, Matsukawa, Ito, Imanishi, Matsuda, Uchiyama, Imai, Ito, Ishida and Suzuki2010) showed that the palmitoyl conjugate yamamarin, C16-Yamamarin (C16-DILRGa), arrested the proliferation of Drosophila S2 cells and decreased the respiration rate of these cells. The strong anti-proliferative activity of yamamarin suggests that this pentapeptide may be involved in cell growth regulation and proliferation, given the fact that it can regulate a number of cells such as hemocytes, which are responsible for insect immunity or processes such as tissue remodeling (for example, during metamorphosis).
Considering the anti-proliferative properties of yamamarin and its involvement in the diapause process, we hypothesize that yamamarin may affects the activity of the insect immune system. Due to the lack of knowledge concerning the physiological function of yamamarin in insects, including its effect on immune system activity, the aim of this study is to investigate how the peptide influences the function of the insects’ immune system by the example of T. molitor, which is generally considered as stored grains pest present all over the world. Furthermore, thanks to its relatively large size and ease of rearing, those insects are often used in biological research. Previous studies, which used the mealworm T. molitor as a model insect demonstrated that heterologous bioassays with T. molitor, are reliable and that yamamarin affects beetles’ physiology (Szymanowska-Dziubasik et al., Reference Szymanowska-Dziubasik, Marciniak, Rosinski and Konopinska2008; Kuczer et al., Reference Kuczer, Dziubasik, Midak-Siewirska, Zahorska, Luczak and Konopinska2010). A better understanding of the physiological function of yamamarin may lead to the commercialized usage of the peptide. The results obtained from tests conducted on T. molitor, may be a starting point for designing, e.g., bioinsecticides, based on yamamarin's chain structure, at a time when insect resistance to conventional insecticides is increasing. Examples of insects chemically modified peptides used in that way are trypsin modulating oostatic factor or allatostatins as bioinsecticides (Borovsky et al., Reference Borovsky, Rabindran, Dawson, Powell, Iannotti, Morris, Shabanowitz, Hunt, DeBondt and DeLoof2006; Chernysh et al., Reference Chernysh, Irina and Irina2012; Chowański et al., Reference Chowański, Kudlewska, Marciniak and Rosiński2014; Chowański et al., Reference Chowański, Adamski, Lubawy, Marciniak, Pacholska-Bogalska, Słocińska, Spochacz, Szymczak, Urbański, Walkowiak-Nowicka and Rosiński2017; Mai et al., Reference Mai, Mauger, Niu, Barnes, Kao, Bergeron, Ling and Tay2017).
Furthermore, knowing how the yamamarin influences the activity of the immune system and taking into account the major problem of rising cancer emergence, which is connected to the actions of the immune system, may indicate that another possible application of the peptide is the creation of anticancer drugs (Tonk et al., Reference Tonk, Vilcinskas and Rahnamaeian2016). Another examples of well-known insects peptides with potential use in medicine like alloferon as an anticancer drug in immunochemotherapy or AMPs as antibactericidal agents. Additionally, a small dose of efficient anticancer drugs and yamamarin's effect on rat hepatoma cells or the hepatocellular carcinoma cell line (dRLh84 cell line) may also be a contributory argument to the conducted studies (Kamiya et al., Reference Kamiya, Sato, Yokoyama, Wang, Aizawa, Kumaki, Mizuguchi, Imai, Demura, Suzuki and Kawano2010).
Materials and methods
Insects
A stock culture of T. molitor L. was maintained at the Department of Animal Physiology and Development, Adam Mickiewicz University in Poznań, as described previously by Rosiński et al. (Reference Rosiński, Wrzeszcz and Obuchowicz1979). To avoid age impact and to reduce fluctuations related to reproductive system development, studies were carried out on 4-day-old adult males, derived from 1-month-old parents, to avoid development disorders in the offspring (Ludwig & Fiore, Reference Ludwig and Fiore1960; Rosiński, Reference Rosiński1995). The control and experimental groups were kept at the same population density in separate boxes. In all bioassays, the tested compound was applied by injection through the ventral membrane, between the thorax and abdomen, using a Hamilton syringe (Hamilton, USA). Before injection, insects were anaesthetized with CO2, disinfected with 70% ethanol and washed with Milli-Q water. In the nodulation bioassay, 4-day-old adult males were used for injection, and after 3 days, nodules were isolated. For each experimental variant n ≥ 8.
