Introduction
Competition may be either direct, when damage to a plant by one herbivore species deprives a second species of that resource, or indirect, when it is plant-mediated (i.e. induced defences) (Ohgushi, Reference Ohgushi2005; van Veen et al., Reference van Veen, Morris and Godfray2006; Kaplan & Denno, Reference Kaplan and Denno2007). Competition appears most likely when herbivores are closely related, sessile and feed on discrete resources (Denno et al., Reference Denno, McClure and Ott1995), but even herbivores feeding on distant portions of the plant may compete (e.g. foliar and root feeders: Bezemer et al., Reference Bezemer, Wagenaar, van Dam and Wäckers2003; Blossey & Hunt-Joshi, Reference Blossey and Hunt-Joshi2003). Plants are assemblages of heterogeneous tissue types and herbivores often specialize on certain plant parts. Accordingly, phloem feeders, leaf chewers, stem borers, seed predators, among others, exploit different resources but rarely consume the whole plant. Morphological or chemical changes in plants in response to damage caused by a guild of herbivores can increase or decrease the attractiveness of the plant to other guilds (Blossey & Hunt-Joshi, Reference Blossey and Hunt-Joshi2003; Jones & Russell, Reference Jones and Russell2009).
Temporal or spatial segregation of consumers (Denno et al., Reference Denno, McClure and Ott1995) or fine-scale resource partitioning (Kagata & Ohgushi, Reference Kagata and Ohgushi2001) are common mechanisms of coexistence in phytophagous insects, and several guilds of herbivores may coexist on the same resource by effectively avoiding each other (Daugherty, Reference Daugherty2009).
Furthermore, local interactions can occur between herbivores belonging to different guilds that are feeding on the same structure of the plant (e.g. leaf). Physical and chemical alteration of leaf tissues caused by a guild may interfere with resource acquisition by another guild (Karban, Reference Karban1986; van Zandt & Agrawal, Reference van Zandt and Agrawal2004; Lynch et al., Reference Lynch, Kaplan, Dively and Denno2006; Kaplan & Denno, Reference Kaplan and Denno2007). Local interactions can also change the spatial distribution of different populations on a small scale (Faeth, Reference Faeth1986; Kagata & Ohgushi, Reference Kagata and Ohgushi2001) and affect performance when space is co-occupied (Oghushi, Reference Ohgushi2005, Reference Ohgushi2008).
The main herbivorous arthropods that feed on the leaves of strawberry (Fragaria X ananassa Duchesne) are the two-spotted spider mite (TSSM), Tetranychus urticae Koch (Acari: Tetranychidae), and several species of aphids, including Aphis gossypii Glover, A. fabae Scopoli, Myzus persicae (Sulzer), Macrosiphum euphorbiae Thomas and Chaetosiphon fragaefolii (Cockerell) (Cédola & Greco, Reference Cédola and Greco2010). TSSM are mesophyll feeders that penetrate epidermal cells and ingest cell contents (Kielkiewicz & van de Vrie, Reference Kielkiewicz and van de Vrie1983; Kielkiewicz, Reference Kielkiewicz1985). Aphids are mobile feeders that suck mainly from the sap flow in phloem sieve elements. Aphids inject watery and solid saliva that remains in leaf tissues after feeding (Dixon, Reference Dixon1998; Powell et al., Reference Powell, Tosh and Hardie2005). The solid saliva deposit on the leaf surface called ‘flange-salival’ is continued within a tubular structure, the ‘stylet-sheath’, that represents the pathways of stylets into leaf tissues (Miles, Reference Miles1972; Tjallingii, Reference Tjallingii1978; Peeters et al., Reference Peeters, Gordon and Read2007). Aphids cause direct feeding damage because of their toxic saliva (van Emden et al., Reference van Emden, Eastop, Hughes and Way1969; Hill, Reference Hill1983; Gharidi, Reference Gharidi2002) and indirect damage from their sugary sticky excreta, the honeydew. Honeydew may decrease photosynthesis, induce senescence (Bardner & Fletcher, Reference Bardner and Fletcher1974) and favour the growth of saprophytic fungi that also may negatively affect photosynthesis (Vereijken, Reference Vereijken1979).