Yamamarin synthesis
Yamamarin synthesis was performed manually with a solid phase method according to a standard Fmoc procedure (Chan & White, Reference Chan and White2000). Amino acids were assembled on the MBHA-Rink amide resin. As a coupling reagent, 2(1Hbenzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate in the presence of 1-hydroxybenzotriazole was used. The N-Fmoc (Fmoc, 9-fluorenylmethoxycarbonyl) group was removed with 20% piperidine in N,N-dimethylformamide. The peptide-resin was cleaved from the resin using a mixture of trifluoroacetic acid/water/triisopropylsilane (95 : 2.5 : 2.5) at room temperature for 2 h. The crude compound was dissolved in water, lyophilized, and purified by reversed-phase high-performance liquid chromatography (RP-HPLC). The yamamarin was purified by preparative RP-HPLC on a Varian ProStar column – Tosoh Biosciences ODS-120T C18 (ODS 300 × 21.5 mm). Water-acetonitrile gradients containing 0.1% trifluoroacetic acid (TFA) at a flow rate of 7 ml min−1 were used for purification with UV detection at 210/254 nm. Analytical HPLC was performed using Thermo Separation Products with a Vydac ProteinRP C18 column (4.6 mm × 250 mm) (Grace, Deerfield, IL, USA) and a linear gradient from 0 to 100% B in 60 min, a flow rate 1 ml min−1, solvent A = 0.1% TFA in water, solvent B = 0.1% TFA in 80% acetonitrile/water, and UV detection at 210 nm. The molecular weight of the synthesized compound was confirmed by ESI-MS using an Apex-Qe Ultra 7T FT-ICR instrument (Bruker Daltonic, Bremen, Germany).
For in vivo bioassays, stock solutions of the yamamarin were created in sterile conditions by dissolving in physiological saline (274 mM NaCl, 19 mM KCl, 9 mM CaCl2, pH 7.0), which was also used in the injection for the control group of insects. Insects were injected with solutions at 10−11, 10−7 and 10−3 M concentrations in a volume of 2 µl to obtain final concentrations of 10−13, 10−9 and 10−5 M peptides in insect hemolymphs, which corresponded to doses at 0.5717 × 10−11, 0.5717 × 10−4 and 0.5717 mg ml−1, respectively.
Determination of PO activity
To determine the changes in the humoral response of T. molitor beetles, the level of PO activity, based on a method described previously by Sorrentino et al. (Reference Sorrentino, Small and Govind2002) and Urbański et al. (Reference Urbański, Czarniewska, Baraniak and Rosinski2014), was measured. Briefly, an hour after yamamarin or physiological saline injection samples of hemolymph (1 µl) were collected using ‘end to end’ microcapillaries, by cutting off the tarsus from the foreleg (Drummond, USA). Hemolymph samples were placed on white Whatman no 52 filter paper, which was soaked in 10 mM sodium phosphate buffer (pH = 6.6) containing L-Dopa (L-3,4-dihydroxyphenylalanine; Sigma Aldrich) at a concentration 2 mg ml−1. Next, the preparations were incubated for 30 min at room temperature and were air-dried and scanned using a Canon Lide110 scanner with conditions 600 dpi, 8 bits, and gray scale. For each insect, three separated replications were collected. The obtained images were transformed by color desaturation, and the darkness of the obtained samples, corresponding to PO activity (the darker sample indicated the higher PO activity), was measured. The images were analyzed with ImageJ (ver. 2) computer software, and the results were expressed as a mean pixel values measured in the central part of each sample. The mean value from three measurements (per insect) was calculated.
Evaluation of hemocytes’ ability to phagocytose
The ability of hemocytes to phagocytose was analyzed in vitro using latex beads (Sigma-Aldrich L1030) according to a method described previously by Urbański et al. (Reference Urbański, Czarniewska, Baraniak and Rosinski2014). One hour after yamamarin injection, the samples of hemolymph (1 µl) were collected by ‘end to end’ microcapillaries from the first pair of legs and incubated at room temperature on a microscope slide covered with poly-L-lysine (Sigma-Aldrich P4707) for 30 min in the dark with latex beads suspended (Sigma-Aldrich L1030) in a mixture of physiological saline and anticoagulant buffer (1 : 10 v/v). Next, the hemocytes were washed to remove the remaining beads and stained with DAPI solution for 5 min in the dark. Preparations were observed using a fluorescence microscope (Nikon Eclipse TE 2000-U). The photos were taken with a digital camera (Nikon DS-1QM) and analyzed with ImageJ software (ver. 2). The data were shown as the percentage of phagocytic cells in the total number of hemocytes.