Adult TSSM females reach potential host plants either by random walking or by passive wind dispersal; hence, if by wind, the probability of finding and colonizing new resources depends in part on the host-plant range and the layout of the crop (Kennedy & Smitley, Reference Kennedy, Smitley, Helle and Sabelis1985). The dispersal of aphids occurs because winged adults are attracted to visual and chemical clues (Powell et al., Reference Powell, Tosh and Hardie2005). On a smaller scale, both TSSM and aphids may increase emigration rates from unsuitable leaflets by changing the patterns of trivial movements (Kareiva, Reference Kareiva and Kogan1986; Andow, Reference Andow1991). When mites have arrived at a leaflet, they can move to other leaflets or to the other surface of the same leaflet if there is injury to the leaf tissue (e.g. injury caused by the feeding of aphids). In turn, aphids may express similar behaviour in response to tissue damage caused by mites.
Although both herbivorous guilds can coexist on the same plant and attain high abundances (Cédola & Greco, Reference Cédola and Greco2010; Greco et al., Reference Greco, Liljesthröm, Ottaviano, Cluigt, Cingolani, Zembo and Sánchez2011), a large number of both species on the same leaflet is uncommon (N.M. Greco, personal observation). Our hypothesis is that there exist negative interactions between TSSM and aphids in strawberries. Leaf tissue injury caused by TSSM feeding could negatively affect aphid feeding, and aphid honeydew may interfere with TSSM feeding and oviposition. If negative interactions influence the spatial distribution and pattern of co-occurrence of TSSM and aphids, we expect to find: (i) a relationship between the spatial co-occurrence at leaflet level and the density and dispersion of each species; (ii) low numbers of individuals of both species coinciding on the same leaflet; (iii) lower population growth rates of both herbivores when together on leaflets; (iv) preference of TSSM for leaflets without honeydew; (v) a higher number of eggs on leaflets without honeydew; (vi) preference of aphids for leaflets without TSSM feeding damage; and (vii) higher numbers of offspring on leaflets with TSSM feeding damage.
The purpose of this study was to determine if there is negative interaction between TSSM and aphids, and whether the interaction is mediated by feeding damage and the presence of honeydew.
Materials and methods
Spatial coincidence
The populations of TSSM and aphids, species mentioned above, were sampled once or twice per month in greenhouses (GH) and from open-field (crop with plastic covered tunnels, CCT) commercial strawberry crops (Aromas) located in La Plata, Province of Buenos Aires, Argentina (38°52'S, 57°59'W). Irrigation and soil management were standard for the region. Granulated fertilizer composed of total nitrogen (N) 15.0%, (ammoniacal nitrogen 8.89%, nitrate nitrogen 6.11%), assimilable phosphorus (P2O5) 15.0% and water soluble potassium (K2O) 15.0% was applied to the soil 20 days before planting. Methyl bromide (Bromopic®, 70% methyl bromide + 30% chloropicrin, Brometan SRL, Burzaco, Argentina, on 12 fields, and Vendaval Fumigante 51®, metam sodium 51%, Síntesis Química SAIC, Buenos Aires, Argentina, on 17 fields) was used to fumigate the soil. The beds were covered with black polyethylene mulch Coverfilm® and irrigated by drip. In all fields, fungicide Benosem 50 PM® (benomyl 50%) was used weekly and acaricide New Mectin® (abamectin 1.8%, Agrimarketing S.A., Buenos Aires, Argentina) was applied when T. urticae reached the economic threshold level (50 active mites per leaflet: Wyman et al., Reference Wyman, Oatman and Voth1979). Insecticides were not applied because densities of other pests, such as thrips, whiteflies and aphids were low. Samplings were performed on 29 strawberry fields. Each field was approx. 1200 m2 (20 beds of 0.70 m width and 50 m length, and 0.5 m between beds). Systematic sampling (Greco et al., Reference Greco, Tetzlaff and Liljesthröm2004) was conducted from May 2006 to January 2007 (17 fields: 6 GH and 11 CCT) and from September 2008 to January 2009 (12 CCT fields). An expanded leaflet was randomly taken from a plant at intervals of 10 m until the end of each row. The number of leaflets per sample varied from 90 to 115. Each sample was put in a plastic bag and the number of TSSM (active mites: larvae, nymphs and adults) and aphids (nymphs and adult females, without discriminating among species) per leaflet, on the abaxial surface, was counted in the laboratory using a binocular microscope (NIKON SMZ 645, 20×).