Evaluation of hemocytes’ F-actin cytoskeleton state
The preparations of hemocytes’ F-actin cytoskeleton were prepared and analyzed according to the method described previously by Czarniewska et al. (Reference Czarniewska, Mrówczynska, Kuczer and Rosiński2012). In brief, hemolymph samples were placed on a microscope slide covered with poly-L-lysine and incubated for 90 min at room temperature in a mixture of physiological saline and anticoagulant buffer (1 : 10 v/v). Next, the remaining fluid was removed, and hemocytes were washed three times with physiological saline. Then, the samples were fixed in 3.7% paraformaldehyde in physiological saline and washed with physiological saline containing 0.1% Triton X-100 for 10 min to permeabilize the cell membrane. Afterwards, Oregon Green 488-phalloidin (Invitrogen) was used to stain the F-actin cytoskeleton (20 min in the dark at room temperature). After incubation, samples were washed three times with physiological saline. For nucleus visualization, hemocytes were stained with DAPI solution for 5 min in the dark. Then, hemocytes were washed and mounted with a mounting medium. Hemocyte preparations were examined with a fluorescence microscope Nikon Eclipse TE 2000-U. The changes in hemocytes morphology, such as the ability to create filopodia, F-actin aggregations or nuclei deformations, were examined. The images were taken with a Nikon DS-1QM camera.
Detection of apoptotic cells
The apoptotic cells in the hemolymph were detected using a Sulforhodamine multiCaspase activity kit (SR-VAD-FMK, ENZO Life Sciences). One hour after yamamarin injection, hemocyte preparations were prepared according to the method previously described by Czarniewska et al. (Reference Czarniewska, Mrówczynska, Kuczer and Rosiński2012). Briefly, samples of hemolymph were placed on a microscope slide covered with poly-L-lysine and incubated for 60 min at room temperature. Next, the remaining fluid was removed, and hemocytes were stained with the sulforhodamine-labeled activated caspase inhibitor probe (SR-VAD-FMK, ENZO Life Sciences) in a mixture of physiological saline and anticoagulant buffer (1 : 10, v/v). After 30 min of staining, additional fluid was removed, and preparations were washed three times with physiological saline (5 min each). Next, the samples were fixed in 3.7% paraformaldehyde in physiological saline (15 min) and washed with wash buffer (ENZO Life Sciences). Then, 0.1% Triton X-100 in physiological saline was added for 15 min to permeabilize the cell membrane. Afterwards, Oregon Green 488-phalloidin (Invitrogen) was used to stain the F-actin cytoskeleton (20 min in the dark). After incubation, samples were washed with wash buffer. To visualize the nucleus, hemocytes were stained with DAPI solution for 5 min in the dark and washed with distilled water. Prepared hemocyte preparations were examined with a fluorescence microscope Nikon Eclipse TE 2000-U. The images were taken with a Nikon DS-1QM camera. Based on the images, the percentage of cells with active caspases was calculated.
Nodulation bioassay
To estimate the immunological activity to create nodules, 4-day-old adult males were injected with yamamarin or physiological saline, and after 3 h, they were injected with a formalin-fixed suspension of non-viable Staphylococcus aureus Wood 46 strain (2 µl per insect). Next, the insects were placed in separate boxes for 3 days. After incubation, insects were anaesthetized, and the nodules were isolated using a precision microsurgical technique. First, the insects were decapitated, and the wings, legs and dorsal membrane of the abdomen were removed. All of the tissues, e.g., intestine, Malpighian tubules, fat body, testes and body walls, were analyzed using a stereomicroscope Olympus SZX 12. To estimate nodulation activity, the number of nodules on the dorsal side of the hemocoel was calculated.
Antibacterial activity
To determine the antibacterial activity of the hemolymph components, an assay with bacterial cultures growing on agar plates was used. The samples of hemolymph were diluted in physiological saline (274 mM NaCl, 19 mM KCl, 9 mM CaCl2) containing anticoagulant buffer (4.5 mM citric acid and 9 mM sodium citrate, 5 : 1 v/v) in ratio 1 : 2 (v/v). The agar plates were prepared as follows: 200 µl of PBS washed Micrococcus luteus (ATCC No. 4698, Sigma) in suspension at OD600 ~ 0.6 was spread over the surface of the agar plates (Trypticasein Soy LAB-AGAR Biocorp) to create a bacterial lawn; in the prepared agar plates, 6–8 wells were made using a sterile cork borer; and in each well, 20 µl of diluted hemolymph was added, and the plates were incubated for 24 h at 30°C. To determine the changes in antibacterial activity of the hemolymph, we measured the diameter of the clear zones formed around each well, in increments of 1 mm, using calipers.