When both populations were in the same field at the same time, the spatial coincidence at leaflet level was estimated using the indices proposed by Griffiths (Reference Griffiths1969):
where IcTSSM is the spatial co-occurrence of TSSM and aphids, TSSMAi is the number of TSSM that occurred with at least one aphid on the ith leaflet, and TSSMi is the number of TSSM on the ith leaflet; n is the number of leaflets in the sample, and
where IcA is the spatial co-occurrence between aphids (A) and TSSM, ATSSMi is the number of aphids that occurred with at least one TSSM on the ith leaflet, and Ai is the number of aphids on the ith leaflet.
Generalized linear/nonlinear models (Lindsey, Reference Lindsey1997) were used to examine the relationship between spatial coincidence indices and TSSM density, aphid density, the percentages of TSSM and aphid-infested leaflets. Time periods (2006–2007 and 2008–2009), the time of sampling (month) and type of crop cover (plastic covered greenhouses or plastic low tunnels) were added as categorical variables to incorporate the structure of sampling in analyses of coincidence. The statistical significance of each variable was tested in turn in the model by a forward step-wise procedure, and those that contributed to the most significant change in deviance from the null model were retained. The change in the deviance was tested using the log-likelihood ratio test considering a Chi-square distribution with a significance level of 0.05. The Wald statistic was used to test the significance of the regression coefficient (Lindsey, Reference Lindsey1997; Fox, Reference Fox2008). Preliminary analyses were performed in order to facilitate pooling of data. Effects of time period, time of sampling and type of crop cover on coincidence indices were analysed by ANOVA. Before analysis, data were checked for normality (normal probability plots) and homogeneity of variance (Levene test). Kruskal-Wallis test was used when the assumptions for an analysis of variance were not met (Zar, Reference Zar1996).
Growth rate of T. urticae and C. fragaefolii (singly and in combination)
Colonies of TSSM and aphids were reared on strawberry leaves in the laboratory under controlled conditions. All experiments were conducted at 24 ± 1 °C, 60–70% RH and 14:10 light:dark. The experimental unit was a new and expanded strawberry leaflet. The petiole of each leaflet was placed in a water-filled tube that was stored in a plastic container covered with plastic film to prevent the escape of individuals. To carry out the experiments, we selected C. fragaefolii because this was the most abundant aphid species during the survey period (Cédola & Greco, Reference Cédola and Greco2010). The following abundances of TSSM and aphids were assayed: 20:0–10:10–0:20. Two-day-old adult females of TSSM and C. fragaefolii were put on the underside of the leaflets with a fine brush. Each treatment was replicated 20 times and the experimental time was seven days. The intrinsic rate, r, of growth of each species was calculated as:
where N 0 = number of individuals at the beginning of the experiment; N t = number of individuals at the end of the experiment and t = seven days. In addition, we recorded the number of individuals of each species per side of leaflet (adaxial or abaxial) once at the end of the seven-day trial in all treatments. Data were analysed by ANOVA, and the Kruskal-Wallis test was used when variances were heteroscedastic (Levene test).