Statistical analysis
For the statistical analysis of the obtained data, we used Graph Pad Prism (version 5) software 191 (Department of Animal Physiology and Development AMU license). Before performing this analysis, we used the Shapiro–Wilk test to assay the normality of the distribution. To check for the homogeneity of variance, Levene's test was used. For statistical comparison of the groups with normal distribution, one-way ANOVA was used, and for nonparametric data, the Kruskal–Wallis test was used. In addition Student's t-test and, for nonparametric data, Mann–Whitney U test were used. The data shown are the mean value of the parameter ±SD.
Results
PO activity
PO activity was measured 1 h after yamamarin injection, and in each concentration, PO activity did not differ compared with the control insects injected with saline.
In the long-term response, 24 h after injection, changes in PO activity were observed at P = 0.05 (Kruskal–Wallis test; H = 12.74; df = 61; P = 0.0052). At a low concentration (10−13 M), yamamarin significantly decreased PO activity (Student's t-test; t = 2.045; df = 30; P < 0.05), whereas at the highest tested concentration (10−5 M), yamamarin caused a significant increase in PO activity (Mann–Whitney test; U = 82.00; df = 32; P < 0.05). No significant changes between PO activity from insects injected with saline and peptide at the 10−9 M concentration were observed (Student's t-test; t = 0.338; df = 26; P = 0.738) (fig. 1).

Fig. 1. The activity of phenoloxidase (PO) in the hemolymph of 7-day-old adult T. molitor beetles 1 and 24 h after peptide injection at concentrations 10−13, 10−9, and 10−5 M. Control insects were injected with saline. PO activity is expressed as the mean (±SD) pixel value. An asterisk means statistically significant changes at P = 0.05 (one-way ANOVA; F = 0.9110; P = 0.4403).
Hemocyte cytoskeleton bioassay
The control hemocytes presented adequate adhesion ability, a well-developed cytoskeleton and long filopodia (fig. 2). The injection of yamamarin at concentrations 10−13 and 10−5 M did not change the properties of hemocytes significantly, and isolated hemocytes possessed well-developed cytoskeletons and filopodia. No symptoms of F-actin aggregation were observed. At concentration 10−9 M, yamamarin caused the aggregation of F-actin, which was observed as clusters of F-actin. The cytoskeleton was well developed, but no filopodia were observed, so the ability of hemocytes to adhesion was reduced. An elongated time of adhesion on poly-lysine did not significantly change hemocytes properties.
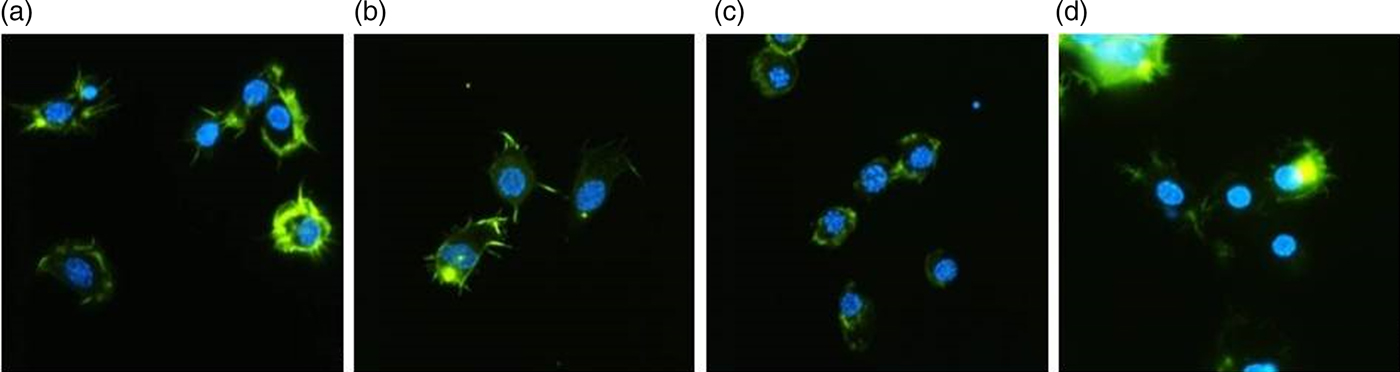
Fig. 2. Representative images of the hemocytes of adult T. molitor obtained by fluorescence microscopy. Hemocytes were stained with DAPI to reveal nuclei (blue) and with Oregon Green®488-phalloidin (Invitrogen) to show F-actin cytoskeletons (green). The scale is 10 µm. (a) Hemocytes of the control insects (injected with saline). (b, c, d) Hemocytes isolated from insects treated with yamamarin at concentrations of 10−13, 10−9, and 10−5 M, respectively. Hemocyte preparations were examined by fluorescence microscopy (Nikon Eclipse TE 2000-U), and the images were taken with a Nikon DS-1QM camera.
Hemocytes phagocytic activity
The phagocytic activity of hemocytes differed after yamamarin injection compared with the control (Kruskal–Wallis test; H = 17.95; df = 48; P = 0.004) (fig. 3). The number of phagocytic cells increased after an injection of yamamarin at each concentration compared with the control samples. Statistically significant changes in phagocytic activity were observed after an injection of the yamamarin at all applied concentrations (Mann–Whitney test; for 10−13 M U = 13.50; df = 19; P = 0.0058; for 10−9 M U = 8.00; df = 23; P = 0.009; for 10−5 M U = 21.5; df = 23; P = 0.0072).

Fig. 3. The phagocytic activity of hemocytes isolated from 4-day-old adult T. molitor beetles injected with saline (control) and yamamarin at concentrations of 10−13, 10−9, and 10−5 M. The data are expressed as the percentage of phagocytic cells after incubation with bacteria. Values are given as the mean ± SD. An asterisk means statistically significant changes (Kruskal–Wallis test; H = 17,95; P = 0.0004).
Pro-apoptotic properties of yamamarin
In the in vivo bioassay on adult beetle T. molitor, we investigated the pro-apoptotic activity of yamamarin (fig. 4). Apoptosis is programmed cell death that is characterized by in changes in cells morphology that results in ultrastructural changes in the F-actin cytoskeleton, filopodia shrinkage, and nucleus degranulation as well as the appearance of the apoptotic bodies and the presence of activated caspases (Vaidyanathan & Scott, Reference Vaidyanathan and Scott2006). In preparation for hemocytes obtained from insects treated with yamamarin, all of the above mentioned symptoms of apoptosis were observed (fig. 5). An injection of yamamarin, at concentrations 10−9 and 10−5 M, significantly increased the number of apoptotic cells (cells with activated caspases) (Mann–Whitney test; for 10−9 M U = 25.00; df = 38; P < 0.0001; for 10−5 M U = 138.00; df = 38; P < 0.05) (fig. 4). In insects treated with the lowest concentration (10−13 M), the number of apoptotic cells did not significantly differ from the control and was maintained at 5% (Mann–Whitney test; U = 172.00; df = 38; P = 0.196). After an application of yamamarin at concentration 10−9 M, the population of apoptotic cells increased up to 67.55%. No apoptotic changes were observed in the control samples.

Fig. 4. The pro-apoptotic activity of yamamarin at concentrations of 10−13, 10−9, and 10−5 M in hemocytes of 4-day-old adult beetles. Values are presented as the mean ± SD. An asterisk means statistically significant changes (Kruskal–Wallis test; H = 39.26; P < 0.0001).
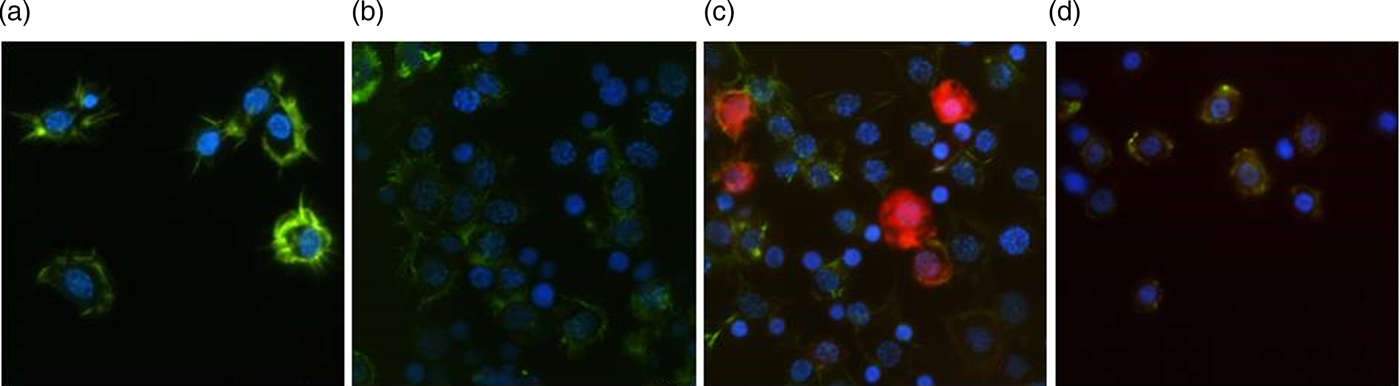
Fig. 5. Representative images showing induced apoptosis in hemocytes obtained from 4-day-old adults of Tenebrio molitor beetles. (a) Hemocytes from the control insects injected with saline, (b, c, and d) hemocytes obtained from insects injected with yamamarin at concentrations of 10−13, 10−9, and 10−5 M, respectively. Hemocytes were stained with DAPI to reveal nuclei (blue) and with Green®488 Phalloidin (Invitrogen) to show F-actin cytoskeletons (green). The active caspases were detected by SR-VAD-FMK reagent (red). The scale is 10 µm.