Preference tests
The experimental unit was a Petri dish (10 cm in diameter) with strawberry leaf discs (1.8 cm in diameter) placed on moistened filter paper. Choice and no-choice experiments were performed with 15 replicates for all conditions. In choice experiments, five TSSM females (48–72-h-old taken from pure cohort) were placed between two discs: one with honeydew on the abaxial side (selected from a strawberry leaf previously infested with approximately 15 females of C. fragaefolii for four days, and then removed) and the other from a similarly-aged leaf without honeydew. The disc acceptance was measured by counting females that settled on each test disc after 48 h and the number of eggs per female. Similarly, five adult aphids (48-h-old taken from pure cohort) were placed between two discs: one selected from a strawberry leaf infested with approximately 25 active TSSM for four days and then removed, and the other without TSSM damage. The disc acceptance was measured by counting aphids that settled on each test disc after 48 h and the number of offspring per female. The proportion of TSSM and C. fragaefolii, and the number of eggs or offspring per female on each kind of disc, were analysed by the Kruskal-Wallis test.
We also performed for TSSM and C. fragaefolii a no-choice test (as control) with each kind of disc (+honeydew, –honeydew, +TSSM damage, –TSSM damage, respectively). The frequency of individuals on each kind of disc and the position on different places of the experimental unit (on abaxial surface or the adaxial surface or on any other place in the Petri dish) were analysed by a 2 (kind of disc) × 3 (different places of the experimental unit) contingency table. Chi-square statistic was used to determine the significance of departure from expected in the contingency table (Zar, Reference Zar1996). The number of eggs and offspring per female of TSSM and C. fragaefolii, respectively, were recorded and analysed by one-way ANOVA.
Results
Spatial coincidence
Populations of TSSM and aphids were recorded together in 82.75% (23/29) of sampled fields. Considering all samplings performed during 2006–2007 (N = 338) and 2008–2009 (N = 50), field coincidence was 29% (98/338) and 98% (49/50), respectively. Coincidence at the leaflet level occurred in 26.53% (26/98) and 87.75% (43/49) of cases in 2006–2007 and 2008–2009, respectively. Preliminary analyses of data indicated that ICTSSM was similar between sampling periods (Kruskal-Wallis test: H(1,N = 70) = 0.28, P = 0.597), between times of sampling in both sampling periods (2006–2007: Kruskal-Wallis test: H(5,N = 26) = 7.94, P = 0.160) (2008–2009: Levene test: F = 1.26, df = 3, 40, P = 0.301; ANOVA: F = 1.42, df = 3, 40, P = 0.250) and between types of crop cover (Kruskal-Wallis test: H(1,N = 70) = 0.58, P = 0.750). ICA was also similar between sampling periods (Levene test: F = 0.71, df = 1, 68, P = 0.402; ANOVA: F = 3.51, df = 1, 68, P = 0.065, log transformation of variable), times of sampling in both periods (2006–2007: Kruskal-Wallis test: H(5,N = 26) = 6.53, P = 0.258) (2008–2009: Levene test: F = 1.87, df = 3, 40, P = 0.150; ANOVA: F = 0.65, df = 3, 40, P = 0.589) and between types of crop cover (Kruskal-Wallis test: H(1,N = 70) = 4.48, P = 0.108).
The TSSM spatial coincidence index was affected by the percentage of TSSM-infested leaflets, the percentage of aphid-infested leaflets and aphid density (fig. 1a–c). The model that included the most significant variable, the percentage of TSSM-infested leaflets, explained 65.09% (deviance change was 13.76 out of 21.14) of the variation in the TSSM spatial coincidence index (table 1). The proportion of TSSM that coincided with at least one aphid decreased as the percentage of leaflets with TSSM increased.

Fig. 1. Spatial coincidence indices: ICTSSM (the spatial coincidence between TSSM and aphids) and IcA (the spatial coincidence between aphids and TSSM). (a) Relationship between ICTSSM and percentage of TSSM-infested leaflets; (b) relationship between ICTSSM and percentage of aphid-infested leaflets; (c) relationship between ICTSSM and aphids density; (d) relationship between ICA and percentage of TSSM-infested leaflets.
Table 1. Summary of the forward step-wise procedure used to build a multiple regression model for the spatial coincidence (IcTSSM) between two-spotted spider mites (TSSM) and aphids (A).