Influence of yamamarin on the nodulation process
The nodulation activity of the hemocytes (fig. 7) varied significantly between the group of control insects and the group of insects injected with the yamamarin (Kruskal–Wallis test; H = 10.68; df = 33; P = 0.0136) (fig. 6). In the control, the average number of nodules was 6. In beetles treated with yamamarin, the nodulation activity of hemocytes at all concentrations decreased equally. At concentrations of 10−13 and 10−9 M, changes were statistically significant (Student's t-test; for 10−13 M t = 8.50; df = 17; P < 0.01; for 10−9 M t = 6.00; df = 15; P < 0.01). The lowest intensity of nodulation was observed at a concentration of 10−13 M where the mean number of nodules was 1.7 per insect. A similar level of inhibition of nodule formation was observed in insects injected with yamamarin at a concentration of 10−9 M. Interestingly, the highest tested concentration of yamamarin (10−5 M) caused the lowest decrease in the number of nodules (mean value: 2.7 per insect) compared with the control. The results obtained at this concentration were not statistically (Mann–Whitney test, U = 20.50; df = 17; P < 0.05).

Fig. 6. The nodulation activity of hemocytes in 7-day-old adult T. molitor beetles. The data are expressed as the average number (±SD) of nodules observed in the insect body 3 days after injection with yamamarin. An asterisk means statistically significant changes (Kruskal–Wallis test; H = 10.68; P = 0.0136).
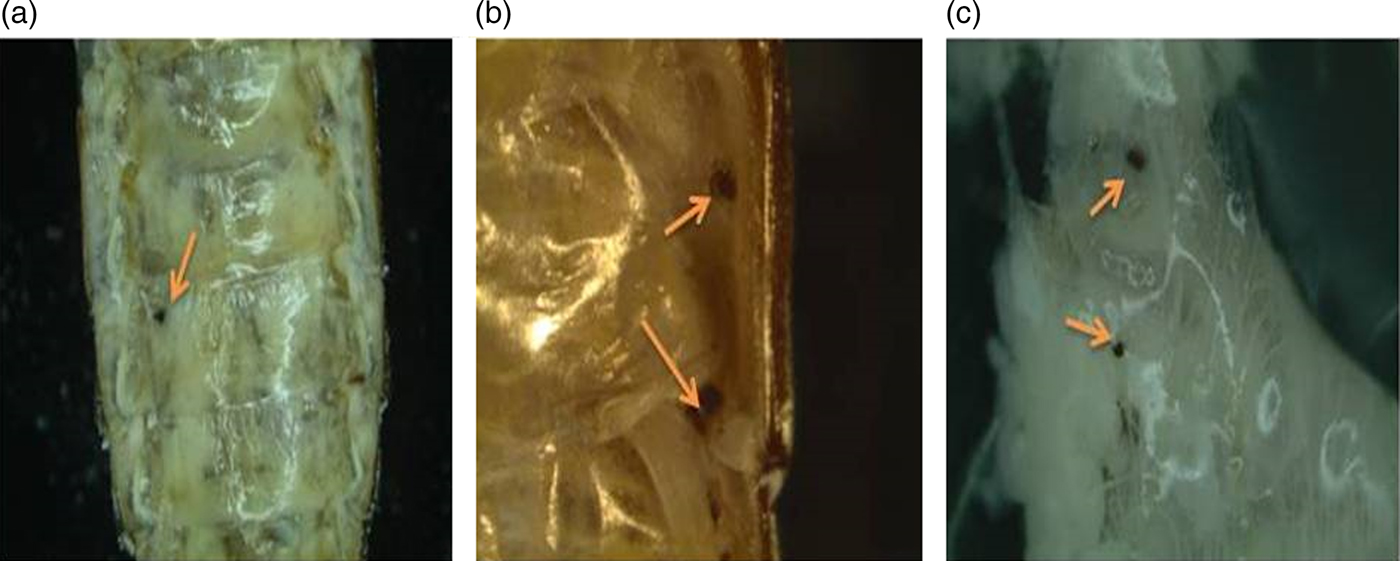
Fig. 7. Sampled photos of T. molitor hemocoel and the inner part of the abdomen taken 3 days after injection. (a) Hemocoel after saline injection. (b, c) Hemocoel and the inner part of the abdomen after yamamarin injection. Yellow arrows show examples of nodules.