The model assumes a poisson distribution of errors and uses the log-link function. The change in deviance after inclusion of a term in the model was tested through a log-likelihood ratio test (P < 0.05).
The spatial coincidence index between aphids and TSSM increased together with the percentage of TSSM-infested leaflets. Complete coincidence (ICA = 1) was registered from 40% of TSSM-infested leaflets (fig. 1d). This was the only significant variable (table 2) when it was added to the normal-log model, demonstrating that this relationship is linear and not curvilinear (r 2 = 0.71, F1,68 = 167.13, P < 0.001). The model explained 64% (total deviance change was 5.30 out of 8.28) of the variation in aphid spatial coincidence index (table 2). Sample period, time of sampling and type of crop cover did not affect the coincidence indices.
Table 2. Summary of the forward stepwise procedure used to build a multiple regression model for the spatial coincidence (IcA) between aphids (A) and two-spotted spider mites (TSSM).
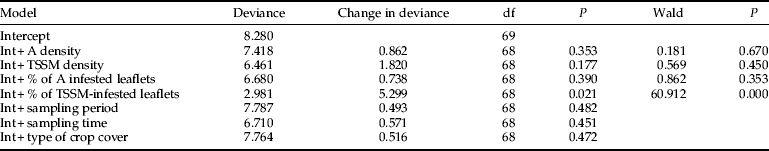
The model assumes a normal distribution of errors and uses the log-link function.
The change in deviance after inclusion of a term in the model was tested through a log-likelihood ratio test (P < 0.05).
When there was spatial coincidence between aphids and TSSM at the leaflet level, each population reached its highest density at the lowest density level of the other (fig. 2a). In most cases, the TSSM coincided with very few aphids on the same leaflet and vice versa (fig. 2b).

Fig. 2. (a) Relationship between aphid density (number of aphids/leaflet) and TSSM density (number of active Tetranychus urticae/leaflet) and (b) relationship between the number of aphids and the number of active T. urticae on the same leaflet.
The intrinsic rate of increase of TSSM and C. fragaefolii (singly and in combination)
The mean rate of increase of TSSM in pure culture (r = 0.39 ± 0.028 measured over seven days) (±SE, N = 20) was marginally but significantly diminished, 3%, in the presence of C. fragaefolii (r = 0.378 ± 0.01, N = 20) (Levene test: F = 0.014 df = 1, 38, P = 0.906; ANOVA: F = 8.08, df = 1, 38, P = 0.0071). In contrast, the mean intrinsic rate of growth of C. fragaefolii in pure culture (r = 0.19 ± 0.009, N = 20) was not reduced in the presence of TSSM (r = 0.20 ± 0.004, N = 20) (Kruskal-Wallis: H(1,N = 49) =0.11, P = 0.74).
The number of individuals settled on the abaxial side of the leaflet was higher than on the adaxial side in pure cultures of T. urticae (Kruskal-Wallis: H(1,N = 40) = 30.41, P < 0.05) and C. fragaefolii (Kruskal-Wallis: H(1,N = 40) = 29.60, P < 0.05). The number of aphids on the adaxial side of leaflets in mixed culture was significant higher than the number of aphids on the adaxial side in pure culture (Levene test: F = 0.81, df = 1, 37, P = 0.37; ANOVA F = 11.78, df = 1, 37, P = 0.001). The number the TSSM on the adaxial side was similar in pure and mixed cultures (Levene test: F = 3.97, df = 1, 38, P = 0.053; ANOVA F = 0.17, df = 1, 38, P = 0.678).
Preference assays
TSSM showed preference for strawberry discs without honeydew (Kruskal-Wallis: H(1,N = 30) = 22.55, P < 0.05) (fig. 3). The number of eggs per TSSM female was higher on discs without honeydew (Kruskal-Wallis: H(1,N = 30) = 17.36, P < 0.05). In some replicates, when discs had honeydew, leaflet hairs were chosen by females to lay the eggs (fig. 4). Strawberry discs without TSSM damage were preferred by aphids (Kruskal-Wallis: H(1,N = 30) = 22.41, P < 0.05) (fig. 4). No aphid offspring were observed on either kind of disc.

Fig. 3. Results of no-choice test showing percentage of TSSM, Tetranychus urticae, and aphid, Chaetosiphon fragaefolii, females on strawberry discs with honeydew and mites damage, respectively. (−HD, discs without honeydew; +HD, disc with honeydew; −D, discs without Tetranychus urticae damage; +D, discs with Tetranychus urticae damage).

Fig. 4. Eggs of Tetranychus urticae on a hair from the abaxial surface of a strawberry leaflet with honeydew.
In no-choice experiments, the location of TSSM females on the abaxial disc surface, the adaxial disc surface or any other place in the Petri dish was not independent of the kind of disc, both with honeydew and without honeydew (X2 = 8.23, df = 2, P = 0.016). Subdividing a contingency table (Zar, Reference Zar1996) and ignoring data of individuals on any other place in the Petri dish, the results indicated that the position of individuals was independent of the kind of disc (X2 = 0.292, df = 1, P = 0.588). This evidenced that there were more individuals on any other place in the Petri dish when discs had honeydew and suggests that, when this occurred, TSSM started wandering in search of another food source. The mean number of TSSM eggs per female on discs without honeydew was higher (11.69 ± 0.69) than that on discs with honeydew (2.96 ± 0.49) (Levene test: F = 3.49 df = 1, 28 P = 0.07; ANOVA: F = 102.58, df = 1, 28, P < 0.05). The location of aphids on the abaxial disc surface, the adaxial disc surface or any other place in the Petri dish was dependent on kind of disc, with honeydew and without honeydew (X2 = 71.29, df = 2, P < 0.001). Subdividing the contingency table and ignoring data of individuals on any other place in the Petri dish, the results indicated that the position of individuals was also dependent on kind of disc (X2 = 61.31, df = 1, P < 0.001). In this case, the results show that more individuals were located on any other place in the Petri dish and on the adaxial surface when discs had TSSM damage. No aphid offspring were observed on either kind of disc.
Discussion
Ecological theory assumes that competition occurs mainly between members of the same guild (Pianka, Reference Pianka1983); however, studies have demonstrated that herbivores in different guilds can exert strong effects on each other (Kaplan & Denno, Reference Kaplan and Denno2007; Kaplan et al., Reference Kaplan, Sardinelli and Denno2009). Indeed, temporally and spatially separated competitive effects mediated by the host plant appear to be common among phytophagous insects (Faeth, Reference Faeth1986; Masters & Brown, Reference Masters, Brown, Gange and Brown1997). Although TSSM and aphids feed on different parts of the leaf and exploit different resources (epidermal cell content and phloem sap, respectively) the results derived from this study suggest a negative interaction between them.
The coincidence between TSSM and aphids populations in strawberry crop was, in general, high. During one of the study periods, the coincidence was low, probably because it covered the winter when these herbivores are absent or at very low density (Greco et al., Reference Greco, Llijesthrom and Sánchez1999; Cédola & Greco, Reference Cédola and Greco2010). In field and greenhouse surveys, the main factor that affected the coincidence indices at the leaflet level was the percentage of TSSM-infested leaflets. The cause of this could be that TSSM would have a greater capacity for population growth and greater ability to move than aphids, so they would occupy new leaflets before aphids do. The proportion of TSSM that coincided with at least one aphid decreased as the percentage of TSSM-infested leaflets increased. The cause for this could be that when TSSM population is higher, the proportion of individuals that coincide with at least one aphid is lower. On the other hand, the proportion of aphids that coincided with at least one TSSM increased as the percentage of TSSM-infested leaflets also increased. Bearing in mind that TSSM density was always higher than aphid density, the probability of an aphid coinciding with at least one TSSM is expected to be higher as TSSM density, and consequently the percentage of mite-infested leaflets, increase. The sampling time did not affect the coincidence at leaflet level; therefore, we found no evidence for seasonal effects independent of density.