Antibacterial activity
In the humoral response against bacteria, proteins, polypeptides and antibacterial agents such as lysozyme, AMPs, polyphenol oxidase, quinones and melanin are involved. The obtained data presented an effect of yamamarin on the antibacterial activity of substances occurring in the hemolymph against Gram(+) bacteria Micrococcus luteus. Based on the diameter of the clear zones in the hemolymph collected 24 h after saline or yamamarin injection, the 10−3 M concentration displayed moderate antibacterial activity. The second set of injections made with additional non-viable bacterial cells (S. aureus) to invoke hypersynthesis of lysozyme in the hemolymph, as expected, resulted in an increased size of the clear zones. Nevertheless, in both cases, no significant effects of yamamarin on the antimicrobial properties of hemolymph were observed (fig. 8).

Fig. 8. Antibacterial activity of hemolymph in the control and yamamarin injected T. molitor beetles. Data are expressed as the mean (±SD) diameter value of the clear zone formed by antimicrobial components of the hemolymph on agar plates covered with bacteria.
Discussion
The current studies regarding yamamarin do not provide much information about the potential role of this peptide in insects. It was shown that this short, five amino-acid peptide possesses cardioinhibitory properties in T. molitor, which was shown by Szymanowska-Dziubasik et al. (Reference Szymanowska-Dziubasik, Marciniak, Rosinski and Konopinska2008). Additionally, yamamarin analogues, e.g., H-[Gln1]-, [Gly1]–[Lys1]–Asp–Ile–Leu–Arg–Gly–NH2 or H–Glp–Ile–Leu–Arg–Gly–NH2, after an application on the myocardium in in vitro conditions, caused a decrease in heart contraction frequency (Szymanowska-Dziubasik et al., Reference Szymanowska-Dziubasik, Marciniak, Rosinski and Konopinska2008). The tested pentapeptide can cause reversible cell-cycle arrest of the Drosophila S2 cell line, and it was found that yamamarin could suppress mitochondrial respiration (Sato et al., Reference Sato, Yang, An, Matsukawa, Ito, Imanishi, Matsuda, Uchiyama, Imai, Ito, Ishida and Suzuki2010). The available literature states that during the diapause process, the metabolism rate is definitely lower, which confirms that yamamarin can be a diapause regulatory factor. Studies with similar results with growth arrest caused by yamamarin were conducted on a murine leukemic cell line where the human gene Bcr/Abl was expressed (Sato et al., Reference Sato, Yang, An, Matsukawa, Ito, Imanishi, Matsuda, Uchiyama, Imai, Ito, Ishida and Suzuki2010). Despite the conducted studies, the physiological function of yamamarin remains unknown. Interestingly, despite the mention results, we found no significant similarities between yamamarin and sequences deposit in public databases using UniProt Peptide Search, except Antheraea jamamai.