Sharing the same leaflet caused disturbances for both species. TSSM showed a lower increase rate when they shared the same leaflet with C. fragaefolii, as well as lower fecundity on strawberry discs with honeydew. Some compound in honeydew or the saliva of aphids may be toxic (Miles, Reference Miles1999) and can cause changes in leaf quality (Dixon & Wratten, Reference Dixon and Wratten1971; Cammell, Reference Cammell1981). An initial piercing before ovipositing allows TSSM to assess host-plant quality; nevertheless, females might settle on an unfavourable resource and have low fecundity (Yano et al., Reference Yano, Wakabayashi, Takabayashi and Takafuji1998).
There are antecedents of negative interactions between phloem feeders and other herbivores. Soroker et al. (Reference Soroker, Grinberg, Adad, Katabi, Perl-Treves and Walling2010) found that whiteflies negatively affect the development of broad mite populations on tomato plants, and some negative effects of broad mites on whiteflies were also observed. Arouni et al. (Reference Arouni, Garrido, Carbonell, Pérez-Panadés, Muñoz, Jacas, Urbaneja and Hermoso de Mendoza2008) observed negative interactions between citric aphids, Aphis spiraecola Patch, and leafminers, Phyllocnistis citrella Stainton, and a short coexistent period in which leafminer survival was lower on leaves with aphid infestations.
The rate of growth of C. fragaefolii did not change, but individuals moved to the other side of the leaflets when they were with TSSM. Furthermore, the damage on strawberry leaflets produced after T. urticae feeding could interfere with the normal stylet pathway of C. fragaefolii in plant tissues. No offspring were produced on leaf discs with TSSM damage; however, the same behaviour was observed in discs without damage, suggesting that the experimental conditions could affect this variable. Many plant species emit volatiles in response to herbivory. Secondary metabolites or allelochemicals (e.g. alkaloids, phenolic compound, glucosinolates) are mediators in the response to herbivores attack (Karban & Baldwin, Reference Karban and Baldwin1997). Tetranychus urticae is a generalist that can feed on several hundred host plant species, and its feeding induces emission of volatiles by certain plants including strawberry (Karban & Baldwin, Reference Karban and Baldwin1997; van den Boom et al., Reference van den Boom, van Beek, Posthumus, de Groot and Dicke2004). The landing of flying aphids on a host plant involves a sequence of visual cues to give specific information, but the landing response may be modified by plant volatiles (Powell et al., Reference Powell, Tosh and Hardie2005). In this study, there were no measurements of volatile compounds, but we suspect that phytochemical cues from T. urticae feeding may determine aphid behaviour in the field and in laboratory experiments.
Phenolic compounds are known to play an important role in mite-strawberry relationships (Luczynski et al., Reference Luczynski, Isman and Raworth1990). Kielkiewicz & van de Vrie (Reference Kielkiewicz and van de Vrie1983) suggested that displacement of phenolics to the places of mite penetration and/or their synthesis ‘de novo’ in these places might be one of the factors which reduce mite injury to leaves. This mechanism could affect aphids such as Zucker (Reference Zucker1982) observed.
Several studies involving temporally or spatially separated species in different feeding guilds (summarized in Kaplan & Denno, Reference Kaplan and Denno2007) suggest that traditional resource-based competition theory may underestimate the frequency and intensity of within trophic level interactions. Negative interactions such as demonstrated herein can affect distribution of herbivorous arthropods (Faeth, Reference Faeth1986). Studies of negative interactions can offer important insights into how interactions structure ecological communities.
Acknowledgements
We thank strawberry growers J. Campagnucci, H. Marengo, S. Parrillo and Evangelina Zembo for permission to carry out the field work at their farms, and M. Roggiero, G. Reboredo, N. Cluigt, S. Ambrosio and M. Basiglio for field assistance. For comments on this work during preparation, we are grateful to N.E. Sánchez. We greatly appreciate critisisms and comments from two anonymous reviewers. This research was supported by grants from Universidad Nacional de La Plata N 453 2005–2008, N 576 2009–2011 and CONICET (PIP 02108/2005, PIP 1182/2008).