As we show above, yamamarin causes specific changes to immune system activity in the mealworm T. molitor. In relation to the humoral response, which operates through immune-related molecules such as PO, no short-term changes of its activity were observed. However, this peptide changed the humoral response, so the activity of PO, of the immunological system after 24 h of incubation. On the short-term, the tested compound changed a few aspects of the cellular response. Yamamarin increased the number of phagocytic cells in the hemolymph, but on the other hand, we observed a decreased nodulation activity of the hemocytes. Nodulation, when considered in a quantitative way, seems to be one of the most important defense mechanism against pathogens such as bacteria, fungi or even viruses (Satyavathi et al., Reference Satyavathi, Minz and Nagaraju2014). The obtained results are especially interesting in comparison with the results obtained by Goldsworthy et al. (Reference Goldsworthy, Mullen, Opoku-Ware and Chandrakant2003) conducted on Locusta migratoria, who proposed that nodule formation involve a localized PO that leads to the melanization of the nodules. However, the observation of the decreased number of nodules in our research was similar to the results obtained by Goldsworthy et al. (Reference Goldsworthy, Mullen, Opoku-Ware and Chandrakant2003), where they tested the immunosuppression activity of dexamethasone, a type of steroid medication that can be used as an immunosuppressant. This relation between the number of occurring nodules and the activity of PO could be explained by the data obtained by Mavrouli et al. (Reference Mavrouli, Tsakas, Theodorou, Lampropoulou and Marmaras2005), which showed that pro-PO is involved in process of phagocytosis. Moreover, Urbański et al. (Reference Urbański, Czarniewska, Baraniak and Rosinski2014) suggested that the higher ability of hemocytes to conduct phagocytosis might be correlated with a higher level of PO activity, which occurred in our studies after the injection of peptide in the higher concentration 10−5 M. We also suggest that the lower level of nodulation activity of those cells 3 days after yamamarin injection is compensated by the higher activity of hemocytes to phagocytosis in a short-term response as an enabled primary defense when pathogens occur. The higher level of PO activity after 24 h may also be an effect of supervene apoptosis. The occurrence of PO in the hemolymph may be caused by releasing enzymes from disintegrated, apoptotic hemocytes where pro-PO is stored. On the other hand, the obtained results may differ from typical immunotropic results, because of usage synthetic beads imitated pathogens to cause phagocytosis and ‘real bacterial pathogens’ to bring up a process of nodulation.
We also showed that the tested peptide caused a heightened portion of hemocytes in apoptotic processes, which can lead e.g. to chill coma activation, so to the process of reversible cessation of movements. Additionally, the formation of F-actin aggregates at a concentration of 10−9 M, which was observed during the evaluation of hemocyte morphology, can also be an indicator of apoptosis; likewise, in research with yamamarin analogue, C16-yamamarin, the addition of palmitic acid was added to increase the ability of the peptide to penetrate through the cell membrane. It was found that this analogue possesses inhibitory properties, causing cell proliferation and mitochondrial respiration arrest (Sato et al., Reference Sato, Yang, An, Matsukawa, Ito, Imanishi, Matsuda, Uchiyama, Imai, Ito, Ishida and Suzuki2010). In research conducted previously by Yang et al. (Reference Yang, Abe, Zhao, An and Suzuki2004), the authors noted that the activation of apoptosis in cells might cause metabolic arrest, which could result in cell death.
In contrast, yamamarin caused no significant changes in the morphology of hemocytes and their cytoskeletons. Both F-actin cytoskeletons and filopodia were well developed after peptide injections at concentrations of 10−13 and 10−5 M. So far, there is no explanatory data of yamamarin's impact on hemocytes. Owing to that fact, the impact of yamamarin on hemocyte morphology remains to be elucidated, and further investigations are required. Similar features of hemocytes can be observed in e.g. cold induced diapauses, and could be explained as competency of hemocytes to adhere to tissues in order to secure tissues against damages.
Based on all obtained data, we hypothesized that yamamarin can be an important controller of immune system function or could probably play a role as a diapause/chill coma controlling factor in groups of diapausing insects. Unfortunately, there seems to be a nonlinear relationship between the tested concentrations of yamamarin and the response of the immune system, but in the case of the phagocytic activity of cells and apoptosis, promising results were obtained after the injection of peptide at concentration 10−9 M. Although, we need to mention that the research is at a very early stage and needs further examination. Further tests needs to be conducted also to find out if the peptide modulates the immune system because it is recognized as a foreign substance, and as fact it would cause toxic effect, or if the peptide modulate the immune system because it is part of endogenous pathways that mediate immune responses.
Conclusions
Research regarding the influence of yamamarin on the function of insect immune system activity was conducted for the first time. The obtained results of our study demonstrated that yamamarin effects of both cellular (firmly) and humoral (moderately) responses, measured using heterologous bioassays of the beetle T. molitor. Yamamarin caused changes in the level of PO and phagocytosis and the nodulation activity of hemocytes, as well as their morphology and apoptosis. The peptide, yamamarin, exerts pleiotropic action in the regulation of immune system function. The obtained data suggest that yamamarin might be involved in the immune system activity in insects.
Acknowledgements
This research was supported by the National Science Centre, Grant No. 2015/19/N/NZ4/00729, to K. Walkowiak-Nowicka.
The authors declare that there is no conflict of interest regarding the publication of this paper.











